Abstract
Klebsiella pneumoniae is a 2,3-butanediol producer, and R-acetoin is an intermediate of 2,3-butanediol production. R-acetoin accumulation and dissimilation in K. pneumoniae was studied here. A budC mutant, which has lost 2,3-butanediol dehydrogenase activity, accumulated high levels of R-acetoin in culture broth. However, after glucose was exhausted, the accumulated R-acetoin could be reused by the cells as a carbon source. Acetoin dehydrogenase enzyme system, encoded by acoABCD, was responsible for R-acetoin dissimilation. acoABCD mutants lost the ability to grow on acetoin as the sole carbon source, and the acetoin accumulated could not be dissimilated. However, in the presence of another carbon source, the acetoin accumulated in broth of acoABCD mutants was converted to 2,3-butanediol. Parameters of R-acetoin production by budC mutants were optimized in batch culture. Aerobic culture and mildly acidic conditions (pH 6–6.5) favored R-acetoin accumulation. At the optimized conditions, in fed-batch fermentation, 62.3 g/L R-acetoin was produced by budC and acoABCD double mutant in 57 h culture, with an optical purity of 98.0 %, and a substrate conversion ratio of 28.7 %.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acetoin (3-hydroxy-2-butanone) exists naturally in dairy products and some fruits, and commercial acetoin is used mainly as an additive to enhance the flavor of food. Some other applications have been reported, such as use in cosmetic products, as a feedstock for synthesis of other chemicals, as a chelating agent, and as a plant growth-promoting molecule [24]. Acetoin can be chemically synthesized from diacetyl (2,3-butanedione) [2], 2,3-butanediol [7], or acetaldehyde [1], and enzymatically synthesized from diacetyl [4], or 2,3-butanediol [11]. Acetoin can be produced by some microorganisms using glucose and other biomass-derived sugars as substrates, and has been considered to be one of the top 30 value-added chemicals from biomass [4].
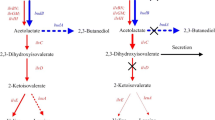
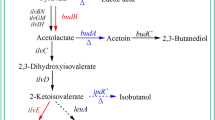
Acetoin is an important metabolic product of microorganisms. The main physiological function of acetoin excretion is energy storage, and acetoin can be used as sole carbon source for the growth of some bacteria [25]. Acetoin is synthesized from pyruvate whereby two molecules of pyruvate condense to yield α-acetolactate and release one molecule of CO2. This reaction is catalyzed by α-acetolactate synthase. α-Acetolactate is then converted to R-acetoin by α-acetolactate decarboxylase. R-acetoin can be further reduced to meso-2,3-butanediol by butanediol dehydrogenase. Genes encoding these enzymes are in the bud regulon and are regulated by BudR in Klebsiella spp. and other Gram-negative bacteria [17]. In Bacillus spp. and other Gram-positive bacteria, the regulon is named als and the AlsR is the transcriptional regulator [25]. 2R,3R-butanediol is synthesized from R-acetoin, employing glycerol dehydrogenase as the catalyzer in Klebsiella pneumoniae [3].
Acetoin dissimilation occurs in bacteria via an oxidative cleavage process which is catalyzed by an acetoin dehydrogenase enzyme system with acetaldehyde and acetyl coenzyme A as the end products. The acetoin dehydrogenase enzyme system consists of thiamine PPi-dependent acetoin dehydrogenase (AoDH E1), dihydrolipoamide acetyltransferase (AoDH E2), and dihydrolipoamide dehydrogenase (AoDH E3), encoded by acoA (encoding the α subunit of AoDH E1), acoB (encoding the β subunit of AoDH E1), acoC, and acoD, respectively [9]. Theses genes are commonly located in aco gene clusters, and the expression of the aco operon is regulated by AcoK in K. pneumoniae [8]. The pathways of acetoin synthesis and use in K. pneumoniae are shown in Fig. 1.
Lactic acid bacteria and yeast have the capacity to produce acetoin as a flavor compound in fermented dairy products. However, acetoin is only produced by these microorganisms at a very low level [10, 15, 16]. Effective acetoin producers are bacteria including Bacillus spp., Paenibacillus spp., and Serratia spp. Some fermentation results are shown in Table 1.
Acetoin is chiral and has two enantiomers. However, the chirality of acetoin produced by fermentation is seldom described. Heterogeneous expression of the bud operon from S. marcescens in E. coli produced R-acetoin, with an optical purity of 97.3 % [26].
K. pneumoniae CGMCC 1.6366 (TUAC01) was isolated for 1,3-propanediol production [6]. In the investigation of 2,3-butanediol stereoisomer formation by this strain, we found that the budC mutant that had lost 2,3-butanediol dehydrogenase activity accumulated R-acetoin in the broth [3]. Hereby, R-acetoin accumulation and dissimilation in K. pneumoniae was investigated in details, and the R-acetoin production by the budC mutant and budC-acoABCD double mutant was investigated in batch and fed-batch fermentation.
Materials and methods
Strains, plasmids, and primers
Bacterial strains and plasmids used in this paper are listed in Table 2.
Construction of K. pneumoniae Δaco and K. pneumoniae ΔbudC–Δaco
For mutant construction, K. pneumoniae and E. coli were grown in Luria–Bertani (LB) medium at 37 °C. The antibiotics used in selective medium were ampicillin (50 μg mL−1), kanamycin (50 μg mL−1), and apramycin (50 μg mL−1).
K. pneumoniae Δaco was constructed according to a previously described method [22]. In brief, acoABCD was amplified from the genome of K. pneumoniae CGMCC 1.6366 using the primer pair aco-s1 (GCCTGGTGACCTTTATTGAGGAGAA)/aco-a1 (ACTGAAATGTCGGCATCCGCAAAGC). The PCR product was then ligated into pMD18-T Simple to generate pMD18-T-aco. Linear DNA with 39 and 40 bp homologous extensions flanking the apramycin resistance gene, aac(3)IV, was amplified from plasmid pIJ773 using the primer pair aco-s2 (TCTTTGAAGCCATCAATATGGCCGTCGTGCTCCAGCTCCATTCCGGGGATCCGTCGACC)/aco-a2 (CGGCAAGGATCGCGCTTTCCAGATCAGAAACAGAGATACGTGTAGGCTGGAGCTGCTTC). pMD18-T-Δaco was constructed by replacing part of acoABCD in plasmid pMD18-T-aco with the aac(3)IV cassette using the Red recombinase system in E. coli. pMD18-T-Δaco was then used as a template for PCR preparation of linear DNA containing aac(3)IV with long homologous regions at either end. The primers used were aco-s1 and aco-a1. Finally, the linear DNA was transformed into K. pneumoniae CGMCC 1.6366 which already host pDK6-red. Homologous recombination between linear DNA and the chromosome was mediated by Red recombinase. K. pneumoniae Δaco was isolated on apramycin plates, and the primer pair Test773 (GCAAATACGGCATCAGTTACC) and aco-s1 was used for PCR confirmation of the mutant. In this process, part of acoA, whole acoB, and part of acoC in the chromosome of K. pneumoniae were replaced by the mark gene.
K. pneumoniae ΔbudC–Δaco was constructed following the same method as K. pneumoniae Δaco, with K. pneumoniae ΔbudC replacing K. pneumoniae CGMCC 1.6366 as the target strain.
Acetoin use
Basic medium was used for acetoin use tests and contained (g/L): racemic acetoin, 4; Na2HPO4, 6; KH2PO4, 3; NH4Cl, 1; NaCl, 0.5; MgSO4∙7H2O, 0.5; and GaCl2.6H2O, 0.5. Klebsiella pneumoniae CGMCC 1.6366 and mutants were inoculated in tubes that contained 3 mL medium and cultured aerobically at 37 °C overnight.
Fermentation medium and culture condition
Fermentation medium contained (g/L): glucose, 100; yeast extract, 2; corn steep liquor, 4; (NH4)2SO4, 5; sodium acetate, 3; KCl, 0.4; and MgSO4, 0.1.
For the seed culture, 250-mL flasks containing 50 mL of LB medium were incubated in a rotary shaker at 37 °C and 200 rpm overnight. 50 mL of seed culture were inoculated into a 5-L bioreactor (BIOSTAT-A plus Sartorius) with a working volume of 3 L. Culture temperature was kept at 37 °C. The culture pH was automatically controlled by the addition of 1 M NaOH and HCl aqueous solutions. In fed-batch culture, a bottle of glucose solution (100 g glucose and 50 g water) was fed to the bioreactor when the glucose level in the medium decreased to 5 g/L.
Optimization of the culture parameters
Culture parameters including oxygen supplementation and culture pH were studied in batch fermentation. A different oxygen supplementation in the fermentation process was achieved by controlled agitation. The air supplement was 4 L/min, and the agitation was set at 300, 400, 500, and 700 rpm, respectively. In this experiment, the culture pH was kept stable at 6. For experiments to study the effect of culture pH, the air supplementation and the stirring agitation were maintained at 4 L/min and 500 rpm, respectively. The culture pH was set at pH 5.5, 6.0, 6.5, or 7.0.
Analytical methods
Biomass concentration was evaluated by determination of optical density (OD) at 600 nm with a NanoDrop-2000C spectrophotometer (Thermo-Scientific, Wilmington, DE). Chemical components in the broth such as glucose, acetoin, meso-2,3-butanediol, 2R,3R-butanediol, 2-ketogluconic acid, succinic acid, acetic acid, and ethanol were quantified by a Shimadzu 20AVP high-performance liquid chromatography system (Shimadzu Corp., Kyoto, Japan) with a RID-10A refractive index detector. The stationary and mobile phases were an Aminex HPX-87H column (300 mm × 7.8 mm) (Bio-Rad, USA) and a 0.005 mol/L H2SO4 solution at 0.8 mL/min flow rate. Chiral isomers of acetoin and 2,3-butanediol in the broth were quantified using a gas chromatography (GC) system (Shimadzu GC 2010) equipped with a flame ionization detector and a Rt®-bDEXse column, as previously described [3].
Results
Acetoin dehydrogenase enzyme system mutation
The acetoin dehydrogenase enzyme system encoding genes acoABCD was knocked out in K. pneumoniae CGMCC 1.6366 and K. pneumoniae ΔbudC to construct K. pneumoniae Δaco and K. pneumoniae ΔbudC–Δaco, respectively. The four strains were cultured in basic medium with acetoin as the sole carbon source. Figure 2 shows the growth of these strains. K. pneumoniae CGMCC 1.6366 and K. pneumoniae ΔbudC grew to turbidity. However, K. pneumoniae Δaco and K. pneumoniae ΔbudC–Δaco did not grow. The wild-type strain can use acetoin as the sole carbon source for growth, but the acoABCD mutants have lost that ability.
Acetoin accumulation and dissimilation in K. pneumoniae ΔbudC
K. pneumoniae ΔbudC was cultured in batch to produce acetoin. Figure 3 shows the results of the culture process.
The whole process of batch fermentation was divided to three phases. Phase I was from the inoculation to the exhaust of glucose, during this period, cells consume glucose for rapid growth, and 28 g/L acetoin was produced. Lower levels of meso-2,3-butanediol, 2R,3R-butanediol, lactic acid, succinic acid, and ethanol were produced. Phase II was from 16 to 24 h culture. During this period, organic acids and ethanol produced in the broth were reused by the cells, and the acetoin level also decreased to about 26 g/L. Phase III was the remaining time of the culture. Acetoin was consumed at a higher speed than that in the phase II. The final metabolic products were meso-2,3-butanediol and 2R,3R-butanediol, with the concentration of 10.7 and 2.6 g/L, respectively.
The effect of the acetoin dehydrogenase enzyme system on acetoin accumulation
K. pneumoniae ΔbudC–Δaco was cultured in batch to produce acetoin compared to K. pneumoniae ΔbudC. The results were shown in Fig. 3.
During phase I and II, cell growth and metabolites production of K. pneumoniae ΔbudC–Δaco were the same as that of K. pneumoniae ΔbudC. However, cell growth, acetoin consumption, meso-2,3-butanediol, and 2R,3R-butanediol production were all stopped during phase III.
The effect of other carbon source supplement on acetoin dissimilation
As about 2 g/L acetoin was consumed by K. pneumoniae ΔbudC and K. pneumoniae ΔbudC–Δaco during phase II. At the same time, ethanol, succinic acid, and lactic acid produced in the broth were all used by the cell. After these chemicals were exhausted, acetoin consumption in K. pneumoniae ΔbudC–Δaco was stopped. We suspected that acetoin dissimilation during this period was the result of ethanol, succinic acid, and lactic acid used by the cell as carbon source and supply NADH to cells.
To test this hypothesis, K. pneumoniae ΔbudC–Δaco was cultured again and extra ethanol was regularly added to the culture in phase III (Fig. 3). When this extra ethanol was supplied, the acetoin level decreased continuously and the meso-2,3-butanediol and 2R,3R-butanediol levels increased continuously. However, the acetoin consumption rate was slower than that of K. pneumoniae ΔbudC.
The effect of oxygen supplementation on acetoin production
As acetoin production was not obviously different between K. pneumoniae ΔbudC and K. pneumoniae ΔbudC–Δaco in the culture before glucose exhaustion, K. pneumoniae ΔbudC was used for culture parameter optimization. In the bioreactor, oxygen supplementation has a positive relationship with agitation. Different agitations were used to study the effect of oxygen supplementation on acetoin production; Fig. 4 shows the fermentation results.
Figure 4a and b shows that cell growth and glucose consumption improved with increased agitation. Glucose was exhausted quickest with agitation at 700 rpm, followed by 500 and 400 rpm. Cell growth was slowest with agitation at 300 rpm, and 50 g/L glucose remained in the broth after culture for 16 h. As well as the slowest growth, 300 rpm agitated culture had the lowest acetoin level. Acetoin levels in 400 rpm and 500 rpm cultures were similar to each other. The culture agitated at 700 rpm had similar acetoin productivity as those at 400 and 500 rpm. However, the conversion ratio was lower (Fig. 4c), owing to accumulation of 2-ketogluconic acid, which is an intermediate of glucose metabolism. The higher the oxygen supplementation, the more 2-ketogluconic acid accumulated (Fig. 4d). Meso-2,3-butanediol production was similar for different agitations and at very low levels in each case (Fig. 4e). Ethanol production had a negative relationship with oxygen supplementation and the 300 rpm agitated culture had the highest ethanol level (Fig. 4f). Succinic acid production had a positive relationship with oxygen supplementation, thus the 700 rpm culture had the highest succinic acid level. Lactic acid is another byproduct of acetoin production; 400 and 500 rpm agitated cultures had the highest lactic acid level. Based on the results in Fig. 4, agitation at 400–500 rpm was the optimum value for culture oxygen supplementation.
The effect of culture pH on acetoin production
The effect of culture pH on acetoin production by K. pneumoniae ΔbudC was investigated and fermentation results are shown in Fig. 5. In the pH range of 5.5–7.0, cell growth and glucose consumption improved with increasing pH (Fig. 5a, b). The highest level of acetoin was achieved at pH 6, while the highest productivity was at pH 6.5 (Fig. 5c). 2-Ketogluconic acid accumulation showed a negative relationship with culture pH, lower pH favoring 2-ketogluconic acid accumulation (Fig. 5d). However, 2-ketogluconic acid accumulated in the broth could be reused by the cells. Meso-2,3-butanediol was only produced at low levels and followed the same tendency as acetoin production (Fig. 5e). Ethanol production had a positive relationship with culture pH; culture at pH 7 resulted in the highest ethanol level, 5.2 g/L (Fig. 5F). Succinic acid and lactic acid production had a positive relationship with culture pH; lower pH inhibited the organic acid production (Fig. 5g, h). Considering the productivity and final acetoin level, a culture pH of 6.0–6.5 was the optimum value.
Acetoin production in fed-batch fermentation
Acetoin production by K. pneumoniae ΔbudC and K. pneumoniae ΔbudC–Δaco was studied in fed-batch fermentation. The agitation and culture pH were set at 500 rpm and pH 6.5, respectively. Figure 6 shows the fermentation results.
Cell growth of the two strains was similar. Cells grew quickly in the first 12 h and reached the highest density at 16–21 h, after which there was some decrease (Fig. 6a). Acetoin production at a high rate coincided with the rapid cell growth phase. After that phase, acetoin was produced at a lower rate. Acetoin production was faster in K. pneumoniae ΔbudC–Δaco than in K. pneumoniae ΔbudC in the first 40 h of culture; 61.0 and 57.4 g/L acetoin were produced by the two strains, respectively. However, the final acetoin levels, after 57 h, were similar; 61.9 and 62.3 g/L for K. pneumoniae ΔbudC and K. pneumoniae ΔbudC–Δaco, respectively (Fig. 6b). The total calculated conversion ratios of glucose to acetoin were 27.9 ± 1.7 and 28.7 ± 1.1 %, respectively. Cells begin synthesis of meso-2,3-butanediol and 2R,3R-butanediol after 9 h of culture, after which their concentrations increased almost linearly. The final concentrations of meso-2,3-butanediol and 2R,3R-butanediol produced by K. pneumoniae ΔbudC were 14.8 and 7.1 g/L, and by K. pneumoniae ΔbudC–Δaco were 14.1 and 7.2 g/L, respectively, (Fig. 6c, d). Ethanol was produced in the rapid cell growth phase and was consumed after that phase (Fig. 6e). Lactic acid was mainly synthesized from 12 to 21 h of culture and after that it was reused by the cells (Fig. 6g). Succinic acid and acetic acid were mainly synthesized after cells stopped growing; unlike ethanol and lactic acid, they were not consumed by the cells (Fig. 6f, h).
Samples of K. pneumoniae ΔbudC and K. pneumoniae ΔbudC–Δaco broth at the end of the culture period were analyzed by GC to test for enantiomers of acetoin and 2,3-butanediol. Table 3 shows the results.
GC analysis showed that the acetoin produced by the two strains was mainly R-acetoin. The optical purity of R-acetoin produced by K. pneumoniae ΔbudC–Δaco was 98.0 %. There was no 2S,3S-butanediol produced by the two strains.
Discussion
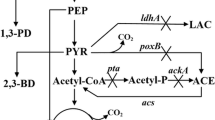
Acetoin is an intermediate in the 2,3-butanediol synthesis pathway. Using glucose as carbon source, the main metabolic product of K. pneumoniae is 2,3-butanediol, accompanied by a low level of acetoin. Butanediol dehydrogenase mutation results in R-acetoin accumulation in culture broth [3]. Wild-type strains of K. pneumoniae can grow on basic medium using acetoin as the carbon source (Fig. 2), and the R-acetoin accumulated in the broth can be reused by the cells (Fig. 3). This is in agreement with the physiological function of acetoin as an energy-storing chemical [25]. acoABCD mutants, however, lost the ability to use acetoin as the carbon source for growth, indicating that oxidative cleavage catalyzed by the acetoin dehydrogenase enzyme system is the sole pathway of acetoin dissimilation in K. pneumoniae. Thus, the additional benefit of K. pneumoniae ΔbudC–Δaco is R-acetion produced during fermentation which can not be dissimilated by themselves.
In the batch culture of K. pneumoniae ΔbudC–Δaco, after glucose was exhausted, about 2 g/L acetoin was consumed. We considered that this fraction of the acetoin was converted to 2,3-butanediol. A possible explanation is that the acoABCD mutation did not affect the acetoin conversion to 2,3-butanediol, but this reaction requires coenzyme NADH. After glucose exhaustion, organic acids and ethanol were reused by the cells. Thus, in this period, R-acetoin in the broth can be converted to 2,3-butanediol with the NADH supplied by organic acid and ethanol catabolism. After the organic acids and ethanol were exhausted, no further acetoin was converted into 2,3-butanediol owing to a lack of NADH. The experiment where extra ethanol was added into the culture of K. pneumoniae ΔbudC–Δaco confirms this hypothesis. During phase II and phase III, 9 g/L 2,3-butanediol was produced as the cells consumed 11 g/L of acetoin, so most of acetoin consumed was converted to 2,3-butanediol. However, only 10 g/L 2,3-butanediol was synthesized by K. pneumoniae ΔbudC cells at the price of 28 g/L acetoin. This indicated a fraction of acetoin was catabolized, like the extra supplemented ethanol, and supplied NADH for other fraction of acetoin conversion to 2,3-butanediol.
Meso-butanediol and 2R,3R-butanediol were the main byproducts in acetoin production by K. pneumoniae ΔbudC and K. pneumoniae ΔbudC–Δaco. The reason is that the other cellular dehydrogenases besides budC-encoded butanediol dehydrogenase catalyze the conversion of acetoin to 2,3-butanediol (Fig. 1). We have demonstrated that a dhaD-encoded glycerol dehydrogenase shows R-enantioselective butanediol dehydrogenase activity [3]. Another glycerol dehydrogenase encoded by gldA also catalyzed the conversion of acetoin to 2,3-butanediol [21]. A short-chain acyl dehydrogenase was identified in K. pneumoniae; this enzyme was an S-enantioselective butanediol dehydrogenase, like BudC [14]. 2,3-Butanediol isomers synthesis was also found in the budC mutant of K. oxytoca [27].
Aerobic conditions favor acetoin production. However, excess oxygen supplementation resulted in 2-ketogluconic acid accumulation. Furthermore, excess oxygen supplementation reduced the conversion of glucose to acetoin. It has been reported that expression of the bud operon was anaerobically induced [13], transcription of genes for acetoin production might be inhibited during aerobic growth of cells, so carbon flux was channeled to other chemicals. In research on acetoin production by Paenibacillus polymyxa, the effect of oxygen supplementation on acetoin production was similar; the highest acetoin level was obtained at a medium agitation [28].
Culture pH is another key parameter in fermentation and mildly acidic conditions favor acetoin synthesis. Beside anaerobiosis, low pH is another factor that induces bud operon expression in K. terrigena [13]. At neutral pH, a large part of the glucose was oxidized to CO2, as in conditions of high oxygen supplementation. At pH 5.5, 2-ketogluconic acid accumulated, an acidic pH-dependent process [20].
In fed-batch fermentation, acetoin level increased quickly in a period of initial 40 h for both K. pneumoniae ΔbudC and K. pneumoniae ΔbudC–Δaco; in extended fermentation, the ratio of meso-2,3-butanediol and 2R,3R-butanediol formation exceeded the acetoin formation, therefore, fermentation should be stopped after 40 h to achieve economical production of acetoin. Compared with acetoin produced by other microorganisms, budC mutants of K. pneumoniae are able to produce more R-acetoin which demonstrates higher optical purity. However, its substrate conversion ratio is rather low, probably due to the formation of 2,3-butanediol. Since all known acetoin producing bacteria also produce 2,3-butanediol as byproduct, with the only exception of a recombinant E. coli which was able to produce R-acetoin with very high substrate conversion rate by expressing a bud operon original from Serratia marcescens [26]. It is also known that the substrate conversion ratio of acetoin from Serratia marcescens H32 increased as the level of 2,3-butanediol was decreasing [18, 19]. Therefore, lower the production of 2,3-butanediol through metabolic engineering should be a promising strategy for further improvement of R-acetoin productivity by K. pneumoniae.
budC mutants of K. pneumoniae are high R-acetoin-producing strains and have the potential to be used in industry. acoABCD mutants block the acetoin dissimilation pathway, and R-acetoin produced during fermentation can not be reused by the cell. K. pneumoniae ΔbudC–Δaco produced 62.3 g/L R-acetoin in fed-batch fermentation. This result is the highest level of R-acetoin produced from glucose by biological methods.
References
Breslow R (1958) On the mechanism of thiamine action 4. Evidence from studies on model systems. J Am Chem 80:3719–3726
Carrara N, Badano J, Bertero N, Torres G, Betti C, Martinez-Bovier L, Quiroga M, Vera C (2014) Kinetics of the liquid phase selective hydrogenation of 2,3-butanedione over new composite supported Pd catalysts. J Chem Technol Biotechnol 89:265–275
Chen C, Wei D, Shi J, Wang M, Hao J (2014) Mechanism of 2, 3-butanediol stereoisomer formation in Klebsiella pneumoniae. Appl Microbiol Biotechnol 98:4603–4613
Gao C, Zhang L, Xie Y, Hu C, Zhang Y, Li L, Wang Y, Ma C, Xu P (2013) Production of (3S)-acetoin from diacetyl by using stereoselective NADPH-dependent carbonyl reductase and glucose dehydrogenase. Bioresour Technol 137:111–115
Gust B, Challis GL, Fowler K, Kieser T, Chater KF (2003) PCR-targeted Streptomyces gene replacement identifies a protein domain needed for biosynthesis of the sesquiterpene soil odor geosmin. P Natl Acad Sci USA 100:1541
Hao J, Lin R, Zheng Z, Liu H, Liu D (2008) Isolation and characterization of microorganisms able to produce 1, 3-propanediol under aerobic conditions. World J Microbiol Biotechnol 24:1731–1740
Hilmi A, Belgsir EM, Leger JM, Lamy C (1997) Electrocatalytic oxidation of aliphatic diols 5. Electro-oxidation of butanediols on platinum based electrodes. J Electroanal Chem 435:69–75
Hsu J-L, Peng H-L, Chang H-Y (2008) The ATP-binding motif in AcoK is required for regulation of acetoin catabolism in Klebsiella pneumoniae CG43. Biochem Biophys Res Commun 376:121–127
Huang M, Oppermann-Sanio FB, Steinbuchel A (1999) Biochemical and molecular characterization of the Bacillus subtilis acetoin catabolic pathway. J Bacteriol 181:3837–3841
Kaneko T, Takahashi M, Suzuki H (1990) Acetoin fermentation by citrate-positive Lactococcus lactis subsp. lactis 3022 grown aerobically in the presence of hemin or Cu2+. Appl Environ Microbiol 56:2644–2649
Kochius S, Paetzold M, Scholz A, Merkens H, Vogel A, Ansorge-Schumacher M, Hollmann F, Schrader J, Holtmann D (2014) Enantioselective enzymatic synthesis of the alpha-hydroxy ketone (R)-acetoin from meso-2,3-butanediol. J Mol Catal B Enzym 103:61–66
Liu Y, Zhang S, Yong Y-C, Ji Z, Ma X, Xu Z, Chen S (2011) Efficient production of acetoin by the newly isolated Bacillus licheniformis strain MEL09. Process Biochem 46:390–394
Mayer D, Schlensog V, Bock A (1995) Identification of the transcriptional activator controlling the butanediol fermentation pathway in Klebsiella terrigena. J Bacteriol 177:5261–5269
Park JM, Hong W-K, Lee S-M, Heo S-Y, Jung YR, Kang IY, Oh B-R, Seo J-W, Kim CH (2014) Identification and characterization of a short-chain acyl dehydrogenase from Klebsiella pneumoniae and its application for high-level production of l-2,3-butanediol. J Ind Microbiol Biotechnol 41:1425–1433
Romano P, Suzzi G (1993) Acetoin production in Saccharomyces cerevisiae wine yeasts. FEMS Microbiol Lett 108:23–26
Romano P, Suzzi G, Zironi R, Comi G (1993) Biometric study of acetoin production in Hanseniaspora guilliermondii and Kloeckera apiculata. Appl Environ Microbiol 59:1838–1841
Stormer F (1975) 2,3-Butanediol biosynthetic system in Aerobacter aerogenes. Methods Enzymol 41:518–533
Sun J-A, Zhang L-Y, Rao B, Shen Y-L, Wei D-Z (2012) Enhanced acetoin production by Serratia marcescens H32 with expression of a water-forming NADH oxidase. Bioresour Technol 119:94–98
Sun J, Zhang L, Rao B, Han Y, Chu J, Zhu J, Shen Y, Wei D (2012) Enhanced acetoin production by Serratia marcescens H32 using statistical optimization and a two-stage agitation speed control strategy. Biotechnol Bioproc E 17:598–605
Sun Y, Wei D, Shi J, Mojović L, Han Z, Hao J (2014) Two-stage fermentation for 2-ketogluconic acid production by Klebsiella pneumoniae. J Microbiol Biotechnol 24:781–787
Wang Y, Tao F, Xu P (2014) Glycerol dehydrogenase plays a dual role in glycerol metabolism and 2, 3-butanediol formation in Klebsiella pneumoniae. J Biol Chem 289:6080–6090
Wei D, Wang M, Shi J, Hao J (2012) Red recombinase assisted gene replacement in Klebsiella pneumoniae. J Ind Microbiol Biotechnol 39:1219–1226
Xiao Z, Liu P, Qin Jy XuP (2007) Statistical optimization of medium components for enhanced acetoin production from molasses and soybean meal hydrolysate. Appl Microbiol Biotechnol 74:61–68
Xiao Z, Lu JR (2014) Strategies for enhancing fermentative production of acetoin: a review. Biotechnol Adv 32:492–503
Xiao Z, Xu P (2007) Acetoin metabolism in bacteria. Crit Rev Microbiol 33:127–140
Xu Q, Xie L, Li Y, Lin H, Sun S, Guan X, Hu K, Shen Y, Zhang L (2015) Metabolic engineering of Escherichia coli for efficient production of (3R)-acetoin. J Chem Technol Biotechnol 90:93–100
Yang TH, Rathnasingh C, Lee HJ, Seung D (2014) Identification of acetoin reductases involved in 2, 3-butanediol pathway in Klebsiella oxytoca. J Biotechnol 172:59–66
Zhang L, Chen S, Xie H, Tian Y, Hu K (2012) Efficient acetoin production by optimization of medium components and oxygen supply control using a newly isolated Paenibacillus polymyxa CS107. J Chem Technol Biotechnol 87:1551–1557
Zhang X, Zhang R, Bao T, Rao Z, Yang T, Xu M, Xu Z, Li H, Yang S (2014) The rebalanced pathway significantly enhances acetoin production by disruption of acetoin reductase gene and moderate-expression of a new water-forming NADH oxidase in Bacillus subtilis. Metab Eng 23:34–41
Zhang X, Zhang R, Yang T, Zhang J, Xu M, Li H, Xu Z, Rao Z (2013) Mutation breeding of acetoin high producing Bacillus subtilis blocked in 2,3-butanediol dehydrogenase. World J Microbiol Biotechnol 29:1783–1789
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (Grant No. 20906076).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, D., Zhou, J., Chen, C. et al. R-acetoin accumulation and dissimilation in Klebsiella pneumoniae . J Ind Microbiol Biotechnol 42, 1105–1115 (2015). https://doi.org/10.1007/s10295-015-1638-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-015-1638-1