Abstract
E. coli has the ability to ferment both C5 and C6 sugars and produce mixture of acids along with small amount of ethanol. In our previous study, we reported the construction of an ethanologenic E. coli strain by modulating flux through the endogenous pathways. In the current study, we made further changes in the strain to make the overall process industry friendly; the changes being (1) removal of plasmid, (2) use of low-cost defined medium, and (3) improvement in consumption rate of both C5 and C6 sugars. We first constructed a plasmid-free strain SSY13 and passaged it on AM1–xylose minimal medium plate for 150 days. Further passaging was done for 56 days in liquid AM1 medium containing either glucose or xylose on alternate days. We observed an increase in specific growth rate and carbon utilization rate with increase in passage numbers until 42 days for both glucose and xylose. The 42nd day passaged strain SSK42 fermented 113 g/L xylose in AM1 minimal medium and produced 51.1 g/L ethanol in 72 h at 89% of maximum theoretical yield with ethanol productivity of 1.4 g/L/h during 24–48 h of fermentation. The ethanol titer, yield and productivity were 49, 40 and 36% higher, respectively, for SSK42 as compared to unevolved SSY13 strain.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
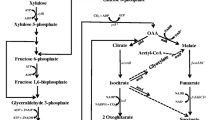
Agricultural biomass represents a promising source of lignocellulosic carbon which can be converted into ethanol using microbial biocatalysts. When compared to gasoline, generation of ethanol from lignocellulosic biomass can result in 60–110% CO2 emission avoidance, while this number is reduced in the range of 10–60% when using corn as an ethanologenic substrate [28]. To make biomass carbon bioavailable for microbial metabolism, it needs to be treated by different physical and chemical methods followed by enzymatic method to release the fermentable sugars [4, 10, 11, 29]. The lignocellulosic biomass consists of cellulose (35–50%), hemicellulose (25–30%) and lignin (15–30%). Cellulose is a linear polymer of glucose while hemicellulose is a branched polymer majorly consisting of xylose with minor amounts of arabinose, mannose, galactose and glucose. Lignin is a phenolic polymer responsible for protecting sugar polymers under changing environmental condition [1, 10, 20, 30]. To make the process economically viable it is necessary to efficiently recover both hexose (mainly glucose) and pentose (mainly xylose) sugars from the biomass. The recovery process involves either alkali or acid pre-treatment to loosen the biomass fibres, followed by addition of both cellulosic as well as hemicellulosic enzymes to hydrolyze the sugar polymers [10, 25, 26].
Usually, naturally occurring microbes are not very efficient at converting both hexose and pentose sugars into ethanol. For example, a typical ethanol-fermenting organism Saccharomyces cerevisiae will only utilize hexose sugar and not pentose sugar. There have been several efforts to engineer microbes to convert both hexose and pentose sugars into ethanol. This has been done by two mechanisms—(1) by introducing pathway for pentose utilization in the native ethanol producer and (2) by introducing or redirecting ethanol production pathway in the native hexose and pentose utilizer. Effort on first mechanism has been mainly directed towards S. cerevisiae [2, 23], while second effort has been mainly implemented in E. coli [2, 8, 12, 31]. However, both these efforts have encountered multiple problems, such as intermediate secretion in the medium, low productivity, instability of the foreign genes introduced, etc. [16]. Researchers have tried to resolve some of these issues by rewiring of endogenous pathways to direct flux towards ethanol production without the need of foreign genes [17, 22]. However, low ethanol productivity on xylose was still an issue.
Evolutionary adaptation had been shown to be a fairly successful strategy in improving the desired trait in the organism of choice [6, 18]. Here, strain improvement is performed by changing the growth environment which places evolutionary pressure on metabolically active cells. It results in heritable changes in the host chromosomal DNA. It was shown that on passaging E. coli strains deficient in key glycolytic gene pgi in M9 medium with glucose, the average increase in growth rate was 3.6-fold over the starting unevolved strain [5]. This strategy was also used to resolve catabolite repression. By alternate switching of carbon source between cellobiose and glucose the cell concentration of Thermobifida fusca increased from 0.31 to 1.43 g/L, specific cellulase activity increased from 2.63 to 3.31 units/mg and the evolved strain showed a generalist phenotype by utilizing xylose, mannose, sucrose, acetate and citrate as carbon source [7]. Separate passaging of E. coli in M9 media using glycerol and lactate as respective carbon source resulted in evolved strains characterized by higher growth rates when grown in acetate, α-ketoglutarate, glucose, glycerol, lactate, malate, pyruvate, ribose and succinate as carbon source [9]. Fermenter-based passaging of ethanologenic E. coli strain in AM1 medium supplemented with xylose and increasing concentration of furfural resulted in an evolved strain which had higher robustness and ethanol productivity in the presence of furfural. MIC of evolved strain to furfural was 2 g/L while that of unevolved strain was 1 g/L and the evolved strain had a higher rate of both xylose consumption and ethanol production in the presence of furfural stress [19]. Evolution of E. coli strain deficient in phosphotransferase system in the presence of glucose resulted in an evolved phenotype where the mutants exhibited improved specific growth rates (0.09–0.13/h) which were comparable to WT with functional PTS (0.11/h) [3]. In another study, passaging araC-deficient E. coli strain with constitutive xylose and arabinose operons on minimal media containing 2 g/L each of arabinose and xylose resulted in an evolved strain capable of co-utilizing glucose and xylose at a faster rate as compared to a ptsG-deficient WT strain [14].
In our earlier studies, we constructed an ethanologenic E. coli strain by internal pathway engineering [22] and used this as host to produce cellulolytic enzymes for conversion of cellobiose into ethanol [21]. In this study we attempted to improve sugar utilization pattern of the ethanologenic E. coli strain via adaptive evolution by successive growth on glucose- and xylose-containing minimal media. This strategy had allowed the cells to co-evolve on both carbon sources and improve the rate of growth, sugar consumption and ethanol formation on both sugars simultaneously.
Materials and methods
Bacterial strains and growth conditions
Basal E. coli strain used in this study was SSY07 (genotype—E. coli B PgapAPDH ΔldhA ΔfrdA), which was a derivative of E. coli B strain having native promoter of pyruvate dehydrogenase (PDH) operon replaced with the promoter of glyceraldehyde-3-phosphate dehydrogenase gene for PDH expression under anaerobic condition and deletion of ldhA and frdA genes for removing lactate and succinate as byproduct (Table 1) [22]. pflB gene deletion was carried out in SSY07 strain using the phage transduction method [27] with the help of single gene knockout Keio strain from CGSC, Yale University, USA as donor. Gene deletion was confirmed via PCR using primers Phage_pflB_fwd and Phage_pflB_rev (Table 1). Resulting strain SSY13 was used for evolutionary adaptation. Initially SSY13 was passaged for 150 days on AM1 agar plates containing 2% xylose and the resultant strain is referred to as SSK01. This evolved strain was subjected to second round of adaptive laboratory experiments as explained in the section below. The representative strains from adaptive laboratory experiments, referred as SSK14, SSK33, SSK42 and SSK56, were selected for further experiments. The last two digits refer to the passage number at which the strain was selected for physiological analysis. Excluding carbon source, the AM1 media components were as follows: (NH4)2HPO4 (19.92 mM), NH4H2PO4 (7.56 mM), KCl (2.00 mM), MgSO4.7H2O (1.50 mM), betaine HCl (1.00 mM), FeCl3.6H2O (8.88 µM), CoCl2.6H2O (1.26 µM), CuCl2.2H2O (0.88 µM), ZnCl2 (2.20 µM), Na2MoO4.2H2O (1.24 µM), H3BO3 (1.21 µM), MnCl2.4H2O (2.50 µM) and CaCl2.2H2O (1.36 µM) [17]. Kanamycin was used at 50 mg/L in both LB and AM1 media.
Adaptive laboratory experiments
The strain passaged on AM1 agar plate, i.e. strain SSK01, was passaged further for 56 days in liquid AM1 medium where the carbon source was alternately switched between 2 g/L glucose and 2 g/L xylose. Passaging was performed under semi-anaerobic condition in 100-mL conical flasks containing 25 mL of AM1 media with either 2 g/L glucose or 2 g/L xylose as carbon source. Cells were allowed to grow till mid-log phase in one carbon source and then the culture was passaged to other carbon source. Following equation was used to determine the starting OD600 of the culture so that OD600 of 0.25 is attained in approximately 24 h at each passage. The dilution of passage culture was adjusted daily to account for enhanced growth rate due to evolutionary adaptation.
where µ is the specific growth rate of the strain in the previous passaging in the same sugar source.
Comparative analysis of evolved strains
We selected SSY13, SSK01 and strain passaged for 14 days (SSK14), 33 days (SSK33), 42 days (SSK42) and 56 days (SSK56) for further analysis of their growth, sugar consumption and product formation rate. Glycerol stock of SSY13 was revived on LB+ 2% xylose plate, while stocks of SSK01, SSK14, SSK33, SSK42 and SSK56 were revived on AM1+ 2% xylose plates. All strains were separately grown overnight in Hungate tubes (18 mL capacity) containing 10 mL of AM1 media with both 2 g/L glucose and 2 g/L xylose as carbon sources in a gyrorotator. This primary culture was used to seed secondary culture in a similar Hungate tube at initial OD600 of 0.1. Samples were extracted at required time points for absorbance and HPLC measurements. Readings were recorded till neither sugar could be detected in the media.
Bioreactor studies
Cultivation was performed in 0.5-L multi-vessel bioreactor (Biostat Q plus, Sartorious) containing 300 mL of either AM1 or LB medium, 10% xylose and 50 mg/L kanamycin under microaerobic conditions. Three strains, i.e. SSY13, SSK01 and SSK42, were selected for fermentation studies in the bioreactor using AM1 medium. For each strain a patch of colonies were suspended in two Hungate tubes (18 mL capacity) each containing 10 mL of AM1 medium with 5% xylose and 50 mg/L kanamycin. Tubes were incubated at 37 °C in a gyrorotator for 5–6 h. The culture was pelleted at 5000 RPM for 2 min and supernatant was discarded. Cell pellet was resuspended in 1 mL of 5% xylose in AM1 medium and inoculated into the primary vessel containing 300 ml of 5% xylose in AM1 medium and grown for 14–16 h to reach an OD600 of ~2. Thereafter, the primary vessel culture was used to seed final bioreactor containing 300 ml of 10% xylose in AM1 media with an initial OD600 of 0.1. Air was only supplied into the headspace at 0.03–0.04 lpm to maintain microaerobic condition. pH was maintained at 6.5 using 2 N KOH. Samples were withdrawn from the bioreactor intermittently for growth and metabolite analyses.
Analytical assay
Cell growth was observed by measuring optical density at 600 nm using spectrophotometer (GE Healthcare). The dry cell mass was calculated by drying cell pellet of defined OD600. An OD600 of 1 corresponded to ~0.5 g/L dry cell mass. The metabolites, such as glucose, xylose, acetic acid and ethanol, were estimated using HPLC as follows. At required time point, samples were withdrawn from the culture, clarified by centrifugation, diluted ten times in 4 mM H2SO4 and filtered via 0.22-µm syringe filter. 10 µL of filtered sample was injected via the autosampler in HPLC machine (Agilent) connected with Aminex HPX 87H (300 × 7.8 mm) column and RI detector. The column temperature was maintained at 40 °C and 4 mM H2SO4 was used as mobile phase at flow rate of 0.3 mL/min. The metabolites were quantitated using reference standard of 1 g/L obtained from Absolute Standards, USA.
Results
Construction of plasmid-free ethanologenic strain
We constructed in our earlier study an E. coli strain SSY09 to produce ethanol by overexpressing pyruvate dehydrogenase enzyme under anaerobic condition through promoter engineering and by deleting ldhA, frdA, ack and pflB genes to prevent accumulation of lactate, succinate, acetate and formate, respectively [22]. However, we observed in that study that growth rate of the cells under anaerobic condition was extremely poor upon deletion of ack gene. We thus introduced ack gene back in SSY09 via pZSack plasmid to regain the growth rate [22]. In this study, we constructed a strain where ack gene in the genome was retained by introducing deletion of pflB in SSY07 strain (Table 1). Thus, the final host SSY13 constructed in this study had a genotype of PgapAPDH ∆ldhA ∆frdA ∆pflB in the background of E. coli B strain. When grown in LB medium containing 105 g/L xylose, this strain produced ~27 g/L of ethanol in 96 h with the yield of 0.36 g/g xylose consumed and 31 g/L xylose remained unutilized (Fig. 1). Ethanol concentration did not rise much beyond 96 h of cultivation and ~ 25 g/L xylose still remained unutilized even after 144 h of cultivation (Fig. 1).
Evolutionary adaptation of ethanologenic E. coli SSY13 strain to improve specific growth rate
The yield and productivity of SSY13 on xylose was considerably lower than the reported values of other xylose-fermenting strains [24, 32]. We thus adopted an evolutionary adaptation approach to improve the growth rate and ethanol productivity. The adaptation was performed in defined AM1 medium to save the fermentation cost. Initially, the colonies were very small on AM1–xylose plate after 24 h. Thus, the cells were first adapted on agar plate containing AM1+ 20 g/L xylose with daily passaging for 150 days. The second set of adaptation was performed in liquid AM1 medium with 2 g/L xylose or glucose as carbon source on alternate days. This was done to achieve high growth rate on both glucose and xylose and to help in co-fermentation of both sugars. The growth curve suggested that cells attained mid-log phase at OD600 of around 0.25 in both glucose and xylose-containing medium (Supplementary Fig S1). Thus, culture was transferred to the fresh medium every time when OD600 reached 0.25. The initial OD600 was maintained such that the desired final OD600 reached at around 24 h. The initial OD600 was calculated based on the specific growth rate of the cells during previous passaging as mentioned in materials and methods section and was readjusted on subsequent passaging as the specific growth rate increased.
An increase in the specific growth rate (µ) as a function of increase in passage number in liquid culture is shown in Fig. 2. After initial lag phase a gradual increase in µ was observed before reaching to saturation towards 50 passaging. Since passaging was always done in mid-log phase, it could be assumed that maximum specific growth rate was maintained throughout the evolution process. A lowest specific growth rate of 0.22/h and the highest specific growth rate of 0.96/h were observed during the passaging event. Wider variation in specific growth rates was observed during the middle phase of evolutionary adaptation, i.e. from 14 to 35 days of passaging, possibly because the mix population of cells were responding with relatively higher variation during the adaptation process. However, as the evolution progresses the favourable population is selected which has narrow variation in the µ values. The strains at various stages of passaging, i.e. 14th passaging (SSK14), 33rd passaging (SSK33), 42nd passaging (SSK42) and 56th passaging (SSK56) were preserved at −80 °C. The number of doublings these strains have gone through are as follows: SSK14—187 doublings, SSK33—432 doublings, SSK42—609 doublings and SSK56—922 doublings. Further analysis of these strains was done to evaluate whether alternate passaging in glucose and xylose-containing liquid medium would improve the metabolizing ability of both these sugars. We also wanted to check if alternate passaging would circumvent the phenomenon of catabolic repression and allow the simultaneous utilization of both types of carbon sources, as was observed in the previous study [7].
Improvement in specific growth rate of E. coli SSY13 with increase in passages in AM1 liquid medium containing either 2 g/L glucose or 2 g/L xylose. The closed circle and open circle represent specific growth of the cells when passaging was done in glucose-containing and xylose-containing medium, respectively
Performance of adapted cells at culture tube level
Cell growth and fermentation profiles of cells with increased passaging
To test the above hypothesis, the strains at different stages of evolutionary adaptation, i.e. SSY13 (unpassaged), SSK01 (plate passaged), SSK14 (14-day liquid passaged), SSK33 (33-day liquid passaged), SSK42 (42-day liquid passaged) and SSK56 (56-day liquid passaged) were grown in AM1 medium having both glucose and xylose at 2 g/L each in Hungate tube and analysed for their growth behaviour and sugar fermenting ability. Relatively higher biomass was formed and sugar fermentation was improved with the increase in passaging (Fig. 3a–f). Around 50% glucose remained in the culture at the initial passaging stage at 6 h of fermentation (Fig. 3a, b), while less than 10% glucose remained during later passaging at this time (Fig. 3e, f). Xylose was utilized completely only by 15 h in SSY13, SSK01 and SSK14 (Fig. 3a–c), while it was completely utilized by 12 h in SSK33 and later passaged strains (Fig. 3d–f). It was observed from the fermentation profiles that xylose utilization started in almost all strains when glucose was nearing exhaustion. The biomass production rate was lowest for SSK01 and highest for both SSK42 and SSK56 (Fig. 4a). The ethanol titer improved with increased passaging and stabilized beyond 33rd passaging (Fig. 3a–f).
Rate of biomass formation (a), glucose consumption (b), xylose consumption (c) and ethanol formation (d) for various E. coli strains as a function of time in Hungate tubes containing AM1 medium with 2 g/L glucose and 2 g/L xylose as carbon sources. The data are presented as average and standard deviation of two independent biological replicates
Rate of glucose utilization across strains with increased passaging
Rate of disappearance of glucose as a function of time was determined in AM1 medium containing both glucose and xylose (Fig. 4b). Interestingly, the lowest consumption rate during 3–6 h of growth was for SSK01, coinciding with lowest biomass formation rate (Fig. 4a), indicating continuous passaging on xylose plate led to reduction in growth rate of SSK01 on glucose as compared to unpassaged strain SSY13. The alternate passaging on glucose and xylose medium subsequently improved the glucose consumption rate of strains, with SSK14 showing similar and SSK33 showing higher consumption rate to SSY13. Both SSK42 and SSK56 showed maximum rate of glucose utilization at 3–6 h of growth (Fig. 4b), indicating that passaging beyond 42 days was not very useful in improving glucose consumption rate. The glucose consumption rate of SSK42 increased by 1.58-fold, i.e. from 0.34 to 0.58 g/L/h, as compared to SSY13 at 6 h. The strains having lower glucose consumption rate during 3–6 h improved their consumption rate during 6–9 h of growth. Thus, the pattern of glucose consumption rate almost reversed during 6–9 h of growth as compared to 3–6 h of growth. No glucose was left unutilized beyond 12 h of growth.
Rate of xylose utilization across strains with increased passaging
Significant xylose utilization started between 6 and 9 h of cultivation (Fig. 4c). Only SSK42 showed some xylose utilization (0.07 g/L/h) during 3–6 h of cultivation. SSY13, SSK01 and SSK14 showed low rate of xylose utilization (0–0.05 g/L/h), while SSK33, SSK42 and SSK56 showed equally high rate of xylose utilization (~0.33 g/L/h) during this period. Strains having low rate of xylose utilization during 6–9 h increased their utilization rate during 9–12 h of cultivation time and finished xylose by 12 h, except SSY13 and SSK01 that continued xylose utilization at the rate of 0.21 and 0.34 g/L/h, respectively, until 15 h (Fig. 4c). This suggested that rate of xylose utilization increased with increase in liquid culture passaging and stabilized near 33rd passaging. However, strain at 42nd passaging had slight edge in initiating the xylose utilization at relatively early stage as compared to all other strains. The xylose consumption rate of SSK42 increased by 5.6-fold, i.e. from 0.06 to 0.34 g/L/h, as compared to SSY13 at 9 h.
Rate of ethanol production of passaged strain
The unpassaged strain SSY13, the plate-passaged strain SSK01 and liquid-passaged SSK14 strains showed very little ethanol formation until 9 h of growth. Ethanol formation rate significantly increased from 33rd passaging onward in liquid and reached maximum upon 42nd passaging as both SSK42 and SSK56 showed similar high ethanol formation rate (0.041 g/L/h) during 3–6 h of growth. The other strains improved their ethanol formation rate during 9–12 h of cultivation. Thus, we observed that with increase in the number of liquid passages ethanol detection could be made in the media at an earlier time point.
Performance of passaged strains in the bioreactor for fermentation of xylose to ethanol
The SSK42 was proven to be the best strain for xylose fermentation based on the performance of strains in Hungate tube. We thus selected SSK42 for fermentation of 10% xylose in AM1 medium in the bioreactor. We used SSY13 and SSK01 as control for comparison purpose. We observed highest rate of xylose utilization for SSK42 (Fig. 5a), with 8.1 mmol/L/h utilized during first 24 h and 20 mmol/L/h during next 24 h of growth (Table 2). The lowest xylose utilization rate during 48 h was observed for SSY13, followed by SSK01. Both SSK01 and SSK42 could consume all xylose (~115 g/L) within 72 h of growth, while 16 g/L xylose remained unutilized in case of SSY13 by this time (Fig. 5a). The increase in cell density as measured by OD600 was highest for SSK42 at 24 h as compared to SSY13 and SSK01 (Fig. 5b). While the cell density of SSK01 picked up at 48 h, the cell density of SSY13 remained low. The final cell yield with respect to xylose was highest for SSK42 and lowest for SSY13 (Table 2). The highest ethanol titer achieved was 1109 mmol/L (51.1 g/L) in case of SSK42, followed by 1028 mmol/L (47.4 g/L) for SSK01 and 744 mmol/L (34.3 g/L) for SSY13 (Fig. 5a; Table 2). This indicated 49% increase in ethanol titer after evolutionary adaptation. The corresponding yield of ethanol for SSK42 also improved reaching 89% of theoretical yield with respect to xylose utilized (Table 2). The volumetric xylose consumption rate and ethanol formation rate was maximum during 24–48 h for all the strains. The SSK42 recorded highest substrate consumption rate as well as ethanol formation rate for both 0–24 and 24–48 h duration (Table 2), with xylose utilization rate and ethanol formation rate reaching 20 mmol/L/h (3 g/L/h) and 30 mmol/L/h (1.4 g/L/h), respectively, during 24–48 h of batch cultivation (Table 2).
Fermentation profiles of E. coli strains SSY13, SSK01 and SSK42 grown in AM1 medium with 115 g/L xylose in the bioreactor. a Profiles for utilization of xylose (solid lines) and formation of ethanol (dotted lines) as function of cultivation time. b Profiles for optical density at 600 nm (dotted lines) and acetic acid formation (solid lines) as function of cultivation time. The data are presented as average and standard deviation of two independent biological replicates
Discussion
E. coli is a model organism which harbours native cellular machinery to metabolize both pentoses and hexoses, which makes it possible to avoid the complexities associated with foreign gene expression. In our previous study, we had taken advantage of this feature and modulated endogenous pathways to divert major carbon flux towards ethanol [22]. In this study, we attempted to improve the properties of the ethanologenic strain SSY09 (pZSack), by (i) removing the plasmid carrying endogenous ack gene, (ii) avoiding use of expensive rich medium for growth and (iii) improving growth rate and productivity in minimal medium by adaptive evolution.
The strain constructed in this study, SSY13, was devoid of any plasmid or foreign gene for ethanol formation. Xylose fermentation in LB medium using SSY13 resulted in low ethanol yield and productivity, thus this strain was evolved in AM1 medium to improve the yield and productivity. The strain was first serially passaged in xylose-containing minimal medium on agar plate, and then shifted to liquid passaging. The liquid passaging was performed alternately in glucose and xylose to retain the metabolizing ability of both sugars. Selected strains from the plate and liquid passage were analysed for metabolizing ability of glucose and xylose. SSY13 and its derivative strains exhibited a classic diauxic sugar utilization pattern where glucose was preferentially metabolized followed by xylose (Fig. 3). With increase in the passage number, metabolism of respective sugar shifted to one earlier time point (Fig. 3a–f). The SSK42 strain showed best utilization rate of both sugars and there appeared no significant advantage with further passaged strain SSK52. SSK14 showed high xylose utilization and ethanol formation rate during later hour of cultivation in Hungate tube, i.e. at 12 h. When this strain was cultivated in the bioreactor with high xylose concentration, both xylose utilization and ethanol formation rates were lower than SSK42 (Supplementary Fig S2).
Serial passaging of SSY13, thus, resulted in selection of a metabolically active cell population which was able to utilize both sugars and produce metabolite of interest at a faster rate. A study along similar experiment lines reported both alleviation of catabolite repression by glucose over cellobiose and increase in specific cellulose activity by Thermobifida fusca strain when passaged alternately on glucose and cellobiose [7]. For the evolved strains we did not detect loss of catabolite repression by glucose over xylose metabolism which would have resulted in co-utilization of both sugars. For a more robust evaluation of physiological performance we tested performance of SSY13, SSK01 and SSK42 at bioreactor level using xylose as sole carbon source.
In the bioreactor studies we observed significant improvement in xylose utilization and ethanol production upon plate passaging of SSY13. At 72 h, xylose was detected only in unevolved SSY13 while SSK01 and SSK42 had completely metabolized the sugar (Fig. 4). Both rate of xylose utilization and ethanol production was higher in the evolved strains evaluated as compared to the unevolved SSY13. Maximum ethanol titer of 1109 mmol/L (51.1 g/L) and yield of 1.47 mmol/mmol xylose (0.45 g/g xylose) was observed in SSK42 which was about 49 and 40%, respectively, higher than unevolved SSY13 (Fig. 4a; Table 2). Further investigation at genetic level is needed to explore the reason for improved characteristics of SSK42. We hypothesize that improved sugar transport efficiency, better redox balance and relieved product inhibition of downstream enzymes, such as pyruvate dehydrogenase, could be some of the factors having beneficial impact on xylose fermentation to ethanol.
Substantial information in the literature exists which highlights the different strategies being adopted to increase the ethanol titers in E. coli strains, but they were mostly done with the help of rich media. Using glucose as carbon source in LB medium with plasmid-based heterologous expression of pdc and adhB genes from Zymomonas mobilis in E. coli resulted in ethanol titers of 750 mM (34.5 g/L) [13], while chromosomal integration of same genes in E. coli with LB medium containing xylose as carbon source resulted in ethanol yield representing 104% of maximum theoretical yield and productivity of 1.3 g/L/h [24].
Among the homoethanologenic pathway-based strategies, deletion of competitive product pathways led to titers of 360 mM (16.58 g/L) ethanol in LB media supplemented with 50 g/L xylose representing 90% of ethanol yield at 192 h [33]. Another study on homoethanol pathway using xylose as a carbon source in complex rich medium reported maximum ethanol productivity of 0.53 g/L/h [15]. We achieved in the present study a higher titer and productivity in minimal media which is relatively more cost effective when compared to complex or rich media. SSK42 produced 1109 mmol/L (51.1 g/L) ethanol in minimal media at 89% of the maximum theoretical yield and productivity of 30 mmol/L/h (1.4 g/L/h) during 24–48 h of batch cultivation. Ethanologenic strains with higher productivity also lower the operation cost of a fermenter thus contributing to the reduced costs of ethanol production.
References
Alonso DM, Bond JQ, Dumesic JA (2010) Catalytic conversion of biomass to biofuels. Green Chem 12:1493–1513
Alper H, Stephanopoulos G (2009) Engineering for biofuels: exploiting innate microbial capacity or importing biosynthetic potential? Nat Rev Microbiol 7:715–723
Balderas-Hernández VE, Hernández-Montalvo V, Bolívar F, Gosset G, Martínez A (2011) Adaptive evolution of Escherichia coli inactivated in the phosphotransferase system operon improves co-utilization of glucose and xylose under anaerobic conditions. Appl Biochem Biotechnol 163:485–496
Bobleter O (1994) Hydrothermal degradation of polymers derived from plants. Prog Polym Sci 19:797–841
Charusanti P, Conrad TM, Knight EM, Venkataraman K, Fong NL, Xie B, Gao Y, Palsson BØ (2010) Genetic basis of growth adaptation of Escherichia coli after deletion of pgi, a major metabolic gene. PLoS Genet. doi:10.1371/journal.pgen.1001186
Conrad TM, Lewis NE, Palsson BØ (2011) Microbial laboratory evolution in the era of genome-scale science. Mol Syst Biol. doi:10.1038/msb.2011.42
Deng Y, Fong SS (2011) Laboratory evolution and multi-platform genome re-sequencing of the cellulolytic Actinobacterium Thermobifida fusca. J Biol Chem 286(46):39958–39966
Dien BS, Cotta MA, Jeffries TW (2003) Bacteria engineered for fuel ethanol production: current status. Appl Microbiol Biotechnol 63:258–266
Fong SS, Joyce AR, Palsson BO (2005) Parallel adaptive evolution cultures of Escherichia coli lead to convergent growth phenotypes with different gene expression states. Genome Res 15:1365–1372
Hendriks ATWM, Zeeman G (2009) Pretreatments to enhance the digestibility of lignocellulosic biomass. Bioresour Technol 100:10–18
Himmel ME, Ding SY, Johnson DK, Adney WS, Nimlos MR, Brady JW, Foust TD (2007) Biomass recalcitrant: engineering plants and enzymes for biofuels production. Science 315:804–807. doi:10.1126/science.1137016
Ingram LO, Aldrich HC, Borges ACC et al (1999) Enteric bacterial catalysts for fuel ethanol production. Biotechnol Progr 15:855–866
Ingram LO, Conway T, Clark DP, Sewell GW, Preston JF (1987) Genetic engineering of ethanol production in Escherichia coli. Appl Environ Microb 53:2420–2425
Kim SM, Choi BY, Ryu YS, Jung SH, Park JM, Kim GH, Lee SK (2015) Simultaneous utilization of glucose and xylose via novel mechanisms in engineered Escherichia coli. Metab Eng 30:141–148
Kim Y, Ingram LO, Shanmugam KT (2007) Construction of an Escherichia coli K-12 mutant for homoethanologenic fermentation of glucose or xylose without foreign genes. Appl Environ Microb 73:1766–1771
Lambertz C, Garvey M, Klinger J et al (2014) Challenges and advances in the heterologous expression of cellulolytic enzymes: a review. Biotechnol Biofuels. doi:10.1186/s13068-014-0135-5
Martinez A, Grabar TB, Shanmugam KT, Yomano LP, York SW, Ingram LO (2007) Low salt medium for lactate and ethanol production by recombinant Escherichia coli B. Biotechnol Lett 29:397–404
McCloskey D, Palsson BØ, Feist AM (2013) Basic and applied uses of genome-scale metabolic network reconstructions of Escherichia coli. Mol Syst Biol. doi:10.1038/msb.2013.18
Miller EN, Jarboe LR, Yomano LP, York SW, Shanmugam KT, Ingram LO (2009) Silencing of NADPH dependent oxidoreductase genes (yqhD and dkgA) in furfural-resistant ethanologenic Escherichia coli. Appl Environ Microb 75(13):4315–4323
Mosier N, Wyman C, Dale B, Elander R, Lee YY, Holtzapple M, Ladisch M (2005) Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour Technol 96:673–686
Munjal N, Jawed K, Wajid S, Yazdani SS (2015) A constitutive expression system for cellulose secretion in Escherichia coli and its use in bioethanol production. PLoS One. doi:10.1371/journal.pone.0119917
Munjal N, Mattam AJ, Pramanik D, Srivastava PS, Yazdani SS (2012) Modulation of endogenous pathways enhances bioethanol yield and productivity in Escherichia coli. Microb Cell Fact 11:145
Nielsen J, Larsson C, Van Maris A, Pronk J (2013) Metabolic engineering of yeast for production of fuels and chemicals. Curr Opin Biotechnol 24:1–7
Ohta K, Beall DS, Mejia JP, Shanmugam KT, Ingram LO (1991) Genetic improvement of Escherichia coli for ethanol production: chromosomal integration of Zymomonas mobilis genes encoding pyruvate deacarboxylase and alcohol dehydrogenase II. Appl Environ Microb 57:893–900
Saha BC, Cotta MA (2010) Comparison of pretreatment strategies for enzymatic saccharification and fermentation of barley straw to ethanol. N Biotechnol 27(1):10–16
Saha BC, Iten LB, Cotta MA, Wu YV (2005) Dilute acid pretreatment, enzymatic saccharification and fermentation of wheat straw to ethanol. Process Biochem 40:3693–3700
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning, a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory, New York
Sims REH, Mabee W, Saddler JN, Taylor M (2010) An overview of second generation biofuel technologies. Bioresour Technol 101:1570–1580
Sun Y, Cheng J (2002) Hydrolysis of lignocellulosic materials for ethanol production: a review. Bioresour Technol 83:1–11
Taherzadeh MJ, Eklund R, Gustafsson L, Niklasson C, Lidén G (1997) Characterization and fermentation of dilute-acid hydrolyzates from wood. Ind Eng Chem Res 36:4659–4665
Warnecke TE, Lynch MD, Karimpour-Fard A, Sandoval N, Gill RT (2008) A genomics approach to improve the analysis and design of strain selections. Metab Eng 10:154–165
Yomano LP, York SW, Ingram LO (1998) Isolation and characterization of ethanol tolerant mutants of Escherichia coli KO11 for fuel ethanol production. J Ind Microbiol Biotechnol 20:132–138
Zhou S, Iverson AG, Grayburn WS (2008) Engineering a native homoethanol pathway in Escherichia coli B for ethanol production. Biotechnol Lett 30:335–342
Acknowledgements
We would like to thank Girish HR for help in HPLC analysis. This work was supported by a Grant (No. BT/PB/Center/03/2011) from Department of Biotechnology, Govt. of India.
Author information
Authors and Affiliations
Corresponding author
Additional information
Syed Bilal Jilani and Siva Sai Krishna Venigalla have contributed equally to this article.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jilani, S.B., Venigalla, S.S.K., Mattam, A.J. et al. Improvement in ethanol productivity of engineered E. coli strain SSY13 in defined medium via adaptive evolution. J Ind Microbiol Biotechnol 44, 1375–1384 (2017). https://doi.org/10.1007/s10295-017-1966-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-017-1966-4