Abstract
The focus of this study was to produce isopropanol and butanol (IB) from dilute sulfuric acid treated cassava bagasse hydrolysate (SACBH), and improve IB production by co-culturing Clostridium beijerinckii (C. beijerinckii) with Clostridium tyrobutyricum (C. tyrobutyricum) in an immobilized-cell fermentation system. Concentrated SACBH could be converted to solvents efficiently by immobilized pure culture of C. beijerinckii. Considerable solvent concentrations of 6.19 g/L isopropanol and 12.32 g/L butanol were obtained from batch fermentation, and the total solvent yield and volumetric productivity were 0.42 g/g and 0.30 g/L/h, respectively. Furthermore, the concentrations of isopropanol and butanol increased to 7.63 and 13.26 g/L, respectively, under the immobilized co-culture conditions when concentrated SACBH was used as the carbon source. The concentrations of isopropanol and butanol from the immobilized co-culture fermentation were, respectively, 42.62 and 25.45 % higher than the production resulting from pure culture fermentation. The total solvent yield and volumetric productivity increased to 0.51 g/g and 0.44 g/L/h when co-culture conditions were utilized. Our results indicated that SACBH could be used as an economically favorable carbon source or substrate for IB production using immobilized fermentation. Additionally, IB production could be significantly improved by co-culture immobilization, which provides extracellular acetic acid to C. beijerinckii from C. tyrobutyricum. This study provided a technically feasible and cost-efficient way for IB production using cassava bagasse, which may be suitable for industrial solvent production.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Isopropanol is an important industrial chemical that has been used as a chemical backbone in synthetic chemical industries, a dissolving reagent in the biochemical field and a cleaning detergent in our daily life [42, 48]. This reagent is also considered a potentially renewable bio-fuel or fuel additive that is similar to butanol, due to its high-energy density and its ability to be produced by biotechnological processes [31, 38]. With the gradual exhaustion of crude oil and the shortage of food-based substrates, the production of these short-chain aliphatic alcohols using microorganisms from an inexpensive biomass has gained the interest of many.
Isopropanol and butanol (IB) can be produced simultaneously by a small percentage of the bacteria, Clostridium beijerinckii (C. beijerinckii). IB is a major product of these strains, which produce none or only trace amounts of acetone and no ethanol [8, 22, 53]. C. beijerinckii can utilize a variety of carbohydrates such as glucose, fructose, xylose, cellobiose, and mannose [17]. In previous studies, a wide variety of agricultural products have been used to produce solvents, including corn starch [19], potato starch [23], cassava starch and molasses [32]. However, the fermentation processes used to produce these solvents have been directly restricted by high substrate costs and limited food supply. For the purpose of lowering the costs involved with these processes, extensive research has been committed to converting agricultural residues and food processing wastes to fermentable sugars, which include maize stalk [12], wheat straw [41], rice straw [7], peanut shells [20], sugarcane bagasse [52], and cassava bagasse [34]. Cassava bagasse is a kind of fibrous residue generated from the industrial processing of cassava starch. This fibrous by-product is generated in large quantities in factories and normally used further as fertilizer or is directly discarded [39, 46]. Cassava bagasse is abundantly rich with starch, cellulose and hemicellulose that can be converted to be glucose and xylose by the process of dilute sulfuric acid hydrolysis, which has been considered as an economical and efficient hydrolysis method of biomass production [16, 33]. In addition, cassava bagasse contains vegetable proteins that can be hydrolyzed into amino acids and used for microorganism growth [1, 39]. Approximately 30 million tons of fibrous residue is generated in starch production from cassava in the south of China each year. Accordingly, we decided to use cassava fibrous residue as the feedstock for IB production in the present study.
Different fermentation strategies have been employed in the production of solvents, including batch fermentation, fed-batch fermentation [18], continuous fermentation [47], immobilized-cell fermentation [25], extractive fermentation [34], and co-culture fermentation [32]. In addition to extractive fermentation, co-culture fermentation could be considered as another effective technique designed to improve solvent production. Co-culture fermentation is defined as the incubation of different specified microbial strains under the same conditions. This type of fermentation appears to be advantageous compared to pure culture fermentation due to the potential for synergistic utilization of the metabolic pathways of all involved microorganisms in the co-culture system [4]. Cheng and Zhu co-cultured the bacterial strain Clostridium thermocellum (C. thermocellum) with Thermoanaerobacterium aotearoense to produce bio-hydrogen from sugarcane bagasse. In this co-culture fermentation, C. thermocellum acted as an important decomposer by degrading the lignocellulosic biomass to fermentable sugars [9]. Dwidar and colleagues co-cultured Bacillus with Clostridium tyrobutyricum (C. tyrobutyricum) to aid C. tyrobutyricum in degrading sucrose in order to produce butyric acid [15]. Li et al. cultured C. tyrobutyricum to C. beijerinckii together to utilize the supply of extracellular butyric acid from C. tyrobutyricum to improve butanol production in co-culture system [32]. The co-culture fermentation processes previously mentioned used a helper bacterial strain to assist the main bacterial strain in obtaining an absorbable substrate from a certain biomass, which the main strain is incapable of directly absorbing. The alternative strategy for using these co-culture fermentations involved the use of a helper bacterial strain to provide the precursor for enhanced production by the main bacterial strain.
For the IB-producing bacterial strain Clostridia, there are two important phases referred to as acidogenesis and solventogenesis in the metabolism of carbon sources [29]. The synthesis of solvents initiates at the end of the acidogenic phase in which acetic acid and butyric acid are synthesized, and then transition into the solventogenic phase form alcohols [11, 54]. In the solventogenic phase, butyric acid synthesized in the acidogenic phase can be reutilized to synthesize butanol [25]. Therefore, co-culturing the butanol-producing Clostridia, with other anaerobic butyric acid-producing microorganisms is an effective way to enhance butanol production, which in turn, can supply extracellular butyric acid directly and effectively [32]. It was also reported that the added acetic acid could be reutilized for butanol synthesis in some other solvent-producing Clostridia [40]. However, further studies are needed to determine whether the synthesis of isopropanol by a bacterial strain that produces isopropanol is affected by the acetic acid produced by the bacterial strain itself or an extraneous strain.
The purpose of the present study was to investigate the feasibility of converting SACBH into IB, and further improve IB production by supplying extracellular acetic acid from C. tyrobutyricum to C. beijerinckii using the immobilized-cell co-culture fermentation.
Materials and methods
Microorganisms, media and growth conditions
Clostridium beijerinckii (ATCC 6014) was purchased from American Type Culture Collection (ATCC, Manassas, VA, USA). C. tyrobutyricum (ATCC 25755) was originally supplied by Professor Shang-Tian Yang at Ohio State University. The two bacterial strains were rejuvenated and propagated in a Reinforced Clostridial Medium (RCM; Oxoid CM149; Oxoid Limited, Hampshire, England) composed of yeast extract at 3.00 g/L, beef extract powder at 10.00 g/L, peptone at 10.00 g/L, soluble starch at 1.00 g/L, glucose at 5.00 g/L, cysteine hydrochloride at 0.50 g/L, sodium chloride at 5.00 g/L and sodium acetate at 3.00 g/L, and incubated at 37 °C in an anaerobic serum bottle. P2 medium [41] was used in the fermentation experiments, it contains carbon sources (glucose, xylose or SACBH), yeast extract at 1.00 g/L, phosphate buffer (KH2PO4 at 0.50 g/L, K2HPO4 at 0.50 g/L, ammonium acetate at 2.20 g/L), mineral salts (MgSO4·7H2O at 0.20 g/L, MnSO4·H2O at 0.01 g/L, FeSO4·7H2O at 0.01 g/L, and NaCl at 0.01 g/L), vitamins (para-aminobenzoic acid at 1.00 mg/L, thiamin at 1.00 mg/L, and biotin at 0.01 mg/L), and pH was maintained at 6.0. The carbon source and yeast extract were sterilized at 115 °C for 20 min. Phosphate buffer, mineral salts and vitamins were sterilized by filtration.
Cassava bagasse hydrolysis preparation
Cassava bagasse was supplied by a cassava starch processing factory (Guangdong, China). The material was dried and milled to a fine powder (approximately 50–300 μm in diameter) and hydrolyzed using 2.50 % (v/v) dilute sulfuric acid with a solid-to-liquid ratio of 1:12 at 120 °C for 120 min. The raw hydrolysate was adjusted to pH 6.0 using solid calcium hydroxide, and then filtered to remove the solid material and further purify the SACBH sample. The SACBH contains 20.70 g/L of total saccharide, which consists of 9.37 g/L of glucose, 7.06 g/L of xylose, 1.53 g/L of mannose, 1.95 g/L of fructose and 0.79 g/L of arabinose. The SACBH was concentrated approximately three-fold to obtain a concentration of 28.98 g/L of glucose, and 23.04 g/L of xylose.
Experimental setup and fermentation process
To analyze the feasibility of cultivating C. beijerinckii using SACBH as an economically favorable substrate, we firstly examined the effects of various single carbon sources on the cell growth and solvent production by feeding C. beijerinckii with 12 types of sugars at a concentration of 30.00 g/L in 100-mL serum bottles with a working volume of 50 mL of P2 medium at 36 °C. Simultaneously, the SACBH was diluted to be equivalent to the concentration of the total sugars and mixed with P2 medium to culture both the C. beijerinckii and C. tyrobutyricum.
The immobilized fermentation system consisted of a 5-L bioreactor (BIOSTA A plus, Sartorius Stedim Biotech, Germany) connected to a 0.5-L fibrous-bed bioreactor (FBB) with a recirculated latex tube loop. The reaction temperature and agitation speed were controlled by an on-line sensing control system. FBB was made of a jacketed glass column packed with a spirally wound cotton towel (35 × 40 cm; about 5 mm thickness; with >95 % porosity). A detailed description of the bioreactor system has been previously described [26, 44].
Cell immobilization was carried out using an autoclaved immobilized cell fermentation system that contained 2.0 L sterile fermentation medium with 30.00 g/L glucose as the carbon source. High purity nitrogen was pumped into the bioreactor for approximately 2 h to obtain an anaerobic environment. The cell was inoculated at a ratio of 5.00 % (v/v) to the bioreactor. The fermentation broth was recirculated through the FBB by the peristaltic pump at a rate of approximately 30 mL/min until the cell optical density (OD600) reached approximately four, and cells were gradually immobilized onto the fibrous matrix. For approximately 12 h of immobilizing the cell by continuous circulation until the cell density no longer decreased and was maintained at a stable level in the broth, the old broth was entirely pumped out to complete a whole cell immobilization process. Next, 2.50 L of fresh P2 medium which contained a specified carbon source, was pumped into the system immediately to start the immobilized batch fermentation. For the repeated batch fermentations, a new cycle was carried out after the old broth of the previous batch fermentation was replaced with fresh medium when there was no fluctuation in the concentrations of the products.
The co-culture fermentation system was constructed by connecting two FBBs in series in the immobilized cell fermentation system (Fig. 1). The upstream FBB was used for immobilizing C. tyrobutyricum and the downstream FBB was used for immobilizing C. beijerinckii. After both strains have been immobilized, the two FBBs were connected and fresh medium was pumped into the co-culture fermentation system to conduct the batch co-culture fermentation.
Analytical methods
Cell density was analyzed by measuring OD600 with a spectrophotometer (UV 2100, Unico). The inhibitor concentrations in SACBH [49], and the sugar concentrations in SACBH or fermentation broth were measured using a high-performance liquid chromatograph (HPLC), (Waters HPLC 2695, Waters Corporation) equipped with an Aminex HPX-87H organic acid analysis column (Bio-Rad, Hercules, CA) operated at 60 °C, and a refractive index detector (Waters Refractive Index Detector 2414) operated at 45 °C. The mobile phase was 2.50 mM H2SO4 at a flow rate of 0.60 mL/min. The concentrations of isopropanol, butanol, acetic acid, and butyric acid were measured by a gas chromatograph (GC) (Agilent 7890A, Agilent Technologies) equipped with a flame ionization detector, an automatic injector (Agilent 7683B series injector, Agilent Technologies), and a DB-FFAP capillary column (30 m, 0.25 mm inside diameter, 0.50 μm film thickness, Agilent Technologies). The oven temperature of the GC was initially maintained at 40 °C for 3 min, then increased to 160 °C at the speed of 10 °C/min, and then increased to 230 °C at the speed of 50 °C/min and maintained at 230 °C for 2 min. The temperatures of the injector and detector were maintained at 200 and 300 °C, respectively.
Results and discussion
IB production from SACBH by C. beijerinckii
To evaluate the effect of saccharides derived from the biomasses on cell growth and solvents production, various single carbon sources were individually supplemented into the P2 medium at a concentration of 30.00 g/L to carry out serum bottle fermentations with C. beijerinckii. Meanwhile, concentrated SACBH was diluted to the equivalent of the total sugar concentration of 30.00 g/L to culture C. beijerinckii and C. tyrobutyricum. C. beijerinckii showed high cell density and solvent concentration when glucose, xylose, mannose, SACBH, fructose and galactose were supplemented as the carbon source (data not shown). Moreover, the highest concentration of isopropanol and butanol at 4.27 and 8.24 g/L, respectively, was achieved using SACBH as the carbon source. However, this bacterial strain could not utilize pectin, arabinose, starch and hemicellulose, and did not efficiently grow when cultured with cellobiose, sucrose and maltose. Isopropanol was undetectable and only trace amounts of butanol was produced under these conditions (data not shown). Furthermore, acetone and ethanol levels were undetectable in the broth according to all of the fermentation test results. The fermentation of C. tyrobutyricum generated 4.67 g/L of acetic acid and 10.92 g/L of butyric acid when cultured with SACBH. Cytotoxicity tests indicated that there were no obvious inhibitory effects found in the two microbial strains when SACBH was added as a substrate even at a sixfold concentration (data not shown). Moreover, the above-mentioned fermentations using SACBH indicated that C. beijerinckii utilized xylose efficiently, which is an advantageous characteristic similar to most solvent-producing Clostridia since these bacterial strains can utilize hexose and pentose [7]. Thus, the utilization of SACBH as a cost efficient substrate for solvent production is feasible.
To investigate IB production by immobilized C. beijerinckii with the concentrated SACBH, batch fermentation was conducted in an immobilized mode using either glucose, a mixture of glucose and xylose, and concentrated SACBH as the carbon source, respectively. The fermentation results in which glucose was used as the carbon source served as the control.
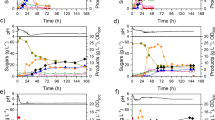
Figure 2a indicates the batch fermentation kinetics when glucose is used as the carbon source. In this experiment, acetic acid and butyric acid were generated during the acidogenesis phase. The acetic acid concentration quickly increased and reached its maximum production level of 5.68 g/L during the first 15 h, and decreased to a relatively stable level of 2.45 g/L after the fermentation phase was switched to the solventogenesis phase. However, the concentration of butyric acid was consistently low and stable at approximately 1.20 g/L and absent of any obvious fluctuation with respect to the concentration. The IB concentration increased rapidly from the 15th to 45th hour. After 63 h of fermentation, the concentrations of isopropanol and butanol were 5.35 and 10.50 g/L, respectively (Table 1). The concentration of acids and IB were similar to those that were previously reported for IB fermentation [47]. The total solvent yield was 0.39 g/g and the volumetric productivity was 0.25 g/L/h with 39.74 % of carbon source conversion. Interestingly, there was no detectable acetone and ethanol in the culture broth, which is similar to the results obtained when fermentation was conducted in the serum bottle. These results differ from the recent report regarding various other strains of C. beijerinckii as well as the original report regarding the similar bacterial strain [8, 47], which generated small amounts of acetone.
Figure 2b indicates the solvent production resulting from the addition of the sugar mixture, glucose and xylose. The growth rate of C. beijerinckii under these conditions was similar to those when glucose was used as the single carbon source. Sugar consumption began with the preferential utilization of glucose. Xylose absorption was slightly delayed due to the cellular preference of glucose compared to xylose [7]. However, xylose decreased to a much lower concentration in the presence of glucose. The final concentrations of isopropanol and butanol were 5.28 and 10.42 g/L, respectively, and were similar to the results mentioned above regarding fermentation with glucose as the carbon source. The total solvent yield was 0.48 g/g and the volumetric productivity was 0.25 g/L/h with 44.63 % total sugar conversion. The experimental results further indicated that both glucose and xylose could be utilized for solvent production by C. beijerinckii when supplied together.
The concentrated SACBH was used as a carbon source in the immobilized batch fermentations in efforts to produce solvents (Fig. 2c). Interestingly, the results showed the production of significantly higher concentrations of isopropanol and butanol, 6.69 and 12.31 g/L, respectively, when concentrated SACBH was used as the carbon source. These concentrations were 25.05 and 17.24 % higher in isopropanol and butanol, respectively, when compared to the results of fermentation with glucose. In addition, isopropanol production improved more than butanol, and was 7.81 % higher in concentration, when using SACBH as the carbon source. The total solvent yield was 0.43 g/g and the volumetric productivity was 0.31 g/L/h, 10.26 and 24.0 % higher, respectively, than the fermentation with glucose. Meanwhile, it was observed that acetic acid reached its maximum concentration of 5.24 g/L, and then the concentration started to decline and the production of isopropanol and butanol initiated simultaneously. The concentration of acetic acid decreased to its lowest level of 1.84 g/L at the end of the batch culture. The butyric acid concentration always kept relatively lower level of approximately 0.80 g/L, with a little fluctuation during solventogenic phase. Furthermore, the final solvent concentrations indicated that there was no evident inhibitory effect in the batch fermentation when concentrated SACBH was used as the carbon source, since only trace amounts of the inhibitors including furfural, 5-Hydroxymethylfurfural and phenol compounds existed in the medium.
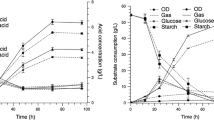
To further evaluate the long-term performance of the solvent production when SACBH was used as the carbon source, the fermentation was repeated for five batches. These batch cultures were conducted under the same conditions using the original immobilized cell in the FBB (Fig. 3). In the five repeated batch fermentations, the production of isopropanol, butanol and acids were relatively stable. The average concentrations of isopropanol and butanol were 6.78 and 12.33 g/L, respectively (Table 1), and the average total solvent yield and volumetric productivity were 0.45 g/g and 0.36 g/L/h, respectively. In the latter four batches, the solvent yield and productivity were slightly higher compared to the first batch. This increase in solvent yield and productivity may be due to an increase in the activity of the solvent-producing cells immobilized on the FBB matrix. Meanwhile, all of the examined fermentation procedures did not display any inhibitory effects, and showed a satisfactory level of stability. Compared to other reports regarding isopropanol-producing strains, 6.78 g/L of isopropanol and 12.33 g/L of butanol were produced using SACBH by immobilized fermentation, whereas, 1.56 or 5.40 g/L isopropanol and 7.56 or 9.70 g/L butanol were produced with batch fermentation using glucose produced by C. beijerinckii [2, 47]. Isopropanol at a concentration of 3.10 g/L and butanol at 7.90 g/L were produced with continuous fermentation using glucose produced by Clostridim isopropylicum [35]. Our immobilized batch fermentation with SACBH indicated higher IB concentrations compare with other reported fermentation results (Table 2). The above-mentioned results further demonstrate the advantages of using concentrated SACBH for IB production, which would be attributed to the presence of a vast variety of sugars and other important nutrient substances such as amino acids and trace elements that might be useful for microorganism growth [1].
Effect of acetic acid on IB production
Acetic acid and butyric acid are the main by-products from Clostridium species. Previous reports show that the acetic acid produced by C. tyrobutyricum can be reutilized for cell growth and butyric acid production [21, 26], and the acetic acid formation yields more ATP to meet the energy demands required for the rapid growth of microorganisms compared to its butyric acid production [36]. In all of the immobilized batch fermentations from this current study, acetic acid concentration was at its peak concentration of approximately 5.00 g/L rapidly in 10 h. However, the concentration of acetic acid decreased shortly and was maintained at a relatively stable level when coupled with the production of isopropanol, butanol and butyric acid. These results suggest a possible acetic acid reutilization of C. beijerinckii during fermentation (Figs. 2, 3). To examine the effect of acetic acid on solvent production by immobilized C. beijerinckii, 3.00 g/L exogenous acetic acid was added to the medium (Fig. 4a), based on the highest concentration of acetic acid in the previously mentioned results was 5.00 g/L, and in other reported fermentation results was 3.00 g/L [32]. The acetic acid concentration increased up to 5.40 g/L on the basic level of 3.00 g/L in the fermentation medium. This result implies that acetic acid was also generated under the presence of extraneous acetic acid. The concentrations of acetic acid began to decrease when the fermentation entered into the solventogenesis phase, and dropped lower than the basic level to its value of 2.67 g/L. These results indicate that both exogenous acetic acid and endogenous acetic acid can be reutilized. When the extraneous acetic acid was provided, the concentrations of isopropanol and butanol increased to 6.32 and 12.07 g/L, which were 18.13 and 14.19 % higher than the control. The total solvent yield was 0.62 g/g and the volumetric productivity was 0.34 g/L/h, 58.97 and 36.00 % higher, respectively, than the control with 26.00 % decreased carbon source consumption. The results of the batch fermentation illustrated that exogenous acetic acids could be reutilized for solvent production by C. beijerinckii, and that the production of isopropanol and butanol were improved simultaneously.
On the other hand, Huang et al., Al-Shorgani et al. and Li et al. verified that the presence of extracellular butyric acid could improve butanol production during fermentation [3, 25, 32]. In addition, Qureshi et al. reported that both acetic acid and butyric acid could be reutilized to aid in butanol production [40]. In our fermentation procedures, the butyric acid concentration was maintained at a relatively low level of approximately 1.20 g/L, due to the verified reutilization by C. beijerinckii. To investigate solvents production in the presence of the exogenous butyric acid during fermentation, 3.00 g/L exogenous acetic acid and 2.00 g/L of butyric acid were added, respectively, to the medium (Fig. 4b). The concentration of butyric acid steadily decreased from its peak concentration of 2.76 g/L at the first 10 h, to 1.45 g/L with minor fluctuation in the concentration at the end. The maximum concentrations of isopropanol and butanol were 6.45 and 12.43 g/L, respectively. The total solvent yield was 0.68 g/g and the volumetric productivity was 0.35 g/L/h, which was, respectively, 74.36 and 40.00 % higher than the control with 30.62 % decreased carbon source consumption. Therefore, the results illustrate that the addition of the two acids could improve IB production when using C. beijerinckii.
IB production by co-culturing C. beijerinckii and C. tyrobutyricum with SACBH
In order to improve IB production by providing C. beijerinckii with acetic acid which was produced synchronously by C. tyrobutyricum in one bioreactor, the co-culture of the two microbial strains was carried out in an immobilized fermentation system which connected two FBBs in series. The upstream FBB was used for immobilizing C. tyrobutyricum and the downstream FBB was used for immobilizing C. beijerinckii. Figure 5a indicates the kinetics of the co-culture fermentation using glucose as the carbon source. This figure shows that the cell density increased quickly, and reached its maximum density in 42 h. Under these conditions, the concentration of the solvents increased at a faster rate and reached the maximum concentration in a shortened fermentation time of 48 h. The concentrations of isopropanol and butanol were, respectively, 6.85 and 12.29 g/L which were 28.04 and 16.38 % higher than the pure culture. The butanol concentration was similar to that reported from butanol production when the Clostridium species were provided butyric acid directly [3]. The total solvent yield and the volumetric productivity were 0.45 g/g and 0.39 g/L/h, which were 15.38 and 56.00 % higher than the pure culture, respectively. Additionally, the acetic acid concentration increased from an initial concentration of 1.48 g/L up to the final concentration of 4.65 g/L, and the butyric acid concentration also increased from 0.38 to 3.53 g/L during the procedure. Whereas in the pure culture, the final concentrations of acetic acid and butyric acid were 2.45 and 1.20 g/L for C. beijerinckii, and those final concentrations of acids were 4.67 and 10.92 g/L for C. tyrobutyricum, respectively. Therefore, it was obvious that most of acids from two species were reutilized for alcohols production in co-culture, but some of these acids still presented in broth and caused the higher final concentrations than those that in pure culture of C. beijerinckii. Furthermore, the total fermentation time was decreased to 48 h, compared to the 63 h used in pure culture fermentation. Based on the results that demonstrate improved solvent production, immobilized co-culture fermentation may enhance IB production by C. beijerinckii by utilizing the extraneous acetic acid produced by C. tyrobutyricum. These results are consistent with the recent report that indicates the addition of acetic acid and butyric acid could significantly increase butanol production [40, 43].
To further improve the immobilized co-culture in IB production, four-fold concentrated SACBH was used as the carbon source of substrate for fermentation (Fig. 5b). As expected, the concentrations of isopropanol and butanol were significantly improved and reached the highest concentration of 7.63 and 13.26 g/L, respectively. The resulting concentrations for isopropanol and butanol were, respectively, 42.62 and 25.45 % higher than the pure culture, and were 11.39 and 8.51 % higher than the co-culture when using glucose as the carbon source. The total solvent yield and the volumetric productivity were 0.51 g/g and 0.44 g/L/h, respectively, which is 13.33 and 12.82 % higher than the co-culture using glucose as the carbon source. These values were the highest compared to each of the above-mentioned fermentation conditions that do not contain extraneous acids. In comparison to other studies, the immobilized co-culture fermentation resulted in 7.63 g/L of isopropanol and 13.26 g/L of butanol production from SACBH, whereas, butanol produced from crystalline cellulose [37], corn cobs [51], rice straw [28], and cellulose [50] by co-culture fermentation obtained lower butanol concentrations of 7.90, 8.30, 6.90 and 3.73 g/L, respectively. Isopropanol production using glucose as the carbon source for bacterial strains that naturally produce isopropanol and most of the engineered strains were also at low concentrations (Table 2). The final concentrations of acetic acid and butyric acid maintained at a relatively high level of 4.23 and 3.45 g/L, respectively. The acids concentration kept a similarly changing trend as observed in the co-culture fermentation using glucose as carbon source. These results suggest that SACBH could be considered as a suitable carbon source and substrate for IB production using co-culture fermentation.
Long-term performance of immobilized co-culture with SACBH was also investigated in our studies (Fig. 6 ). Five repeated batch fermentations showed that the concentrations of isopropanol and butanol always maintained a stable level of approximately 7.47–7.63 and 12.92–13.26 g/L, respectively, as shown in Table 1. The concentration of the acids, solvent yields and volumetric productivities in the latter four batches maintained a similarly changing trend as observed in the first batch. Both bacterial strains maintained growth activities in the multiple repeated batch fermentations, due to the basic tolerance of solvents and acids [54]. As such, further improvements towards the co-culture processes are warranted to optimize IB production.
Conclusions
This study investigated the feasibility of producing isopropanol and butanol from dilute sulfuric acid treated cassava bagasse hydrolysate by the immobilized culture of C. beijerinckii and by the co-culture of C. beijerinckii with C. tyrobutyricum. A significant amount of isopropanol and butanol was produced and reached concentrations as high as 6.78 and 12.33 g/L, respectively, and were achieved using immobilized fermentation under pure culture procedures with C. beijerinckii. Isopropanol and butanol production were further improved with an increase in IB concentrations up to 7.63 and 13.26 g/L, respectively, by the co-culture process when concentrated SACBH was added as the carbon source. The results suggest that SACBH can be used as the carbon source for efficient IB production, and that this production can be enhanced by co-culturing isopropanol-producing Clostridia with an acetic acid or a butyric acid-producing bacterial strain.
References
Aaslyng MD, Martens M, Poll L, Nielsen PM, Flyge H, Larsen LM (1998) Chemical and sensory characterization of hydrolyzed vegetable protein, a savory flavoring. J Agr Food Chem 46:481–489
Ahmed I, Ross RA, Mathur VK, Chesbro WR (1988) Growth rate dependence of solventogenesis and solvents produced by Clostridium beijerinckii. Appl Microbiol Biot 28:182–187
Al-Shorgani NKN, Ali E, Kalil MS, Yusoff WMW (2012) Bioconversion of butyric acid to butanol by Clostridium saccharoperbutylacetonicum N1-4 (ATCC 13564) in a limited nutrient medium. Bioenerg Res 5:287–293
Bader J, Mast-Gerlach E, Popović MK, Bajpai R, Stahl U (2010) Relevance of microbial coculture fermentations in biotechnology. J Appl Microbiol 109:371–387
Bankar SB, Jurgens G, Survase SA, Ojamo H, Granström T (2014) Enhanced isopropanol-butanol-ethanol (IBE) production in immobilized column reactor using modified Clostridium acetobutylicum DSM792. Fuel 136:226–232
Bankar SB, Jurgens G, Survase SA, Ojamo H, Granström T (2015) Genetic engineering of Clostridium acetobutylicum to enhance isopropanol-butanol-ethanol production with an integrated DNA-technology approach. Renew Energ 83:1076–1083
Bellido C, Pinto ML, Coca M, González-Benito G, García-Cubero MT (2014) Acetone-butanol-ethanol (ABE) production by Clostridium beijerinckii from wheat straw hydrolysates: efficient use of penta and hexa carbohydrates. Bioresour Technol 167:198–205
Chen JS, Hiu SF (1986) Acetone-butanol-isopropanol production by Clostridium beijerinckii (synonym, Clostridium butylicum). Biotechnol Lett 8:371–376
Cheng J, Zhu M (2013) A novel anaerobic co-culture system for bio-hydrogen production from sugarcane bagasse. Bioresour Technol 144:623–631
Collas F, Kuit W, Clément B, Marchal R, López-Contreras AM, Monot F (2012) Simultaneous production of isopropanol, butanol, ethanol and 2,3-butanediol by Clostridium acetobutylicum ATCC 824 engineered strains. Amb Express 2:1–10
Du Y, Jiang W, Yu M, Tang IC, Yang ST (2015) Metabolic process engineering of Clostridium tyrobutyricum Δ ack-adhE2 for enhanced n-butanol production from glucose: effects of methyl viologen on NADH availability, flux distribution and fermentation kinetics. Biotechnol Bioeng 112:705–715
Du T, He A, Wu H, Chen J, Kong X, Liu J, Jiang M, Ouyang P (2013) Butanol production from acid hydrolyzed corn fiber with Clostridium beijerinckii mutant. Bioresour Technol 135:254–261
Dai Z, Dong H, Zhu Y, Zhang Y, Li Y, Ma Y (2012) Introducing a single secondary alcohol dehydrogenase into butanol-tolerant Clostridium acetobutylicum Rh8 switches ABE fermentation to high level IBE fermentation. Biotechnol Biofuels 5:1–10
Dusséaux S, Croux C, Soucaille P, Meynial-Salles I (2013) Metabolic engineering of Clostridium acetobutylicum ATCC 824 for the high-yield production of a biofuel composed of an isopropanol/butanol/ethanol mixture. Metab Eng 18(1):1–8
Dwidar M, Kim S, Jeon BS, Um Y, Mitchell RJ, Sang BI (2013) Co-culturing a novel Bacillus strain with Clostridium tyrobutyricum ATCC 25755 to produce butyric acid from sucrose. Biotechnol Biofuels 6:1–10
Esteghlalian A, Hashimoto AG, Fenske JJ, Penner MH (1997) Modeling and optimization of the dilute-sulfuric-acid pretreatment of corn stover, poplar and switchgrass. Bioresour Technol 59:129–136
Ezeji T, Blaschek HP (2008) Fermentation of dried distillers’ grains and solubles (DDGS) hydrolysates to solvents and value-added products by solventogenic Clostridia. Bioresour Technol 99:5232–5242
Ezeji TC, Qureshi N, Blaschek HP (2004) Acetone butanol ethanol (ABE) production from concentrated substrate: reduction in substrate inhibition by fed-batch technique and product inhibition by gas stripping. Appl Microbiol Biot 63:653–658
Ezeji T, Qureshi N, Blaschek HP (2007) Production of acetone-butanol-ethanol (ABE) in a continuous flow bioreactor using degermed corn and Clostridium beijerinckii. Process Biochem 42:34–39
Fang ZF, Liu KL, Chen FS, Zhang LF, Guo Z (2014) Cationic Surfactant-assisted Microwave-NaOH Pretreatment for Enhancing Enzymatic Hydrolysis and Fermentable Sugar Yield from Peanut Shells. Bioresources 9:1290–1302
Fayolle F, Marchal R, Ballerini D (1990) Effect of controlled substrate feeding on butyric acid production by Clostridium tyrobutyricum. J Ind Microbiol Biot 6:179–183
George HA, Johnson JL, Moore WEC, Holdeman LV, Chen JS (1983) Acetone, isopropanol, and butanol production by Clostridium beijerinckii (syn. Clostridium butylicum) and Clostridium aurantibutyricum. Appl Environ Microb 45:1160–1163
Grobben NG, Eggink G, Cuperus FP, Huizing HJ (1993) Production of acetone, butanol and ethanol (ABE) from potato wastes: fermentation with integrated membrane extraction. Appl Microbiol Biot 39:494–498
Hanai T, Atsumi S, Liao JC (2007) Engineered synthetic pathway for isopropanol production in Escherichia coli. Appl Environ Microb 73:7814–7818
Huang WC, Ramey DE, Yang ST (2004) Continuous production of butanol by Clostridium acetobutylicum immobilized in a fibrous bed bioreactor. Appl Biochem Biotech 115:887–898
Jiang L, Wang J, Liang S, Wang X, Cen P, Xu Z (2009) Butyric acid fermentation in a fibrous bed bioreactor with immobilized Clostridium tyrobutyricum from cane molasses. Bioresour Technol 100:3403–3409
Jojima T, Inui M, Yukawa H (2008) Production of isopropanol by metabolically engineered Escherichia coli. Appl Microbiol Biot 77:1219–1224
Kiyoshi K, Furukawa M, Seyama T, Kadokura T, Nakazato A, Nakayama S (2015) Butanol production from alkali-pretreated rice straw by co-culture of Clostridium thermocellum and Clostridium saccharoperbutylacetonicum. Bioresour Technol 186:325–328
Kumar M, Gayen K (2011) Developments in biobutanol production: new insights. Appl Energ 88:1999–2012
Lee J, Jang YS, Choi SJ, Im JA, Song H, Cho JH, Seung DY, Papoutsakis ET, Bennett GN, Lee SY (2012) Metabolic engineering of Clostridium acetobutylicum ATCC 824 for isopropanol-butanol-ethanol fermentation. Appl Environ Microb 78:1416–1423
Lee SY, Park JH, Jang SH, Nielsen LK, Kim J, Jung KS (2008) Fermentative butanol production by Clostridia. Biotechnol Bioeng 101:209–228
Li L, Ai H, Zhang S, Li S, Liang Z, Wu ZQ, Yang ST, Wang JF (2013) Enhanced butanol production by coculture of Clostridium beijerinckii and Clostridium tyrobutyricum. Bioresour Technol 143:397–404
Lloyd TA, Wyman CE (2005) Combined sugar yields for dilute sulfuric acid pretreatment of corn stover followed by enzymatic hydrolysis of the remaining solids. Bioresour Technol 96:1967–1977
Lu C, Zhao J, Yang ST, Wei D (2012) Fed-batch fermentation for n-butanol production from cassava bagasse hydrolysate in a fibrous bed bioreactor with continuous gas stripping. Bioresour Technol 104:380–387
Matsumura M, Takehara S, Kataoka H (1992) Continuous butanol/isopropanol fermentation in down-flow column reactor coupled with pervaporation using supported liquid membrane. Biotechnol Bioeng 39:148–156
Michel-Savin D, Marchal R, Vandecasteele JP (1990) Control of the selectivity of butyric acid production and improvement of fermentation performance with Clostridium tyrobutyricum. Appl Microbiol Biot 32:387–392
Nakayama S, Kiyoshi K, Kadokura T, Nakazato A (2011) Butanol production from crystalline cellulose by cocultured Clostridium thermocellum and Clostridium saccharoperbutylacetonicum N1-4. Appl Environ Microb 77:6470–6475
Palsson BO, Fathi-Afshar S, Rudd DF, Lightfoot EN (1981) Biomass as a source of chemical feedstocks: an economic evaluation. Science 213:513–517
Pandey A, Soccol CR, Nigam P, Soccol VT, Vandenberghe LPS, Mohan R (2000) Biotechnological potential of agro-industrial residues. II: cassava bagasse. Bioresour Technol 74:81–87
Qureshi N, Meagher MM, Huang J, Hutkins RW (2001) Acetone butanol ethanol (ABE) recovery by pervaporation using silicalite-silicone composite membrane from fed-batch reactor of Clostridium acetobutylicum. J Membrane Sci 187:93–102
Qureshi N, Saha BC, Hector RE, Hughes SR, Cotta MA (2008) Butanol production from wheat straw by simultaneous saccharification and fermentation using Clostridium beijerinckii: part I-Batch fermentation. Biomass Bioenerg 32:168–175
Rassadin VG, Shlygin OY, Likhterova NM, Slavin VN, Zharov AV (2006) Problems in production of high-octane, unleaded automotive gasolines. Chem Tech Fuels Oils 42:235–242
Regestein L, Doerr EW, Staaden A, Rehmann L (2015) Impact of butyric acid on butanol formation by Clostridium pasteurianum. Bioresour Technol 196:153–159
Silva EM, Yang ST (1995) Kinetics and stability of a fibrous-bed bioreactor for continuous production of lactic acid from unsupplemented acid whey. J Biotechnol 41:59–70
Soma Y, Inokuma K, Tanaka T, Ogino C, Kondo A, Okamoto M, Hanai T (2012) Direct isopropanol production from cellobiose by engineered Escherichia coli using a synthetic pathway and a cell surface display system. J Biosci Bioeng 114:80–85
Sriroth K, Chollakup R, Chotineeranat S, Piyachomkwan K, Oates CG (2000) Processing of cassava waste for improved biomass utilization. Bioresour Technol 71:63–69
Survase SA, Jurgens G, Heiningen AV, Granström T (2011) Continuous production of isopropanol and butanol using Clostridium beijerinckii DSM 6423. Appl Microbiol Biot 91:1305–1313
Tamakawa H, Mita T, Yokoyama A, Ikushima S, Yoshida S (2013) Metabolic engineering of Candida utilis for isopropanol production. Appl Microbiol Biot 97:6231–6239
Theobald A, Müller A, Anklam E (1998) Determination of 5-hydroxymethylfurfural in vinegar samples by HPLC. J Agr Food Chem 46:1850–1854
Wang Z, Cao G, Zheng J, Fu D, Song J, Zhang J, Zhao L, Yang Q (2015) Developing a mesophilic co-culture for direct conversion of cellulose to butanol in consolidated bioprocess. Biotechnol Biofuels 8:1–9
Wen Z, Wu M, Lin Y, Yang L, Lin J, Cen P (2014) Artificial symbiosis for acetone-butanol-ethanol (ABE) fermentation from alkali extracted deshelled corn cobs by co-culture of Clostridium beijerinckii and Clostridium cellulovorans. Microb Cell Fact 13:1–11
Wei D, Liu X, Yang ST (2013) Butyric acid production from sugarcane bagasse hydrolysate by Clostridium tyrobutyricum immobilized in a fibrous-bed bioreactor. Bioresour Technol 129:553–560
Yan RT, Zhu CX, Golemboski C, Chen JS (1988) Expression of solvent-forming enzymes and onset of solvent production in batch cultures of Clostridium beijerinckii (“Clostridium butylicum”). Appl Env Microb 54:642–648
Yu M, Zhang Y, Tang IC, Yang ST (2011) Metabolic engineering of Clostridium tyrobutyricum for n-butanol production. Metab Eng 13:373–382
Acknowledgments
This work was supported by the Natural Science Foundation of China (21276093) and the Planned Science and Technology Project of Guangdong Province, China (2015A050502014).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, S., Qu, C., Huang, X. et al. Enhanced isopropanol and n-butanol production by supplying exogenous acetic acid via co-culturing two clostridium strains from cassava bagasse hydrolysate. J Ind Microbiol Biotechnol 43, 915–925 (2016). https://doi.org/10.1007/s10295-016-1775-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-016-1775-1