Abstract
Renewable energy, including biofuels such as ethanol and butanol from syngas bioconversed by Clostridium carboxidivorans P7, has been drawing extensive attention due to the fossil energy depletion and global eco-environmental issues. Effects of zinc on the growth and metabolites of C. carboxidivorans P7 were investigated with model syngas as the carbon source. The cell concentration was doubled, the ethanol content increased 3.02-fold and the butanol content increased 7.60-fold, the hexanol content increased 44.00-fold in the medium with 280 μM Zn2+, when comparing with those in the control medium [Zn2+, (7 μM)]. Studies of the genes expression involved in the carbon fixation as well as acid and alcohol production in the medium with 280 μM Zn2+ indicated that fdhII was up-regulated on the second day, acs A, fdhII, bdh35 and bdh50 were up-regulated on the third day and bdh35, acsB, fdhI, fdhIII, fdhIV, buk, bdh10, bdh35, bdh40 and bdh50 were up-regulated on the fourth day. The results indicated that the increased Zn2+ content increased the alcohol production through increase in the gene expression of the carbon fixation and alcohol dehydrogenase.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Currently, renewable energy has been drawing extensive attention due to the excess exploitation and use of fossil fuels, which not only threatens energy safety, but also causes serious environmental pollution [7, 35]. On the other hand, there are millions of tons of waste produced in the industrial society and in our daily life, such as stalk, food waste, waste gas etc. So, waste-to-energy was recognized as a good way to generate bioenergy along with solving the environmental problems.
Some industrial processes, such as oil refining [15], steelmaking and the production of carbon black, methanol and coke [16], gasification and pyrolysis [1, 17], will release a large amount of waste gases, and the main components are CO and H2 (syngas). These waste gases can be used as raw materials for biological conversion into biofuels [30] through fermentation. Syngas fermentation is a process by which microorganisms use a mixture of H2, CO, and CO2 to produce fuels and chemicals such as ethanol, butanol, hexanol, acetic acid, butyric acid, and methane [5, 20, 22, 29, 38]. The industrial utilization of syngas can accelerate the recovery of carbon resources and decrease the dependence on fossil fuels. Syngas fermentation may also meet the strategic needs of the low-carbon development path. It not only has remarkable economic effects, but demonstrates significant ecological and environmental benefits. Therefore, the studies for syngas fermentation are particularly necessary.
A class of anaerobic microorganisms named acetogens can autotrophically use H2 + CO2 or/and CO as carbon sources and can synthesize chemicals, such as ethanol, and butanol, making it an ideal catalyst for microbial syngas fermentation [13]. Acetogens use acetyl coenzyme A (acetyl-CoA) pathway, also known as the Wood–Ljungdahl pathway, to convert C1 substrates into acetyl-CoA. This approach is considered the oldest discovered carbon fixation pathway. This pathway uses an important CO2/CO synthetic intermediate metabolite, acetyl-CoA, and CO or CO2 fixation can be achieved. This is accompanied by the transfer of energy during carbon fixation, whereas the carbon-immobilized product, acetyl-CoA, participates in the formation of the cytoskeleton [9]. The methyl and carbonyl groups obtained from the two branches of the Wood–Ljungdahl pathway bind to coenzyme A to produce an important intermediate, acetyl-CoA, which is further converted to organic acids and alcohols. Acetyl-CoA can be directly converted to acetic acid and ethanol, whereas butyric acid and butanol are directly produced from butyryl-CoA [11].
Well-studied acetogens in the laboratory and industry include Butyribacterium methylotrophicum, Clostridium autoethanogenum, Clostridium carboxidivorans, Clostridium ljungdahlii, Clostridium ragsdalei, and Eubacterium limosum [36]. Among these commonly used strains, C. carboxidivorans can use syngas to produce ethanol and higher alcohols, i.e., butanol and hexanol [6, 10]. However, biofuels from C. carboxidivorans P7 remains far from industrialization, which is attributed by reasons such as low gas–liquid mass transfer efficiency [12], difficulties in expanding the scale of fermentation, low rate of CO absorption and poor efficiency of conversion to alcohols [5, 37]. So far, efforts have been focused on solving these problems by increasing gas velocity, gas–liquid interface and gas solubility (by pressure and surfactant) to improve the efficiency of gas–liquid mass transfer [4]. In addition, domesticated strains with shortened fermentation times and improved CO tolerance will improve the fermentation efficiency.
Zinc is an essential nutrient for all the living organisms, which affects both cell growth and metabolism. Although zinc plays an important role in bacteria, high concentrations of zinc can have toxic effects on the bacteria. First, excess zinc ions compete with other metal ion-binding sites to inactivate the protein. Second, zinc can also form hydroxyl radicals, and cause damage to the DNA, protein and lipids. In addition, high concentrations of zinc ions directly inhibit the electron transport chain during electron transfer, causing respiratory effects that cause damage [14]. Therefore, in bacteria, zinc ions must be tightly regulated [27].
There were several studies reported that suitable zinc supplementation in the culture medium would be beneficial for ethanol production [28, 42]. Zhao et al. [42] found that the ethanol tolerance, thermal tolerance and ethanol production were significantly improved by zinc supplements. However, no study on the gene expression changes in C. carboxidivorans P7 resulting from zinc supplementation along with ethanol fermentation was reported.
The effects of zinc stress on C. carboxidivorans P7 growth, CO uptake and chemicals production were studied in detail, as well as the expression levels of related genes were studied. This study will help to elucidate the effects and mechanism of zinc on bio-alcohols production and will provide a theoretical basis for the synthesis of bio-alcohols.
Materials and methods
Strain, medium and seed culture
Clostridium carboxidivorans P7 was a kind gift from the Professor Yang Gu. Laboratory stocks and they were maintained at – 80 °C. It was inoculated into the Wilkins Chalgren Anaerobe Broth, containing tryptone 10 g/L, fish peptone 10 g/L, yeast extract 5 g/L, glucose 2 g/L, NaCl 5 g/L, l-arginine 1 g/L, sodium pyruvate 1 g/L, l-cysteine 0.3 g/L (Adjust pH to 6.0 with solid NaOH), and 1 mL/L stock c (0.1 g NaHCO3 + 100 mL H2O), 10 mL/L stock a (0.5 g of hemin + 10 mL 1 M NaOH + 990 mL H2O), and 200 µL/L stock b (0.05 mL vitamin K1 solution + 20 mL 95% ethanol) [26] (filter sterilized, and medium components added after sterilization) [19, 26]. The inoculation ratio was 5% (v/v), and the seed culture was incubated upright in the anaerobic chamber at 37 °C for 36 h until exponential phase. Subculture was performed for approximately 12 h to achieve the exponential phase of cell growth. The gas fermentation medium contained NaCl 2.4 g/L, NH4Cl 3 g/L, KCl 0.3 g/L, KH2PO4 0.3 g/L, MgSO4 0.6 g/L, CaCl2 0.12 g/L, l-cysteine·HCl 0.2 g/L, l-cysteine 0.2 g/L, yeast extract 0.5 g/L, MES 5 g/L, trace element solution (nitrilotriacetic acid 10 g/L, MnSO4 5 g/L, (NH4)2SO4·FeSO4·6H2O 4 g/L, CoCl2·6H2O 1 g/L, CuCl2·2H2O 0.1 g/L, NiCl2·6H2O 0.1 g/L, Na2MoO4·2H2O 0.1 g/L, Na2SeO4 0.1 g/L, Na2WO4·2H2O 0.1 g) 2 mL/L, (adjust pH to 6.0 with NaOH), and 1 mL/L vitamin solution (VB6 0.1 g/L, thiamine 0.05 g/L, VB2 0.05 g/L, calcium pantothenate 0.05 g/L, thioacetic acid 0.05 g/L, p-aminobenzoic acid 0.05 g/L, nicotinic acid 0.05 g/L, VB12 0.05 g/L, biotin 0.02 g/L, and folic acid 0.02 g/L) (filter sterilized through a 0.22 μM filter). The inoculation ratio was 5% (v/v). All the media were sterilized at 121 °C for 20 min, if there is no more statement of filter sterilization.
Fermentation
To study the effect of Zn2+ on the syngas fermentation of strain P7, different concentrations of ZnSO4·7H2O (0, 7, 70, 140, 280 µM) were added to the fermentation medium.
Serum bottles containing 20 mL of the fermentation media was inoculated with 5% (v/v) of C. carboxidivorans P7 seed culture. Artificial syngas (50% CO/35% CO2/15% H2) was purged with a headspace pressure of 0.2 MPa after inoculation and every 24 h afterwards, and 1.5 mL samples were collected every 24 h before re-purge of the syngas in 4 days. The OD at 600 nm of 0.5 mL fermented broth was determined by a spectrophotometer (UV-1800) and expressed as OD600 for cell concentration. And 1 mL samples were centrifuged at 6000 rpm, 4 °C for 10 min. The supernatants were used to determine the content of alcohol and acid. And the pellet from the fermentation medium with 280 µM of zinc as the experimental group and 7 µM of zinc as the control group were transferred to – 80 °C and kept for RNA extraction and Q-RT-PCR analysis.
RNA extraction and cDNA synthesis
The extraction of C. carboxidivorans P7 total RNA was performed by using RNAprep pure Cell/Bacteria Kit (TIANGEN BIOTECH, CN). Residual of DNA was evaluated by PCR with universal primers of bacteria 16s rRNA sequence [31]. First chain of cDNA was obtained by using FastQuant RT Super Mix (TIANGEN BIOTECH, CN) and stored at – 20 °C.
Gene expression detection via Q-RT-PCR
Quantitative real-time PCR (Q-RT-PCR) was performed for selected genes including carbon fixation, acidogenesis and solventogenesis pathways (supplemental materials S1) in different samples. The reactions were conducted with SYBR® Select Master Mix (Applied Biosystems, Austin, USA) for supporting the validity of the RNA-seq data. The LightCycler 96 real-time PCR system (Roche Diagnostics, Mannheim, Germany) was used to amplify and quantify the PCR of the products. The program was as follows: 5 min at 95 °C, followed by 40 amplification cycles of 10 s at 95 °C, 30 s at 60 °C. Transcript abundance of the target genes were normalized with the reference gene guk encoding guanine kinase [21]. Relative levels of transcript abundance of the studied genes were calculated with the 2−∆∆CT method [23]. Quantitative real-time PCR of each gene was performed with three reactions in parallel. The experiments were confirmed with biological triplicates.
Determination of the products
Fermentation metabolites, including ethanol, butanol, hexanol, acetic acid, and butyric acid, were measured with an internal standard method using a gas chromatograph equipped with a flame ionization detector and capillary column (Zebron ZB-WaxPlus, Phenomenex, USA) [18, 41]. Samples from fermentation were prepared by centrifuging at 12,000×g for 5 min and filtrated with 0.22 µm filters. The program was as follows: oven temperature 150 °C, injector temperature 180 °C, and detector temperature 180 °C. The internal standards were isobutanol, isobutyric acid and hydrochloric acid (acidified with 1 M HCl).
Determination of CO utilization
The CO concentration (C 0) was determined by gas chromatography with a TDX-01 80/100 column and thermal conductivity detector. The program was as follows: oven temperature 180 °C, detector temperature 150 °C, and injector temperature, 80 °C. Samples with known concentrations of CO were used to construct a standard curve.
The CO gas volume (V) was determined using the drainage method at room temperature and atmospheric pressure. A 200 mL test tube full of water was upside down in a beaker full of water. And the gas in the serum bottle after fermentation was introduced to the test tube and the liquid surface of the test tube and serum bottles were kept at the same level until stable. The CO volume (V) was calculated as the sum of the gas volume in the serum bottle and the gas volume in the test tube which was introduced from the serum bottles.
The total CO utilization was calculated as follows:
The product conversion rate (P rate) was defined as the ratio of the maximum alcohol content and the CO utilization, as indicated by the following equation:
where c max, x max and M max are the maximum product yield, the time corresponding to the maximum yield of the product, and the total amount of CO absorbed at this time, respectively.
Analysis of the data
All the experiments were conducted three times and the results presented were the mean values of the three samples and the standard deviation was showed.
Results and discussion
Effects of zinc on CO utilization and product yield by C. carboxidivorans P7
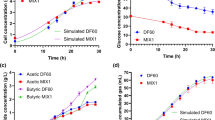
The cell concentration (Fig. 1), CO utilization (Fig. 2a), product yield (Fig. 2b, c) and CO conversion rate (Fig. 2d) of C. carboxidivorans P7 in different zinc concentrations have been studied. The cell concentration, product yield and CO conversion rate of C. carboxidivorans P7 improved by increasing the zinc concentration. The cell concentration, ethanol production, butanol production, hexanol production and total CO conversion rate in medium with 280 μM Zn2+ increased 1.03 times, 3.02 times, 7.60 times, 44.00 times and 1.83 times, when compared with the control medium with 7 μM Zn2+.
Similar results was also found in C. acetobutylicum. Production of acetone, ethanol, and butanol was improved with zinc supplementary, which was attributed to the rapid acid’s re-assimilation for more efficient ABE production [39].
Effects of zinc on gene expression of C. carboxidivorans P7
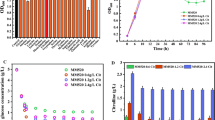
Figure 3 shows the gene expression of C. carboxidivorans P7 metabolic pathway associated with the CO dehydrogenase-related gene, in which expression of gene coocII was not detected. The CO-related genes were significantly down-regulated (P < 0.01) in medium containing 280 μM Zn2+ compared with control medium, except for the acsB gene (Fig. 3a, c, f). In the early growth phase (the 1st day), the effect of Zn2+ was not obvious in the low cell concentration (Fig. 1). The next day, the expression levels of all the genes decreased (P < 0.05), and this trend was likely due to the depletion of glucose which was carried in by inoculation and was adapting to the carbon source of CO [3]. On the 3rd day, the expression levels of all the genes increased, and the expression of the acsA gene (Fig. 3a) was significantly up-regulated in the high Zn2+ treatment (P < 0.05). However, the expression of the coocI (Fig. 3f) gene was significantly down-regulated (P < 0.05). Until the 4th day, CO-related gene (acsA, acsD, acsE) expression decreased significantly, but the expression of the acsB gene (Fig. 3b) was significantly up-regulated in medium with 280 μM Zn2+ (P < 0.01). In general, changes in the expression levels of the CO-related genes under different zinc treatments were similar to those of the control group. The significant increase of the acsA gene on the 3rd day and acsB gene expression content on the 4th day were the main reasons for the improvement of CO utilization (Fig. 3a, b).
Figure 6 shows the relative expression of the formate dehydrogenase-related gene in the C. carboxidivorans P7 metabolic pathway. The formate dehydrogenase-related genes under different Zn2+ treatments of the 1st day were significantly inhibited (P < 0.05). The expression of the fdhII (Fig. 4b) gene was significantly increased (P < 0.05) on the 2nd day, and the other genes, such as fdhI, fdhIII, fdhIV, and fdhV (Fig. 4a, c–e) did not change significantly (P > 0.05) in the high Zn2+ treatment, when comparing to that with the control treatment. On the 3rd day of the cultivation, only the expression of fdhI and fdhII (Fig. 4a, b) increased significantly (P < 0.05). On the 4th day, the expression of fdhIII and fdhIV (Fig. 4c, d) were significantly up-regulated (P < 0.05), and among them, fdhIII (Fig. 4c) was very significant (P < 0.01). The results indicated that the gene for formate dehydrogenases up-regulated was expressed under the high Zn2+ treatment. Thus, the high Zn2+ treatment has a sustained stimulating effect on the formic acid dehydrogenase gene, whereas the control group gene expression trend did not have a obvious change during the studies.
FDH is the key enzyme for the reduction of CO2 to formate in C. formicoaceticum and C. thermoaceticum [2, 40]. Five types of FDH have been found in C. carboxidivorans P7 and most of them were up-regulated, and then attributed to the capacity for carbon fixation.
Figure 5 shows the relative expression of the acetic acid/butyric acid kinase-related gene in the C. carboxidivorans P7 metabolic pathway. The trend for the acetic acid kinase gene expression of the control group first increased, whereas high Zn2+ treatments were basically unchanged (Fig. 5a). Expression of the acetic acid kinase gene was significantly down-regulated on the 1st day compared to the control group (P < 0.05). The expression of the butyric acid kinase gene (Fig. 5b) in the control group was stable. However, it increased under the high zinc treatment from the 2nd to 4th day, except the significant decrease on the 1st day (P < 0.05). The relative expression of the gene on the 3rd day and the 4th day was significantly increased (P < 0.05).
More ATPs can be produced during the organic acid formation comparing with that during alcohol formation, which will be helpful for cell growth [33]. The acetic acid kinase gene expression did not change after zinc supplementation, which was verified by the acetic acid production. The expression of the butyric acid kinase gene was up-regulated than that of the control, however, the butyric acid production did not change during cultivation significantly. This may be caused by the up-regulation of the gene expression that did not lead to the up-regulation of the butyric acid kinase activity, or there may be more butyric acid produced which was utilized for butanol production.
Figure 6 shows the relative expression of the alcohol dehydrogenase and acetyl-CoA transferase-related genes in C. carboxidivorans P7 metabolic pathway. The alcohol dehydrogenase genes (Fig. 6a) in the control group and experimental group have changed similarly, first increasing and then decreasing. The relative expression of acetyl-CoA transferase (Fig. 6f) did not change significantly, but gene expression under high zinc treatment showed an increasing trend. The genes bdh10, bdh35, bdh40, bdh50 (Fig. 6b–e) related to butanol dehydrogenase except bdh10 were significantly lower on the 1st day for the high zinc treatment, when compared with the control group (P < 0.05), and the others showed no significant change in the first 2 days. The expression levels of bdh35 (Fig. 6c) and bdh50 (Fig. 6e) were up-regulated on the 3rd day and 4th day, and the expression of the butanol dehydrogenase gene was significantly up-regulated on the 4th day, compared with the control group (P < 0.05). In particular, the bdh35 gene (Fig. 6c) was significantly different on the 4th day (P < 0.05).
Alcohol dehydrogenase (Fig. 6a) and acetyl coenzyme A transferase gene (Fig. 6f) expression levels did not change significantly, compared with the control group, whereas the production increased. The synthesis of ethanol and the acetic acid precursor acetyl-CoA content were significantly increased, comparing with that of the control group, suggesting that carbon fixation was improved under the high zinc treatment.
In general, high zinc levels led to delayed growth on the 1st day, however, most of the metabolic processes and gene expression levels were significantly higher than in the control on the following days, indicating increased fermentation activity.
A large number of protein constituents are active in the presence of zinc, in which some of the zinc is involved in the active center of the cell signaling pathway that constitutes the transcription factor [34]. The zinc supplementation is beneficial to the production of ethanol, but the mechanism of high production is different in different references [8, 24, 32].
Studies considered that zinc not only contributes to the rapid use of glucose and increase the cell growth, but also promotes the re-assimilation of acid and butanol production, and promotes the initiation of C. acetobutylicum solvent generation [39]. They found that zinc has a significant impact on the central carbon metabolism of hydrazine, aad, adhE2, cac3292, cap0059, thLB and other genes, suggesting that zinc can significantly enhance the acetone butanol process, thereby promoting acid generation and solvent generation [39].
Our results indicated that zinc supplementation stimulated cell growth and alcohol production. The quantitative fluorescence analysis results indicated that zinc supplementation also stimulated most of the metabolic fermentation pathways of the genes, such as acsA (Fig. 3a), acsB (Fig. 3b), fdhI (Fig. 4a), fdhII (Fig. 4b), fdhIII (Fig. 4c), fdhIV (Fig. 4d), buk (Fig. 5b), bdh10 (Fig. 6b), bdh35 (Fig. 6c), bdh40 (Fig. 6d) and bdh50 (Fig. 6e), thus affecting the synthesis of alcohol.
Zinc supplementation increased the product yield maybe due to the promotion of carbon fixation. From the genetic level, the expression of the alcohol dehydrogenase gene (Fig. 6a), the acetyl-CoA transferase gene (Fig. 6f) and acetate kinase (Fig. 5a) were not increased significantly by zinc supplementation. However, it stimulated the ethanol production (Fig. 2b). Zinc can increase the amount of acetyl-CoA, and the increase in acetyl-CoA is due to carbon fixation. The increase of CO absorption and the increase of the conversion rate indicated that zinc supplementation promotes the uptake and transformation of CO, leading to an increase of acetyl-CoA content in the precursor of synthetic ethanol, which eventually leads to the increase of ethanol content.
Zinc is a key factor affecting the biological function of butanol dehydrogenase, which is a key enzyme responsible for the synthesis of butanol [39]. The results indicated that zinc supplementation also stimulated the expression of butanol dehydrogenase gene (Fig. 6b–e) and butyrate kinase gene (Fig. 5b). Thus, butyric acid, whose production was increased by the increased expression of butyrate kinase gene, was converted to butanol. Then, the increased ratio of butanol was higher than that of ethanol.
Zinc supplementation maybe double-edged, i.e., some enzyme activities can be improved, but some maybe inhibited [25]. We found that after adding zinc, most of the genes on the 1st day are inhibited to varying degrees, and the need for an adaptive process of gene expression can be restored to normal levels and even significantly increased.
These results indicated that zinc supplementation contributes to carbon fixation and the synthesis of alcohol-related gene expression. Zinc supplementation can change the ratio of acid and alcohol in the fermentation broth; therefore, it is important for increasing the substrate utilization, reducing production costs, and improving biofuel production.
Conclusions
Through the combinational analysis of CO concentration, fermentation metabolites and gene expression, we demonstrated for the first time that, zinc regulates the de novo synthesis of alcohols rather than acid re-assimilation in this strain. This knowledge helps fine-tune the medium composition to improve alcohol production, and also identifies potential genetic targets for future bio-engineering to optimize the C1-based bio-chemical synthesizing platform.
References
Al-Rahbi AS, Williams PT (2017) Hydrogen-rich syngas production and tar removal from biomass gasification using sacrificial tyre pyrolysis char. Appl Energy 190:501–509
Andreesen JR, El Ghazzawi E, Gottschalk G (1974) The effect of ferrous ions, tungstate and selenite on the level of formate dehydrogenase in Clostridium formicoaceticum and formate synthesis from CO2 during pyruvate fermentation. Arch Microbiol 96:103–118
Boeriu CG, Corici LN, Frissen AE, Verhoeven HA, Beekwilder MJ (2015) Process for reducing carbon dioxide to methanol. WO/2015/097020.
Bredwell MD, Srivastava P, Worden RM (1999) Reactor design issues for synthesis-gas fermentations. Biotechnol Prog 15:834–844
Brown RC, Brown TR (2013) Biorenewable resources: engineering new products from agriculture. Wiley, New York
Bruant G, Lévesque MJ, Peter C, Guiot SR, Masson L (2010) Genomic analysis of carbon monoxide utilization and butanol production by Clostridium carboxidivorans strain P7. PLoS One 5:e13033
Campbell CJ, Laherrère JH (1998) The end of cheap oil. Sci Am 278:78–83
Chandrasena G, Walker GM, Staines HJ (1997) Use of response surfaces to investigate metal ion interactions in yeast fermentations. Am Soc Brew Chem 55:24–29
Drake HL, Gössner AS, Daniel SL (2008) Old acetogens, new light. Ann N Y Acad Sci 1125:100
Fernández-Naveira Á, Abubackar HN, Veiga MC, Kennes C (2017) Production of chemicals from C1 gases (CO, CO2) by Clostridium carboxidivorans. World J Microbiol Biotechnol 33:43
Hädicke O, Grammel H, Klamt S (2011) Metabolic network modeling of redox balancing and biohydrogen production in purple nonsulfur bacteria. BMC Syst Biol 5:150
Henstra AM, Sipma J, Rinzema A, Stams AJM (2007) Microbiology of synthesis gas fermentation for biofuel production. Curr Opin Biotechnol 18:200–206
Kai S, Müller V (2016) Energetics and application of heterotrophy in acetogenic bacteria. Appl Environ Microbiol 82:4056–4069
Iqbal M, Kenney PB, Al-Humadi NH, Klandorf H (2000) Relationship between mechanical properties and pentosidine in tendon: effects of age, diet restriction, and aminoguanidine in broiler breeder hens. Poult Sci 79:1338–1344
Johansson D, Franck P-Å, Berntsson T (2012) Hydrogen production from biomass gasification in the oil refining industry—a system analysis. Energy 38:212–227
Johansson MT, Söderström M (2011) Options for the Swedish steel industry—energy efficiency measures and fuel conversion. Energy 36:191–198
Li D, Chen L, Zhang X, Ye N, Xing F (2011) Pyrolytic characteristics and kinetic studies of three kinds of red algae. Biomass Bioenergy 35:1765–1772
Li D, Zhang K, Chen L, Ding M, Zhao M, Chen S (2017) Selection of Schizochytrium limacinum mutants based on butanol tolerance. Electron J Biotechnol 30:58–63
Li N, Yang J, Chai C, Yang S, Jiang W, Gu Y (2015) Complete genome sequence of Clostridium carboxidivorans P7 T, a syngas-fermenting bacterium capable of producing long-chain alcohols. J Biotechnol 211:44–45
Liou JSC, Balkwill DL, Drake GR, Tanner RS (2005) Clostridium carboxidivorans sp. nov., a solvent-producing clostridium isolated from an agricultural settling lagoon, and reclassification of the acetogen Clostridium scatologenes strain SL1 as Clostridium drakei sp. nov. Int J Syst Evol Microbiol 55:2085–2091
Liu J, Tan Y, Yang X, Chen X, Li F (2013) Evaluation of Clostridium ljungdahlii DSM 13528 reference genes in gene expression studies by qRT-PCR. J Biosci Bioeng 116:460–464
Liu K, Atiyeh HK, Stevenson BS, Tanner RS, Wilkins MR, Huhnke RL (2014) Continuous syngas fermentation for the production of ethanol, n-propanol and n-butanol. Bioresour Technol 151:69–77
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408
Maddox IS, Hough JS (2013) Effect of zinc and cobalt on yeast growth and fermentation. J Inst Brew 76:262–264
Maret W, Yetman CA, Jiang LJ (2001) Enzyme regulation by reversible zinc inhibition: glycerol phosphate dehydrogenase as an example. Chem Biol Interact 130–132:891–901
Matson EG, Zhang X, Leadbetter JR (2010) Selenium controls transcription of paralogous formate dehydrogenase genes in the termite gut acetogen, Treponema primitia. Environ Microbiol 12:2245–2258
Patzer SI, Hantke K (1998) The ZnuABC high-affinity zinc uptake system and its regulator Zur in Escherichia coli. Mol Microbiol 28:1199–1210
Phillips JR, Atiyeh HK, Tanner RS, Torres JR, Saxena J, Wilkins MR, Huhnke RL (2015) Butanol and hexanol production in Clostridium carboxidivorans syngas fermentation: medium development and culture techniques. Bioresour Technol 190:114–121
Phillips JR, Clausen EC, Gaddy JL (1994) Synthesis gas as substrate for the biological production of fuels and chemicals. Appl Biochem Biotechnol 45–46:145–157
Saha BC, Woodward J (1997) Fuels and chemicals from biomass, vol 666. American chemical society, Washington, DC
Skillman LC, Evans PN, Strömpl C, Joblin KN (2006) 16S rDNA directed PCR primers and detection of methanogens in the bovine rumen. Lett Appl Microbiol 42:222–228
Tosun A, Ergun M (2007) Use of experimental design method to investigate metal ion effects in yeast fermentations. J Chem Technol Biotechnol 82:11–15
Ukpong MN, Atiyeh HK, De Lorme MJ, Liu K, Zhu X, Tanner RS, Wilkins MR, Stevenson BS (2012) Physiological response of Clostridium carboxidivorans during conversion of synthesis gas to solvents in a gas-fed bioreactor. Biotechnol Bioeng 109:2720–2728
Vallee BL, Auld DS (1993) Zinc: biological functions and coordination motifs. Acc Chem Res 26:543–551
Vasudevan PT, Gagnon MD, Briggs MS (2010) Environmentally sustainable biofuels—the case for biodiesel, biobutanol and cellulosic ethanol. Springer, Amsterdam, pp 43–62
Wilkins MR, Atiyeh HK (2011) Microbial production of ethanol from carbon monoxide. Curr Opin Biotechnol 22:326–330
Worden RM, Bredwell MD, Grethlein AJ (1997) Engineering issues in synthesis-gas fermentations. ACS Symposium Series, vol 666. American Chemical Society, pp 320–335
Worden RM, Grethlein AJ, Jain MK, Datta R (1991) Production of butanol and ethanol from synthesis gas via fermentation. Fuel 70:615–619
Wu YD, Xue C, Chen LJ, Bai FW (2013) Effect of zinc supplementation on acetone–butanol–ethanol fermentation by Clostridium acetobutylicum. J Biotechnol 165:18–21
Yamamoto I, Saiki T, Liu S-M, Ljungdahl LG (1983) Purification and properties of NADP-dependent formate dehydrogenase from Clostridium thermoaceticum, a tungsten-selenium-iron protein. J Biol Chem 258:1826–1832
Zhang K, Chen L, Liu J, Gao F, He R, Chen W, Guo W, Chen S, Li D (2017) Effects of butanol on high value product production in Schizochytrium limacinum B4D1. Enzym Microb Technol 102:9–15
Zhao XQ, Xue C, Ge XM, Yuan WJ, Wang JY, Bai FW (2009) Impact of zinc supplementation on the improvement of ethanol tolerance and yield of self-flocculating yeast in continuous ethanol fermentation. J Biotechnol 139:55–60
Acknowledgements
The authors would like to thank Professor Yang Gu for the bacteria strain and original cultivation recipe. The present study was supported by the National High-tech Research Development Program China and the Ministry of Science and Technology of the People’s Republic of China (2015AA020202), the Youth Innovation Promotion Association CAS, the Key Research and Development Program of Shandong Province (2016GSF121030), the Natural Science Foundation of Shandong province (ZR2011DM006, ZR2011CQ010), the Supporting Project for Young Teachers in Shandong University of Technology (4072–110045, 4072–114021).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, D., Meng, C., Wu, G. et al. Effects of zinc on the production of alcohol by Clostridium carboxidivorans P7 using model syngas. J Ind Microbiol Biotechnol 45, 61–69 (2018). https://doi.org/10.1007/s10295-017-1992-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-017-1992-2