Abstract
Furfural is a toxic by-product formulated from pretreatment processes of lignocellulosic biomass. In order to utilize the lignocellulosic biomass on isobutanol production, inhibitory effect of the furfural on isobutanol production was investigated and combinatorial application of two oxidoreductases, FucO and YqhD, was suggested as an alternative strategy. Furfural decreased cell growth and isobutanol production when only YqhD or FucO was employed as an isobutyraldehyde oxidoreductase. However, combinatorial overexpression of FucO and YqhD could overcome the inhibitory effect of furfural giving higher isobutanol production by 110 % compared with overexpression of YqhD. The combinatorial oxidoreductases increased furfural detoxification rate 2.1-fold and also accelerated glucose consumption 1.4-fold. When it compares to another known system increasing furfural tolerance, membrane-bound transhydrogenase (pntAB), the combinatorial aldehyde oxidoreductases were better on cell growth and production. Thus, to control oxidoreductases is important to produce isobutanol using furfural-containing biomass and the combinatorial overexpression of FucO and YqhD can be an alternative strategy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
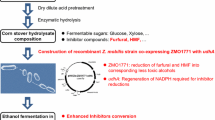
Lignocellulosic biomass contains more than 50 % of carbohydrates in its composition, and it is considered as a potential and sustainable carbon source [25, 28, 30]. To extract the sugars from this lignocellulosic biomass, pretreatment process has to be implemented due to complex structure of the biomass [7, 30]. Various pretreatment processes such as acid hydrolysis, ammonia hydrolysis, and hot water hydrolysis have been developed to purify the sugar, which has been extracted from the complex [15, 29]. However, biological fermentation gets hampered because of toxic compounds such as furfural, 5-hydroxymethylfurfural (HMF), acetate, and aromatic polymers produced in various pretreatment [8, 21, 22]. Therefore, effective detoxification strategies have to be developed for the efficient utilization of this hydrolysate [22].
Among the by-products, furfural has been reported to be produced during harsh pretreatment process like acid hydrolysis [22]. During acid hydrolysis pretreatment, about 10 mM of furfural, though it may differ upon characteristics of biomass, is formed. It damages the DNA and inhibits glycolysis, thereby affecting the metabolisms of sugar in microorganisms [5, 9, 14, 22]. Furfural is reduced by aldehyde oxidoreductases (AORs) into less toxic compound, furfuryl alcohol [34]. However, this process also decreases cell growth, because activated AOR is mostly NADPH dependent [31, 32], and the intracellular consumption of NADPH results in decreased cell growth [17, 18]. Therefore, one solution to improve furfural tolerance is to delete NADPH-dependent AORs like YqhD [18]. As another solution, the furfural active, NADH-dependent 1,2-propanediol oxidoreductase (FucO) has also shown furfural reduction activity, and it could be alternatively used for furfural detoxification. Membrane-bound transhydrogenase (PntAB) also increases furfural tolerance by expanding the availability of NADPH [17, 19, 32].
Isobutanol is produced by valine biosynthesis pathway and Ehrlich pathway. α-Ketoisovalerate is synthesized by the valine pathway, and it is converted to isobutyraldehyde by kivD gene in Lactococcus lactis spp., which encodes α-ketoisovalerate decarboxylase [1]. Finally, the isobutyraldehyde is reduced to isobutyl alcohol by AORs. One of effective AORs showing good activity against isobutyraldehyde is YqhD from Escherichia coli, which is NADPH dependent [3, 12]. One of the successful methods of isobutanol (2-methylpropan-1-ol) production involves the use of engineered E. coli strains [1]. Engineering of E. coli could achieve high isobutanol productivity and yield using glucose-based synthetic media [1]. However, when E. coli produces isobutanol with lignocellulose, fermentation efficiency would be interfered by the toxic compounds. The problem is expected based on characteristic of YqhD that it has broad substrate specificity to isobutyraldehyde and furfural [18]. As an alternative of the NADPH-dependent YqhD, NADH-dependent AdhA from L. lactis was suggested and engineered to improve enzyme specificity on isobutyraldehyde among other substrates such as acetaldehyde [3, 16].
As mentioned above, inhibitory effects of the furfural on ethanol fermentation have been well studied [11, 17, 26, 31, 33, 34]. However, unlike ethanol production, we have little information about the production of isobutanol in the presence of furfural. In this study, we have described a combinatorial application of two oxidoreductases showing activity on isobutyraldehyde and furfural to improve isobutanol production using glucose minimal media containing 15 mM of furfural.
Materials and methods
Bacterial strains, media, and culture conditions
The strains and plasmids used in this study are listed in Table 1. Bacillus subtilis and L. lactis were cultured aerobically at 30 °C. As given in Table 1, E. coli DH5α and E. coli DSM01 were used as host strains for gene cloning and production, respectively. For cell preparation and selection of transformants, these strains were cultured in lysogeny broth (LB) agar and/or liquid broth. LB agar was prepared by dissolving 10 g of tryptone, 5 g of yeast extract, 10 g of NaCl, and 20 g of agar in 1 L of distilled water. For isobutanol production, the transformants were cultured in M9 minimal medium containing 20 g/L of glucose and 5 g/L of yeast extract, which had initial pH 6.8. Appropriate antibiotics (100 μg/mL of spectinomycin, 100 μg/mL of ampicillin, and 25 μg/mL of chloramphenicol for transformation of E. coli) and 0.1 mM IPTG were also added when required. For preculture, a single colony of strain from an LB agar plate was used to inoculate 3 mL of LB medium. The culture was incubated overnight in a shaking incubator at 37 °C, 200 rpm. To conduct flask culture, the grown cells were inoculated into 100 mL of production media taken in a 250-mL screwed cap flask at 1:100 (v/v) dilutions; the initial OD of this medium was 0.01. This flask was then sealed with the screwed cap. Isopropyl β-d-1-thiogalactopyranoside (IPTG) was added initially to the culture medium to induce protein expression. The culture was continuously shaken in a shaking incubator at 200 rpm. The temperature of this incubator was maintained at 30 °C. Aliquots were removed intermittently from the culture for carrying out further steps. Test tube culture was carried out using 5 mL of the production media containing the same concentration of glucose and yeast extract. This tube was sealed to create microaerobic condition.
Reagents
Restriction enzymes and polymerase were bought from Enzynomics (Daejeon, Korea). Plasmid extraction and gel purification kits were purchased from GeneAll (Seoul, Korea). Media components were purchased from Bacto or Difco (NJ, USA). Furfural and furfuryl alcohol were bought from Sigma-Aldrich (MO, USA).
DNA manipulations
Gene cloning was conducted as a general molecular biology method [23]. In brief, the target gene was amplified by PCR using the primers at Table S1. The amplified gene was purified before subjecting it to double restriction endonuclease digestion. The digested fragment was ligated to a vector plasmid, which was also digested by the same restriction enzymes. Then, the E. coli DH5α was transformed by heat-shock method. The intended plasmids were used for further study only when they were confirmed by sequencing.
Analysis techniques
The concentration of isobutanol was determined by gas chromatography (GC; Young Lin Tech, Korea); the chromatographic technique was performed using a DB-Wax column (30 m × 0.32 mm × 0.5 μm) (Agilent Technologies, CA, USA) and a flame ionization detector (FID). The split ratio was 1:20. Two microliters of the sample were injected into the column. Helium was used as a carrier gas; its flow rate was maintained at 3.0 mL/min. The oven was held at 40 °C for 5 min; then, it was heated to 230 °C at a rate of 12 °C/min; the temperature of the oven was maintained at 230 °C for 5 min. The culture samples were centrifuged at 4000 rpm for 10 min, and the isobutanol that was dissolved in the supernatant was extracted using chloroform. The same volume of chloroform was added, and the mixture was voltexed for 5 s followed by separation by centrifuge 13,000 rpm for 1 min. The beneath chloroform fraction was used for isobutanol determination. The concentration of furfural and furfuryl alcohol was also determined under these conditions. The residual glucose concentration was calculated by 3,5-dinitrosalicylic acid (DNS) method with slight modification. The color changes were read using the 96-well microplate reader at a wavelength of 540 nm.
Furfural tolerance
Furfural resistance was determined using 5 mL of M9 minimal medium, which contained 20 g/L of glucose and 5 g/L of yeast extract. Furfural concentration was adjusted from 0 to 40 mM by adding appropriate volume of furfural stock solution. Initial pH of all media was 6.8 ± 0.1. Cultivation was completed under microaerobic condition, and the culture media were sampled after 48 h to measure cell growth. The cell growth was measured in terms of cell density using 96-well microplate reader (TECAN, Switzerland).
Measurement and calculation of parameters
Growth inhibition and production inhibition were determined using 15 mM of furfural. Cell growth inhibition and relative growth rate were calculated by measuring OD at a wavelength of 595 nm. The relative growth rate was calculated by comparing the slope derived after plotting OD in mid-log phase. After 96 h, the inhibitory effect on isobutanol production, final isobutanol titer, and yield was determined from the isobutanol accumulated in the media. The residual furfural was quantified to determine the detoxification rate, which is defined as reduced concentration of furfural per hour (mM/h).
Results
Finding of synergistic effects of two oxidoreductases on isobutanol production in the presence of furfural
To construct T7 promoter-based isobutanol-producing strains, we amplified metabolic flux through a valine biosynthesis pathway. Acetolactate synthase (alsS) from B. subtilis was heterologously expressed because of its high affinity to pyruvate [2]. Ketol-acid reductoisomerase (ilvC) and dihydroxyacid dehydratase (ilvD) from E. coli were overexpressed, and α-ketoisovalerate decarboxylase (kivD) from L. lactis was additionally expressed generating E. coli HM60 using E. coli DSM01 [4]. Starting from the HM60, YqhD and FucO were additionally overexpressed and their furfural tolerance was investigated (Fig. 1). All the strains could not grow over 35 mM furfural, which agrees with other reports [33]. HM60::fucO (HM502) showed robust cell growth along with increasing furfural concentration compared to other strains, which implies FucO was successfully overexpressed. However, overexpression of YqhD made cell more sensitive to furfural. Growth of HM60::yqhD (HM501) was inhibited by low concentration (10 mM) of furfural, while growth of HM60 and HM60::fucO (HM502) was not inhibited. Moreover, overexpression of YqhD decreased cell density even when furfural was not added (Fig. 1). The inhibitory effect of furfural on cell growth is attributed to the depletion of cellular NADPH that consumed during detoxification [17, 18]. Accordingly, furfural was expected to inhibit isobutanol production requiring NADPH in the biosynthesis pathway [1]. To investigate the inhibitory effects on growth and isobutanol production at specific concentration, cell growth and isobutanol in the presence of 15 mM furfural were measured (Fig. 2). With 15 mM furfural, cell density of the HM60, HM60::yqhD (HM501), and HM60::fucO (HM502) decreased 26, 14, and 7 %, respectively. In case of isobutanol production, inhibitory effect was more dramatic (Fig. 2b). In particular, furfural decreased 51 % of isobutanol production by HM60::yqhD (HM501). Although the inhibition on HM60::yqhD (HM501) was distinctive, it was most productive among the strains in the absence of furfural, suggesting that YqhD is helpful to produce isobutanol despite the inhibitions on growth. Based on the results presented above, we designed a strain overexpressing both YqhD and FucO, designated by HM60::yqhD::fucOi (HM601), and it showed better growth and more production of isobutanol than any other strains with and without furfural (Fig. 2). The HM601 also exhibited cell robustness against furfural as much as HM60::fucO (HM502) (Fig. 1). To determine the improvement, further studies on effect of the combinatorial oxidoreductases were carried.
Effects of furfural on cell growth and isobutanol production. The inhibitory effects of furfural on the recombinant cells were investigated. All the results were delivered after 48 h of culture cultivation. The error bars represent standard deviation of three replicates. a Growth inhibition by furfural. b Inhibition of isobutanol production by furfural
Isobutanol production of HM60::yqhD (HM501) and HM60::yqhD::fucO (HM601) in the presence of furfural
To check the differences between the strains HM501 and HM601, we cultured and monitored isobutanol production by the two strains for 72 h (Fig. 3). Only HM60::yqhD (HM501) was selected to compare with HM60::yqhD::fucO (HM601) because HM60::fucO (HM502) did not produce isobutanol as much as HM501 (Fig. 2b). After 72 h, the isobutanol accumulated by HM601 was almost double of that accumulated by HM501. Also, furfural detoxification rate of HM601 was 2.1-fold greater than that of HM501 and the both strains entirely converted furfural to furfuryl alcohol (Table 2; Fig. 3b). HM601 reached higher cell density at early phase and also exhibited 1.4-fold greater sugar consumption rate than that of HM501 (Table 2). Interestingly, HM601 converted furfural from furfuryl alcohol after 48 h, which would be attributed to the reversibility of FucO [6] (Figs. 3b, S1). In summary, we found out that the combination of FucO and YqhD showed 110 % increased isobutanol (4.3 g/L) in the presence of furfural, which was similar amount to the isobutanol production without furfural (Table S2). Both FucO and YqhD are active to isobutyraldehyde and furfural [12, 24, 31]. Therefore, overexpressing the reductases improves furfural detoxification and isobutanol production by accelerating conversion rate of furfural into furfuryl alcohol and isobutyraldehyde into isobutanol (Table 2).
Comparisons of HM60::yqhD (HM501) and HM60::yqhD::fucO (HM601). The differences between the recombinant overexpressing FucO and YqhD (HM601) and the recombinant overexpressing YqhD (HM501) were monitored for 3 days. The error bars represent standard deviation of two replicates. a Cell growth, accumulated isobutanol and glucose consumption. b Residual furfural and furfuryl alcohol
Comparison of isobutanol production with known PntAB system
Increasing NADPH availability using membrane-bound transhydrogenase (PntAB), which balances the redox cofactor by initiating transhydrogenation between NADP(H) and NAD(H), is known to increase furfural tolerance in ethanologenic E. coli [17, 27, 32]. In order to compare the combinatorial system with the known PntAB system, we constructed strains overexpressing the membrane-bound transhydrogenase from E. coli (Fig. 4). PntAB improved furfural tolerance, resulting in 20 % increased cell density (Fig. 4a). Also, HM501::pntAB produced 64 % increased isobutanol compared to HM501::pACYC, which has empty pACYC vector. This supports other reports that supplementing NADPH increases furfural tolerance and productions [17, 32]. Compared with the HM501::pntAB, HM601::pACYC showed higher cell density and isobutanol production (Fig. 4). In addition, the combinatorial system totally prevented inhibition of furfural on final isobutanol titer while 9 % of isobutanol was reduced at HM501::pntAB by furfural (Table S2). When PntAB was additionally overexpressed to the HM601 (HM601::pntAB), there was no notable improvement (Table 3).
Effects of additional transhydrogenase (PntAB) overexpression to growth and isobutanol. 15 mM furfural was added to the initial media, and cell growth and the isobutanol titer were measured after 96 h. The error bars represent standard deviation of three replicates. a Effect of transhydrogenase on growth. b Effect of transhydrogenase on isobutanol production
Discussion
When lignocellulosic biomass is hydrolyzed by the pretreatment process, a certain amount of furfural is usually formed [7, 28]. This aldehyde impedes cell growth and also increases the toxicity of other compounds [33]. E. coli reduces furfural to the less toxic furfuryl alcohol using oxidoreductases, and it has been reported that the reduction is mainly NADPH dependent [10]. The reductase YqhD has low K m value for NADPH, which might cause the depletion of NADPH during cell growth [12]. Therefore, silencing the NADPH-dependent reductase was preferred [18, 32]. On the other hand, NADH-dependent 1,2-propanediol oxidoreductase (FucO), which is involved in fucose metabolism, was overexpressed, and it increased furfural tolerance [6, 31].
In this study, we employed each of oxidoreductases to produce isobutanol in the presence of furfural. When YqhD was employed, furfural inhibited isobutanol production up to 51 % while it helped to produce isobutanol in the presence or absence of furfural (Fig. 2b). Instead of YqhD, FucO was employed but furfural still inhibited isobutanol production while it increased furfural tolerance, resulting in better growth. To overcome the inhibitions by furfural, we applied both YqhD and FucO, and the results clearly showed that the combinatorial system could increase cell growth and production of isobutanol under the influence of furfural. When combinatorial system was compared to the other known system, which supplements cellular NADPH (Fig. 4), combinatorial oxidoreductases entirely prevented the inhibition of furfural on isobutanol production while the overexpression of PntAB exhibited 9 % of isobutanol inhibition by furfural. When PntAB was additionally overexpressed to the strain expressing FucO and YqhD (HM601), it did not show further improvement, suggesting that there is no additional effect by supplementing cellular NADPH.
The possible explanation of the combinatorial oxidoreductases can be hinted from other reports. YqhD is helpful enzyme in the production of isobutanol having low K m for isobutyraldehyde (1.8 mM), but it has low K m for NADPH (0.008 mM), resulting in growth inhibition in the presence of furfural [3, 18]. Compared with YqhD, FucO is better for furfural reduction because of lower K m for furfural with low K m for NADH (0.003 mM) [13, 20, 31]. But, FucO has lower activity than YqhD to produce isobutanol (Fig. 2b). Hence, combinatorial application of the FucO and YqhD improves the detoxification rate of furfural and isobutanol productivity, resulting in higher cell growth and greater isobutanol production (Tables 2, 3; Fig. 3). Again, the results suggest that the combinatorial reductase system is effective on isobutanol production in the presence of furfural not only because of balanced use of redox cofactors, but also because of the additional enzymatic activities on isobutyraldehyde to isobutanol by the two oxidoreductases.
Conclusion
In this study, a novel approach to improve isobutanol production using media containing furfural was investigated. Because the furan derivative is one of the most toxic compounds formulated during treatment of lignocelluloses, this strategy can be further applied to isobutanol fermentation using lignocellulosic hydrolysates. The presented results suggest that control of aldehyde oxidoreductase would be important on isobutanol production with lignocelluloses containing furfural. Greater improvements can be achieved by engineering oxidoreductases to lower K m value for furfural or other toxic furan derivatives such as HMF.
References
Atsumi S, Hanai T, Liao JC (2008) Non-fermentative pathways for synthesis of branched-chain higher alcohols as biofuels. Nature 451:86–89. doi:10.1038/nature06450
Atsumi S, Li Z, Liao JC (2009) Acetolactate synthase from Bacillus subtilis serves as a 2-ketoisovalerate decarboxylase for isobutanol biosynthesis in Escherichia coli. Appl Environ Microbiol 75:6306–6311. doi:10.1128/AEM.01160-09
Atsumi S, Wu TY, Eckl EM, Hawkins SD, Buelter T, Liao JC (2010) Engineering the isobutanol biosynthetic pathway in Escherichia coli by comparison of three aldehyde reductase/alcohol dehydrogenase genes. Appl Microbiol Biotechnol 85:651–657. doi:10.1007/s00253-009-2085-6
Baek JM, Mazumdar S, Lee SW, Jung MY, Lim JH, Seo SW, Jung GY, Oh MK (2013) Butyrate production in engineered Escherichia coli with synthetic scaffolds. Biotechnol Bioeng 110:2790–2794. doi:10.1002/bit.24925
Barciszewski J, Siboska GE, Pedersen BO, Clark BF, Rattan SI (1997) A mechanism for the in vivo formation of N6-furfuryladenine, kinetin, as a secondary oxidative damage product of DNA. FEBS Lett 414:457–460
Boronat A, Aguilar J (1979) Rhamnose-induced propanediol oxidoreductase in Escherichia coli: purification, properties, and comparison with the fucose-induced enzyme. J Bacteriol 140:320–326
Carroll A, Somerville C (2009) Cellulosic biofuels. Annu Rev Plant Biol 60:165–182. doi:10.1146/annurev.arplant.043008.092125
Gonzalez-Benito G, Rodriguez-Brana L, Bolado S, Coca M, Garcia-Cubero MT (2009) Batch ethanol fermentation of lignocellulosic hydrolysates by Pichia stipitis. Effect of acetic acid, furfural and HMF. New Biotechnol 25:S261
Gorsich SW, Dien BS, Nichols NN, Slininger PJ, Liu ZL, Skory CD (2006) Tolerance to furfural-induced stress is associated with pentose phosphate pathway genes ZWF1, GND1, RPE1, and TKL1 in Saccharomyces cerevisiae. Appl Microbiol Biotechnol 71:339–349. doi:10.1007/s00253-005-0142-3
Gutierrez T, Ingram LO, Preston JF (2006) Purification and characterization of a furfural reductase (FFR) from Escherichia coli strain LYO1—an enzyme important in the detoxification of furfural during ethanol production. J Biotechnol 121:154–164. doi:10.1016/j.jbiotec.2005.07.003
Horvath IS, Taherzadeh MJ, Niklasson C, Liden G (2001) Effects of furfural on anaerobic continuous cultivation of Saccharomyces cerevisiae. Biotechnol Bioeng 75:540–549
Jarboe LR (2011) YqhD: a broad-substrate range aldehyde reductase with various applications in production of biorenewable fuels and chemicals. Appl Microbiol Biotechnol 89:249–257. doi:10.1007/s00253-010-2912-9
Jarboe LR, Liu P, Kautharapu KB, Ingram LO (2012) Optimization of enzyme parameters for fermentative production of biorenewable fuels and chemicals. Comput Struct Biotechnol J 3:e201210005. doi:10.5936/csbj.201210005
Khan QA, Shamsi FA, Hadi SM (1995) Mutagenicity of furfural in plasmid DNA. Cancer Lett 89:95–99
Kumar P, Barrett DM, Delwiche MJ, Stroeve P (2009) Methods for pretreatment of lignocellulosic biomass for efficient hydrolysis and biofuel production. Ind Eng Chem Res 48:3713–3729. doi:10.1021/Ie801542g
Liu X, Bastian S, Snow CD, Brustad EM, Saleski TE, Xu JH, Meinhold P, Arnold FH (2012) Structure-guided engineering of Lactococcus lactis alcohol dehydrogenase LlAdhA for improved conversion of isobutyraldehyde to isobutanol. J Biotechnol 164:188–195. doi:10.1016/j.jbiotec.2012.08.008
Miller EN, Jarboe LR, Turner PC, Pharkya P, Yomano LP, York SW, Nunn D, Shanmugam KT, Ingram LO (2009) Furfural inhibits growth by limiting sulfur assimilation in ethanologenic Escherichia coli strain LY180. Appl Environ Microbiol 75:6132–6141. doi:10.1128/AEM.01187-09
Miller EN, Jarboe LR, Yomano LP, York SW, Shanmugam KT, Ingram LO (2009) Silencing of NADPH-dependent oxidoreductase genes (yqhD and dkgA) in furfural-resistant ethanologenic Escherichia coli. Appl Environ Microbiol 75:4315–4323. doi:10.1128/AEM.00567-09
Miller EN, Turner PC, Jarboe LR, Ingram LO (2010) Genetic changes that increase 5-hydroxymethyl furfural resistance in ethanol-producing Escherichia coli LY180. Biotechnol Lett 32:661–667. doi:10.1007/s10529-010-0209-9
Obradors N, Cabiscol E, Aguilar J, Ros J (1998) Site-directed mutagenesis studies of the metal-binding center of the iron-dependent propanediol oxidoreductase from Escherichia coli. Eur J Biochem 258:207–213
Palmqvist E, Hahn-Hagerdal B (2000) Fermentation of lignocellulosic hydrolysates. I: inhibition and detoxification. Bioresour Technol 74:17–24
Palmqvist E, Hahn-Hagerdal B (2000) Fermentation of lignocellulosic hydrolysates. II: inhibitors and mechanisms of inhibition. Bioresour Technol 74:25–33
Phylactides M (1997) Molecular biology series 3. Tools of molecular biology: gene cloning. Br J Hosp Med 57:49–50
Rodriguez GM, Atsumi S (2014) Toward aldehyde and alkane production by removing aldehyde reductase activity in Escherichia coli. Metab Eng 25:227–237. doi:10.1016/j.ymben.2014.07.012
Rubin EM (2008) Genomics of cellulosic biofuels. Nature 454:841–845. doi:10.1038/nature07190
Sarvari Horvath I, Franzen CJ, Taherzadeh MJ, Niklasson C, Liden G (2003) Effects of furfural on the respiratory metabolism of Saccharomyces cerevisiae in glucose-limited chemostats. Appl Environ Microbiol 69:4076–4086
Sauer U, Canonaco F, Heri S, Perrenoud A, Fischer E (2004) The soluble and membrane-bound transhydrogenases UdhA and PntAB have divergent functions in NADPH metabolism of Escherichia coli. J Biol Chem 279:6613–6619. doi:10.1074/jbc.M311657200
Service RF (2007) Cellulosic ethanol. Biofuel researchers prepare to reap a new harvest. Science 315:1488–1491. doi:10.1126/science.315.5818.1488
Sun Y, Cheng J (2002) Hydrolysis of lignocellulosic materials for ethanol production: a review. Bioresour Technol 83:1–11
van der Weijde T, Alvim Kamei CL, Torres AF, Vermerris W, Dolstra O, Visser RG, Trindade LM (2013) The potential of C4 grasses for cellulosic biofuel production. Front Plant Sci 4:107. doi:10.3389/fpls.2013.00107
Wang X, Miller EN, Yomano LP, Zhang X, Shanmugam KT, Ingram LO (2011) Increased furfural tolerance due to overexpression of NADH-dependent oxidoreductase FucO in Escherichia coli strains engineered for the production of ethanol and lactate. Appl Environ Microbiol 77:5132–5140. doi:10.1128/AEM.05008-11
Wang X, Yomano LP, Lee JY, York SW, Zheng H, Mullinnix MT, Shanmugam KT, Ingram LO (2013) Engineering furfural tolerance in Escherichia coli improves the fermentation of lignocellulosic sugars into renewable chemicals. Proc Natl Acad Sci USA 110:4021–4026. doi:10.1073/pnas.1217958110
Zaldivar J, Martinez A, Ingram LO (1999) Effect of selected aldehydes on the growth and fermentation of ethanologenic Escherichia coli. Biotechnol Bioeng 65:24–33
Zaldivar J, Martinez A, Ingram LO (2000) Effect of alcohol compounds found in hemicellulose hydrolysate on the growth and fermentation of ethanologenic Escherichia coli. Biotechnol Bioeng 68:524–530
Acknowledgments
The authors thank to Prof. Oh, Min-Kyu for kind gift of E. coli strain, DSM01. The study was partially supported by the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2015M1A5A1037196) and Advanced Production Technology Development Program, Ministry of Agriculture, Food and Rural Affairs, Republic of Korea (1201349190011). This work was also supported by the R&D Program of MOTIE/KEIT (10048350, 10049674) and the Energy Efficiency and Resources of the Korea Institute of Energy Technology Evaluation and Planning (KETEP) grant funded by the Korea Government Ministry of Trade, Industry and Energy (20133030000300).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors claimed that there is no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Seo, HM., Jeon, JM., Lee, J.H. et al. Combinatorial application of two aldehyde oxidoreductases on isobutanol production in the presence of furfural. J Ind Microbiol Biotechnol 43, 37–44 (2016). https://doi.org/10.1007/s10295-015-1718-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-015-1718-2