Abstract
Microbial carbonate precipitation has emerged as a promising technology for remediation and restoration of concrete structures. Deterioration of reinforced concrete structures in marine environments is a major concern due to chloride-induced corrosion. In the current study, halophilic bacteria Exiguobacterium mexicanum was isolated from sea water and tested for biomineralization potential under different salt stress conditions. The growth, urease and carbonic anhydrase production significantly increased under salt stress conditions. Maximum calcium carbonate precipitation was recorded at 5 % NaCl concentration. Application of E. mexicanum on concrete specimens significantly increased the compressive strength (23.5 %) and reduced water absorption about five times under 5 % salt stress conditions compared to control specimens. SEM and XRD analysis of bacterial-treated concrete specimens confirmed the precipitation of calcite. The present study results support the potential of this technology for improving the strength and durability properties of building structures in marine environments.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Strength and durability are often regarded as the most important criteria in concrete structure design [26]. Poor concrete durability and corrosion of reinforced concrete (RC) bars are the primary cause of structural deterioration. It has become increasingly apparent that attack by aggressive agents such as chloride ions leading to corrosion of embedded steel may cause a structure to deteriorate. Chloride-induced corrosion of reinforcing bars is the primary cause of deterioration of RC structures in onshore and offshore marine environments [39]. Thus, the corrosion of reinforcing steel in concrete due to chloride transport in marine environment has received increasing attention in recent years because of its wide spread occurrence and high cost repair [35]. Corrosion is initiated by chloride contamination, often in conjunction with inadequate cover or poor quality concrete, which leads to cracking and spalling. To improve the durability for RC structures built in marine environment, high performance concrete, increased concrete cover and use of admixtures are recommended, which tend to increase the initial cost of structures, but do not completely eliminate the risk of corrosion [39]. This has paved the way for more radical, sustainable and holistic approaches.
In the last few years, use of microbial-induced calcium carbonate precipitation (MICP) has emerged as an attractive and successful alternative for remediation and restoration of different building materials [12, 15]. This technology offers the benefits of being eco-friendly and straight forward. MICP in nature has been a widely recognized phenomenon reported in different environments such as sea water, freshwater, industrial wastewaters and soil [9, 16, 21]. The precipitation of carbonates is governed mainly by four factors: (1) calcium concentration, (2) carbonate concentration, (3) pH of the environment and (4) presence of nucleation sites [21]. Different mechanisms have been proposed for precipitation of carbonates in alkaline environments rich in Ca2+ ions [29]. Precipitation of calcium carbonate by ureolytic bacteria is one such mechanism where urea is hydrolysed into ammonium and bicarbonate. The Ca2+ ions subsequently react with the CO3 2− ions, leading to the precipitation of CaCO3 at the cell surface that serves as a nucleation site.
Precipitation of carbonates by carbonic anhydrase (CA) is another mechanism [14]. This enzyme has been found to have the most potential biological catalyst for hydration of CO2 leading to formation of CaCO3 in presence of calcium source [24]
If HCO3 − is the source of dissolved inorganic carbon (DIC), CA may catalyze its conversion into CO2
If CO2 is the source of DIC, CA may catalyze its conversion into HCO3 −
Recent studies have confirmed the efficacy of bacterial CA for CO2 sequestration and calcium carbonate precipitation [41].
Several genera of halophilic bacteria have been reported to precipitate carbonates in natural marine habitats, which include Halomonas, Deleya, Flavobacterium, Acinetobacter and Salinivibrio [17, 27, 28]. These bacteria have the potential to grow in wide range of osmotic concentrations, which makes them very useful for studying the effect of different salt concentrations on carbonate precipitation efficacy. Halomonas halophila is reported to have a salinity range between 2 and 30 % NaCl with its optimum at 7.5 % [33]. Halophilic biomineralizing bacteria have been reported to have different strategies to overcome the stress of high salt concentrations either by maintaining cytoplasmic KCl concentration similar to environment or by using organic osmolytes, which balance the osmotic pressure and maintain high intracellular turgor [20]. The potential of such halophilic bacteria has already been explored in various industrial applications including salt-tolerant enzymes isolation and recovery of saline soils [33]. Precipitation of calcite by halotolerant, alkaliphilic Bacillus sp. VS1 significantly reduced the seepage rate of sand line model pond by sealing the sand particles [36, 37]. Alkali-tolerant and halotolerant Sporosarcina sp. HY008 and Bacillus sp. JH7 isolated from concrete samples showed calcium carbonate precipitation abilities [22]. Though many bacteria have been reported to play an important role in enhancing the durability of building structures, no reports are available on the application of marine halophilic bacteria for offshore concrete structures, which might be more promising for such environments. The biotechnological potential of halophilic bacteria isolated from marine sources may prove to bear high potential in remediation of structures adjoining sea shores and coastal regions with high salts through MICP.
In the present investigation, the biomineralization efficacy of Exiguobacterium mexicanum (MSR1), an halophilic bacterial isolate recovered from sea water, was tested for its ability to grow and produce urease and carbonic anhydrase enzymes under different salt stress conditions. Further, this isolate was applied on concrete specimens to improve the strength and water absorption properties of concrete materials under salt stress conditions.
Materials and methods
Isolation of bacteria
Sea water samples were collected from Nellore district of Andhra Pradesh, India and stored at 4 °C. The sea water samples were analyzed and determined to have the following characteristics: pH 7.6, Electrical conductivity (μS/cm) 1824, major ion concentrations (mg/L); chloride 19.29, Na 10.71, Mg 1.3, sulfate 2.65, Ca 0.42 and potassium 0.39. Sea water samples were filtered through 0.22 µm Millipore membrane filter and the material captured on the filters were transferred into 50 mL sea water medium (SWM) (Medium composition g/L: 27.5 NaCl, 5.0 MgCl2, 2.0 MgSO4, 0.5 KCl, 0.001 FeSO4, 5.0 peptone, 1.0 yeast extract, pH 7.5) with 2 % urea and incubated at 37 °C for 5 days under shaking conditions (130 rpm) to enrich urea degraders. Sub- culturing was done for four generations. Ureolytic bacteria were enumerated using the serial dilution technique by total plate count method on sea water agar plates. The plates were incubated at 37 °C overnight. For isolation of ureolytic bacteria, all the cultures were screened on urea agar base (Hi Media, India), a urease selective medium, to check the production of urease. One of the isolates MSR1 was selected for further studies based on its ability to produce high amount of urease.
Growth and enzyme activities
To study the effect of salinity on the growth, urease, and carbonic anhydrase production of E. mexicanum, sea water medium (SWM) was supplemented with different concentrations of NaCl (3.6, 5, 7.5 and 10 %). The final pH of the medium was adjusted to 8.0. The growth was determined by measuring the optical density at 600 nm. To determine the urease production, 5.0 μM nickel chloride along with 2 % urea was amended in SWM [11]. Carbonic anhydrase production was determined by supplementing 10 μM zinc sulfate and 25 mM NaHCO3 in SWM. The culture was incubated at 37 °C for 96 h under shaking conditions (120 rpm) and tested for the growth, urease and carbonic anhydrase activities at different time intervals (1, 2, 3 and 4 days). The urease activity was determined by measuring the amount of ammonia released from urea according to phenol–hypochlorite assay method [1]. One unit of urease was defined as the amount of enzyme hydrolyzing 1 μmole of urea per minute. Carbonic anhydrase activity was determined as described in Yadav et al. [40]. One unit of carbonic anhydrase activity was defined as the amount of enzyme required to form 1 µmole of p-nitrophenol per minute. Triplicates were maintained for each treatment.
Precipitation of CaCO3 in different salt stress conditions
To test the CaCO3 precipitation ability of E. mexicanum, the bacteria were grown in SWM supplemented with 2 % urea, 5 % NaCl and 25 mM CaCl2. Control set was prepared without bacterial inoculation. The culture was incubated at 37 °C in rotating shaker at 120 rpm for 24 h. The aliquots from flasks were taken at regular time intervals of 2 h and broth was centrifuged to quantify soluble Ca2+ in the supernatant by EDTA titration method as described in Stocks-Fischer et al. [38]. Precipitation of CaCO3 by this isolate in different salt stress conditions (3.6, 5, 7.5 and 10 %) was also determined by growing the bacteria for 96 h. The contents of the flasks were filtered through 0.45 µm Whatman filter paper, washed with phosphate buffered saline and dried at 37 °C overnight. Precipitated CaCO3 was measured by EDTA titration method [2]. Triplicates were maintained for each treatment.
Effect of bacteria on concrete specimens
Compressive strength
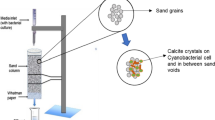
To study the potential of isolated halophilic bacteria in improving the strength properties of concrete specimen, the E. mexicanum was grown in SWM. Ordinary Portland cement of grade 43 confirming to IS 4031 was used to make cement mortar cubes. Dry, clean, well-graded, river sand was used as fine aggregate. Mortar mixes were prepared using binder/sand ratio of 1:3 by weight. For control mixes, water/binder ratio was taken as 0.47 (mL/g) and for bacterial-treated mixes, equivalent amount of bacterial culture OD600 = 1.5 was used instead of water. Mortar cubes of size 70.6 mm × 70.6 mm × 70.6 mm were cast as per BIS: 4031-1988 [4]. All the cubes were cast and compacted on a vibrating table to ensure good consolidation. After de-molding, all the specimens were cured in SWM with 2 % urea and 25 mM CaCl2 along with 5 % NaCl at room temperature. Compression testing was performed as per BIS 516: 1959 [5] using automatic compression testing machine, COMPTEST 3000 at 3-, 7- and 28-day intervals. The cubes were tested perpendicular to the face of casting, and load was applied at the pace rate of 0.4 KN/s. The average of five specimens was taken as the compressive strength of the mix.
Water absorption
To determine the increase in resistance towards water penetration, a sorptivity test, based on the RILEM 25 PEM, was carried out on concrete specimens. The concrete specimens were prepared exactly as mentioned in the compressive strength and cured for 28 days. The specimens were then dried at 45 °C in a ventilated oven, establishing a mass equilibrium of less than 0.1 % between two measurements at 24 h interval. The specimens were then exposed to 10 ± 1 mm of water (water level just 2.0 mm above the base of specimen). This was done in an atmosphere of 20 °C and relative humidity of 60 %. At regular time intervals (15, 30 min, 1, 1.5, 3, 5, 8, 24, 72, 96, 120 and 144 h), the specimens were removed from water and weighed, after drying the surface with the wet towel. Immediately after the measurement, the specimens were submerged again. The sorptivity coefficient, k (cm s−1/2), was obtained by using the following expression:
where Q is the amount of water absorbed (cm3); A is the cross section of the specimen that was in contact with water (cm2); t is the time(s), Q/A was plotted against the square root of time. Five replicates were maintained for each treatment.
SEM and XRD analysis
The morphology and chemical constituents of pure bacterial crystals as well as concrete specimens were analyzed with SEM and XRD. For the SEM analysis, pure crystals were fixed overnight in 2.5 % glutaraldehyde in 0.1 M sodium phosphate buffer at 4 °C, rinsed in 0.2 M phosphate buffer saline solution (pH 7.4) for 1 h and dehydrated in a series of graded ethyl alcohol. In case of mortar samples, 1 cm3 samples were cut from the surface, dried at room temperature and analyzed. SEM observation was done under the following analytical conditions: EHT 20.00 kv, WD 10–11 mm. X-ray diffraction spectra (XRD) was obtained using X’ Pert PRO diffractometer with a Cu anode (40 kV and 30 mA) and scanning from 3° to 60° 2θ. X-ray diffraction identified different crystalline phases of calcium carbonates formed. The components of the sample were identified by comparing them with standards established by the International Centre for Diffraction Data.
Statistical analysis
The data are presented as mean ± standard deviation. Data of growth, enzyme activities, compressive strength and water absorption were statistically analyzed by analysis of variance (ANOVA) and the means were compared by Tukey’s honestly significant different test. All the analyses were performed by using Graph pad prism (5.1)® software.
Results and discussion
Isolation and identification of bacteria
Growth of halophilic ureolytic bacteria on urea agar plates was detected by color change of the medium to dark pink. Quantitative urease production was investigated in 20 colonies and one of the most efficient isolates (MSR1) was selected based on its ureolytic activity. This isolate was identified as Exiguobacterium mexicanum based on its morphological and 16S rDNA sequence analysis. The 16S rDNA sequence obtained in this study was submitted to GenBank (NCBI) under the accession number KX345944.
Growth and enzyme activities
The growth, urease and carbonic anhydrase activities of E. mexicanum were studied under different salt stress conditions. E. mexicanum showed more growth at 7.5 and 10 % salt concentrations than 3.6 and 5 % (Fig. 1a). Urease production was significantly higher at lower concentrations of salt (3.6 and 5 %) than at higher concentrations (7.5 and 10 %) grown at different time intervals. Maximum urease production was observed in 5 % salt concentration on 4th day (Fig. 1c). When compared to 7.5 and 10 % salt concentrations, higher urease production was recorded with 7.5 % salt stress than 10 % (Fig. 1b). Carbonic anhydrase production also increased with time in 3.6 and 5 % salt stress compared to higher concentrations. Maximum carbonic anhydrase production was observed on 4th day at 5 % salt stress. When compared with high concentrations, the enzyme production was higher in 7.5 % salt stress than 10 % (Fig. 1c).
Growth and enzyme activities of E. mexicanum grown under different salt stress conditions at different time intervals a optical density at 600 nm, b urease activity and c carbonic anhydrase activity. Mean values (n = 3) are plotted, with error bars representing ± one standard deviation. The difference between mean values sharing a common letter is not statistically significant at P < 0.05
Constitutive production of urease is a common feature of many species of soil bacteria [6] but efficacy of enzyme production under saline environments needs to be investigated as highly saline conditions cause osmotic stress, and result in bacterial cell lysis and loss of both intra- as well as extracellular enzyme production [19]. In this case, the potential of isolates to produce significant enzymes indicates their adaptability for such conditions. Two mechanisms have been found responsible for this osmo-adaptation; (a) through maintaining cytoplasmic KCl concentration similar to environmental condition and (b) by using organic osmolytes to balance osmotic pressure and maintain high intracellular turgor [33].
Calcium carbonate precipitation
The concentration of soluble Ca2+ decreased significantly up to 6 h (from 25 to 7.4 mM) then slowly decreased up to 10 h (3.6 mM) due to CaCO3 precipitation and remained in the same levels thereafter (Fig. 2a). The abiogenic precipitation of calcium carbonate (without bacteria) has been ruled out since insignificant carbonate precipitate was observed in the control suggesting the involvement of bacteria in carbonate precipitation. The precipitation of CaCO3 decreased with increasing salt concentration and the maximum carbonate precipitation was observed at 5 % salt concentration (155 mg/100 mL) followed by 3.6 % salt (131 mg/100 mL). However, the CaCO3 precipitation decreased significantly at 10 % salt stress conditions (Fig. 2b). Few studies have reported the negative influence of excess salts on carbonate biomineralization [3, 17]. But some moderately halophilic bacterial strains have been reported to have an optimal salt concentration between 10 and 20 % for biomineralization of carbonates and formation of inorganic crystals is suppressed at low salt concentrations [33]. Hammes and Verstraete [21] found that microbes influence most mineralization factors such as pH, calcium and dissolved inorganic carbon. In the present study, E. mexicanum formed higher amounts of crystals at 5 and 3.6 %, but had shown more growth at 7.5 and 10 % salt concentrations. These results confirm with the previous findings of Ferrer et al. [18] that inhibitory effects of salts is milder in halophilic bacteria compared to non-halophilic ones.
Precipitation of CaCO3 by E. mexicanum a soluble Ca2+ ion levels in sea water medium supplemented with 5 % NaCl for different time intervals and b calcium carbonate crystals precipitated under different salt stress conditions after 96 h. Mean values (n = 3) are plotted, with error bars representing ± one standard deviation. The difference between mean values sharing a common letter is not statistically significant at P < 0.05
Effect of E. mexicanum on concrete specimens
Compressive strength
The compressive strength of cement mortar is the most common performance measure to estimate the durability of concrete structures. Figure 3a summarizes the positive effect of E. mexicanum on strength of concrete mortar specimens over different time intervals treated with 5 % salt. During the initial 3 and 7 days, there was a small, but not statistically significant increase in compressive strength of bacterial-treated mortar specimens (8.2, 14.6 MPa) compared to control samples (7.4, 12.5 MPa), which might be due to slow acclimatization of the bacterial cells to concrete environment. At the end of 28 days of curing, E. mexicanum-treated specimens showed a statistically significant increase (23.5 %) of compressive strength of mortar specimens compared to control (Fig. 3a). Earlier studies have reported significant improvement of strength (up to 30 %) in concrete and mortar specimens by different non-halophilic bacterial isolates ([11] and references therein). Stabnikov et al. [36] reported the potential of halotolerant alkaliphilic bacteria Bacillus sp. VS1 and VUK 5 in cementation of sand grains. The increase in strength has been attributed to the formation of carbonates on the exterior surface of the concrete as well as within the pores of cement matrix [12]. The pores in the matrix are plugged due to accumulation of carbonates, which act as a binder to enhance the strength of the specimens. The flow of nutrients and oxygen to bacterial cells stops due to carbonate accumulation on the surface of the bacteria, which eventually lead to the death of cells or formation of endospores [25].
Influence of E. mexicanum on a compressive strength of cement mortar at different days of curing and b water absorption of cement mortar cubes treated with 5 % NaCl. Mean values (n = 5) are plotted, with error bars representing ± one standard deviation. The difference between mean values sharing a common letter is not statistically significant at P < 0.05
Water absorption
The water absorption test was carried out at the end of 28 days of bacteria curing. Significant decrease of water uptake was observed compared to control specimen (Fig. 3b). Nearly five times lesser water absorption was recorded in case of bacterial-treated specimens with respect to the control ones. The deposition of bacterial calcite crystals on the surface of concrete specimens acts as bio-sealant layer. Similar studies have been carried out by non-halophilic bacterial isolates wherein significant reduction of porosity has been reported [8, 10, 12]. The deposition of carbonate layer on the surface of concrete specimens under marine conditions resulted in decrease of the sorptivity paving way to blockage of water and other substances. Our earlier studies also reported the formation of CaCO3 crystals within sand plugs and mud blocks by B. megaterium [13].
SEM and XRD analysis of carbonate crystals
The carbonate crystals formed by E. mexicanum were characterized by SEM and XRD. The crystal size varied from 20 to 50 µm and rod-shaped bacterial cells were clearly seen embedded inside the crystals as well as in close packing association (Fig. 4a). X-ray diffraction results showed that calcite is the major phase formed followed by vaterite and aragonite (Fig. 4b). SEM analysis of the bacterial-treated mortar specimens revealed the presence of dense matrix of crystals with visible rod-shaped bacterial cells in close association with the crystals (Fig. 5a, b). The association of calcite crystals with bacterial cells indicates that bacterial cells served as nucleation sites during the biomineralization process [38]. XRD analysis of mortar specimens showed that the major phase in bacterial-treated specimens is calcite (Fig. 5c).
Several studies have been conducted to understand the enigma behind the formation of a variety of carbonate polymorphs, which are found to be dependent on different factors such as growth media, substrate type, pH, temperature, bacterial species, organic matter and saturation index to [Ca2+]/[CO3 2−] ratio [31]. Ferrer et al. [17] reported that the ionic strength of the medium affects different groups of halophilic microorganisms in different ways. High salt concentrations promote the formation of monohydrocalcite in few microorganisms. The formation of carbonate polymorphs such as vaterite and aragonite have been reported under unfavorable growth conditions while calcite and dolomite have been reported under optimal growth conditions [31]. Some authors also reported that, in case of saline environments, calcite formation is inhibited and aragonite formation increases with increasing concentration of Mg [7, 23, 34]. In the present study, E. mexicanum successfully formed calcite, indicating strain specificity during biomineralization. Another concern is that the minerals formed in high salt conditions are richer in Ca2+ than Mg2+. Rosen [32] reported that bacteria pump Ca2+ towards the exterior of the cell while Mg2+ is pumped towards the interior. On negatively charged surfaces of bacterial cells, Ca2+ is adsorbed with greater intensity than Mg2+ due to its greater ionic selectivity [30]. Though the precipitation of minerals in saline environments is a complex phenomenon, the absence of precipitation in control set without bacteria highlighted the importance of bacterial metabolic activity.
Conclusions
Halophilic bacterial isolate E. mexicanum isolated from sea water has been efficiently shown to produce high amounts of urease and carbonic anhydrase enzymes along with precipitation of carbonates under high salt concentrations up to 10 %. Application of E. mexicanum on concrete specimens significantly increased its compressive strength (23.5 %) and reduced water absorption (five times) compared to control specimens. The current study proved the feasibility of halophilic bacterial isolates in remediation and restoration of construction materials in marine environments. The outcome of the current study supports the potential of this technology for applications in several fields such as remediation of concrete structures, stabilization of beach sands, stability of embankments, strengthening of tailing dams, improving resistance of offshore structures, controlling erosions as well as mitigation of submarine sediment liquefaction near the sea shores.
References
Achal V, Mukherjee A, Basu PC, Reddy MS (2009) Lactose mother liquor as an alternative nutrient source for microbial concrete production by Sporosarcina pasteurii. J Ind Microbiol Biotechnol 36:433–438
APHA (1989) Standard methods for the examination of water and wastewater, 17th edn. American Public Health Association, Washington, DC
Billy C (1980) Problèmes posés par le métabolisme de quelques bactéries calcifiantes aérobies. I. Étude d’une association bacterienne halophile productice d’aragonite en milieu marin. Vieux Milieu 30:165–169
BIS: 4031 (Part 6) (1988) Bureau of Indian Standards—methods of physical tests for hydraulic cement—determination of compressive strength of hydraulic cement other than masonry cement
BIS: 516 (1959) Bureau of Indian Standards—Methods of tests for strength of concrete, New Delhi (Reaffirmed 2004)
Burbank MB, Weaver TJ, Green TL, Williams BC, Crawford RL (2011) Precipitation of calcite by indigenous microorganisms to strengthen liquefiable soils. Geomicrobiol J 28:301–312
Cailleau P, Dragone D, Girou A, Humbert L, Jacquin C, Roques H (1977) Etude experimentale de la pr ´ ecipitation des ´ carbonates de calcium en presence de l’ion magn ´ esium. Bull Soc Fran Miner Crystallogr 100:81–88
Dejong J, Burbank M, Kavazanjian E, Weaver T, Montoya B, Hamdan N, Bang S, Esnault-Filet A, Tsesarsky M, Aydilek A, Ciurli S, Tanyu B, Manning DAC, Larrahondo J, Soga K, Chu J, Cheng X, Kuo M, Al Qabany A, Seagren EA, Van Paassen LA, Renforth P, Laloui L, Nelson DC, Hata T, Burns S, Chen CY, Caslake LF, Fauriel S, Jefferis S, Santamarina JC, Inagaki Y, Martinez B, Palomino A (2013) Biogeochemical processes and geotechnical applications: progress, opportunities and challenges. Géotechnique 63:287–301
Delgado G, Delgado R, Párraga J, Rivadeneyra MA, Aranda V (2008) Precipitation of carbonates and phosphates by bacteria in extract solutions from a semi-arid saline soil. Influence of Ca2+ and Mg2+ concentrations and Mg2+/Ca2+ molar ratio in biomineralisation. Geomicrobiol J 25:1–13
De Muynck W, De Belie N, Verstraete W (2010) Microbial carbonate precipitation in construction materials: a review. Ecol Engn 36:118–136
Dhami NK, Mukherjee A, Reddy MS (2013) Bacillus megaterium mediated mineralization of calcium carbonate as biogenic surface treatment of Green building materials. World J Microbiol Biotechnol 29:2397–2406
Dhami NK, Reddy M, Mukherjee A (2012) Biofilm and microbial applications in biomineralized concrete. In: Advanced topics in biomineralization (Ed Jong Seto). InTech, p 137–164
Dhami NK, Reddy M, Mukherjee A (2013) Biomineralization of calcium carbonate polymorphs by the bacterial strains isolated from calcareous sites. J Microbiol Biotechnol 23:707–714
Dhami NK, Reddy MS, Mukherjee A (2014) Synergistic role of bacterial urease and carbonic anhydrase in carbonate mineralization. Appl Biochem Biotechnol 172:2552–2561
Dhami NK, Reddy MS, Mukherjee A (2016) Significant indicators for biomineralisation in sand of varying grain sizes. Constr Build Mater 104:198–207
Ehrlich HL (2002) Geomicrobiology, 4th edn. Marcel Dekker, New York, p 768
Ferrer MR, Quevedo-Sarmiento J, Rivadeneyra MA, Bejar V, Delgado R, Ramos-Cormenzana A (1988) Calcium carbonate precipitation by two groups of moderately halophilic microorganisms at different temperatures and salt concentrations. Curr Microbiol 17:221–227
Ferrer MR, Quevedo-Sarmiento J, Bejar V, Delgado R, Ramos-Cormenzana A, Rivadeneyra MA (1988) Calcium carbonate formation by Deleya halophila: effect of salt concentration and incubation temperature. Geomicrobiol J 6:49–57
Frankenberger WT, Bingham FT (1982) Influence of salinity on soil enzyme activities. Soil Sci Soc Am J 46:1173–1177
Galinski EA, Trüper HG (1994) Microbial behaviour in salt-stressed ecosystems. FEMS Microbiol Rev 15:95–108
Hammes F, Verstraete W (2002) Key roles of pH and calcium metabolism in microbial carbonate precipitation. Rev Environ Sci Biotechnol 1:3–7
Kim HJ, Eom HJ, Park C, Jung J, Shin B, Kim W, Chung N, Choi IG, Park W (2016) Calcium carbonate precipitation by Bacillus and Sporosarcina strains isolated from concrete and analysis of the bacterial community of concrete. J Microbiol Biotechnol 26:540–548
Kitano Y, Akira T, Arakaki T (1979) Magnesium calcite synthesis from calcium bicarbonate solution containing magnesium and barium ions. Geochem J 13:181–185
Li W, Liu LP, Chen W, Yu LJ, Li W, Yu H (2010) Calcium carbonate precipitation and crystal morphology induced by microbial carbonic anhydrase and other biological factors. Proc Biochem 45:1017–1021
Ramachandran SK, Ramakrishnan V, Bang SS (2001) Remediation of concrete using microorganisms. Am Con Inst Mat J 98:3–9
Ramli M, Kwan WH, Abas NF (2013) Strength and durability of concrete–fiber–reinforced concrete in aggressive environments. Constr Build Mater 18:554–566
Rivadeneyra MA, Delgado R, Delgado G, Moral A, Ferrer MR, Ramos-Cormenzana A (1993) Precipitation of carbonate by Bacillus sp. isolated from saline soils. Geomicrobiol J 11:175–184
Rivadeneyra MA, Delgado R, Párraga J, RamosCormenzana A, Delgado R (2006) Precipitation of minerals by 22 species of moderately halophilic bacteria in artificial marine salts media. Influence of salt concentration. Folia Microbiol 51:445–453
Rivadeneyra MA, Delgado R, Quesada E, Ramos-Cormenzana A (1991) Precipitation of calcium carbonate by Deleyahalophila in media containing NaCl as sole salt. Curr Microbiol 22:185–190
Rivadeneyra MA, Parraga J, Delgado R, Ramos-Cormenzana A, Delgado G (2004) Biomineralization of carbonates by Halobacillus trueperiin solid and liquid media with different salinities. FEMS Microbiol Ecol 48:39–46
Rodriguez-Navarro C, Jroundi F, Schiro M, Ruiz-Agudo E, González-Muñoz MT (2012) Influence of substrate mineralogy on bacterial mineralization of calcium carbonate: implications in stone conservation. Appl Environ Microbiol 78:4017–4029
Rosen BP (1987) Bacterial calcium transport. Biochem Biophys Acta 906:101–110
Rothenstein D, Baier J, Schreiber TD, Barucha V, Bill J (2012) Influence of zinc on the calcium carbonate biomineralization of Halomonas halophile. Aquat Biosyst 8:31
Sayoko Y, Kitano Y (1985) Transformation of aragonite to calcite through heating. Geochem J 19:245–249
Song HW, Lee CH, Ann KY (2008) Factors influencing chloride transport in concrete structures exposed to marine environments. Cem Concr Compos 30:113–121
Stabnikov V, Naeimi M, Ivanov V, Jian C (2011) Formation of water-impermeable crust on sand surface using biocement. Cem Concr Res 41:1143–1149
Stabnikov V, Jian C, Ivanov V, Li Y (2013) Halotolerant, alkaliphilic urease-producing bacteria from different climate zones and their application for biocementation of sand. World J Microbiol Biotechnol 29(8):1453–1460
Stocks-Fischer S, Galinat JK, Bang SS (1999) Microbiological precipitation of CaCO3. Soil Biol Biochem 31:1563–1571
Val DV, Stewart MG (2003) Life-cycle cost analysis of reinforced concrete structures in marine environments. Struct Saf 25:343–362
Yadav R, Labhsetwar N, Kotwal S, Rayalu S (2011) Single enzyme nanoparticle for biomimetic CO2 sequestration. J Nanopart Res 13:263–271
Zhu T, Dittrich M (2016) Carbonate precipitation through microbial activities in natural environment, and their potential in biotechnology: a review. Front Bioeng Biotechnol 4:4
Acknowledgments
This research received funding from Department of Science and Technology, Gov. of India under the scheme SB/S3/CEE/0063/2013.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bansal, R., Dhami, N.K., Mukherjee, A. et al. Biocalcification by halophilic bacteria for remediation of concrete structures in marine environment. J Ind Microbiol Biotechnol 43, 1497–1505 (2016). https://doi.org/10.1007/s10295-016-1835-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-016-1835-6