Abstract
Heterotrophic denitrifying enriched culture (DEC) from a lab-scale high-rate denitrifying reactor was discovered to perform nitrate-dependent anaerobic ferrous oxidation (NAFO). The DEC was systematically investigated to reveal their denitrification activity, their NAFO activity, and the predominant microbial population. The DEC was capable of heterotrophic denitrification with methanol as the electron donor, and autotrophic denitrification with ferrous salt as the electron donor named NAFO. The conversion ratios of ferrous-Fe and nitrate-N were 87.41 and 98.74 %, and the consumption Fe/N ratio was 2.3:1 (mol/mol). The maximum reaction velocity and half saturation constant of Fe were 412.54 mg/(l h) and 8,276.44 mg/l, and the counterparts of N were 20.87 mg/(l h) and 322.58 mg/l, respectively. The predominant bacteria were Hyphomicrobium, Thauera, and Flavobacterium, and the predominant archaea were Methanomethylovorans, Methanohalophilus, and Methanolobus. The discovery of NAFO by heterotrophic DEC is significant for the development of wastewater treatment and the biogeochemical iron cycle and nitrogen cycle.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Iron, as one of the most significant elements in microbial evolution, provided energy for the synthesis of ATP, the formation of heme, and some other essential metabolic processes in microorganism by the oxidation from ferrous to ferric [25]. Nitrate-dependent anaerobic ferrous oxidation (NAFO) with ferrous salt as electron donor and nitrate as electron acceptor was a significant discovery in environmental microbiology. The first iron-oxidizing nitrate-reducing bacterium was discovered by Straub et al. [32], which gave rise to a number of important studies on NAFO. A number of bacterial species had been reported to have the ability of NAFO [6, 34], but the biochemistry and enzymology of NAFO are still unclear [7]. Samples of enriched culture from natural habitat such as town ditches and hyper saline sediment were studied for determining the abundance and activity of NAFO in the environment [5, 9]. So far, the distribution of NAFO bacteria have been found in classes Alphaproteobacteria, Betaproteobacteria, Gammaproteobacteria, and Deltaproteobacteria, while NAFO archaea have been classified in Euryarchaeota and Crenarchaeota [10, 13, 14, 33].
In traditional denitrification, organics were essential as an electron donor [23], which not only decreased the cost of wastewater treatment but was also a source of secondary pollution. As the forth most abundant element of the earth’s crust, iron was of great application value [14]. NAFO that reduced nitrate with ferrous salt as electron donor was the solution to treat the unnecessary waste of organics and secondary pollution. In conclusion, the study of NAFO is a significant and new development in the field of biotechnology for simultaneous removal of nitrogen and iron from wastewater since the bioreaction can convert nitrate into nitrogen gas and ferrous salt into ferric salt. The study of NAFO is also helpful to get an insight into the biogeochemical iron and nitrogen cycles due to the fundamental role of biological ferrous conversion in modern and ancient environmental systems on the earth.
Recently, an enriched culture from a lab-scale high-rate denitrifying reactor [21] has been discovered to possess the ability for NAFO. This is the first time to report NAFO of methanol-utilization denitrifying enriched culture (DEC). The objective of this work was to reveal the heterotrophic denitrification activity, the autotrophic NAFO activity, and to investigate the predominant microbial population of the DEC.
Methods
DEC
DEC was obtained from a high-rate denitrifying reactor in the lab. The reactor was operated with methanol as the electron donor and nitrate as the electron acceptor. The volumetric chemical oxygen demand loading rate and volumetric nitrogen loading rate were 181 and 35 kg/(m3 day), respectively.
Synthetic wastewater
The mineral medium contained 0.320 g/l MgCl2·6H2O, 0.350 g/l CaCl2, 0.270 g/l KCl, 0.453 g/l KHCO3, 0.040 g/l KBr, 0.011 g/l H3BO3, 0.001 g/l NaF, 0.010 g/l Na2HPO3·12H2O, 0.005 g/l sodium silicate [13, 19, 33]. In heterotrophic denitrification culture, methanol and nitrate were supplemented to the mineral medium as electron donor and electron acceptor. In NAFO culture, ferrous sulfate and nitrate were supplemented to the mineral medium as electron donor and electron acceptor.
Determination of NAFO activity
The DEC sample was washed twice using 0.9 % NaCl solution prior to the experiment. Ten milliliters of washed DEC sample was put into 65-ml serum bottles containing 40 ml of mineral medium. The control groups were set as follows: CK01 was a reaction system with methanol and nitrate but without DEC; CK11 was a reaction system with ferrous salt and nitrate but without DEC; CK02 was a reaction system with methanol, nitrate, and DEC. The experimental group (NF11) was a reaction system with ferrous salt, nitrate, and DEC. Detailed information has been shown in Table 1. Both the control and experiment groups were in duplicate, and they were cultivated in a shaking table at 30 °C, at 160 rpm. The concentrations of ferrous-Fe and nitrate-N were tested after 48 h.
Determination of NAFO kinetics
A DEC sample was washed twice with 0.9 % NaCl solution. Ten milliliters of washed DEC sample was put into 65-ml serum bottles containing 40 ml of mineral medium. The gradient N concentrations were set as 10, 50, 100, 250, 500, 750, 1,000, and 1,500 mg/l and the gradient Fe concentrations were set as 1,500, 3,000, 7,000, 10,000, 20,000, and 30,000 mg/l.
Analysis of predominant microbial population
Before the DNA extraction, the DEC sample was washed several times using phosphate buffer solution. It was transferred into a beaker flask. Then, the DEC sample was shaken with some glass beads for a few hours to scatter the microbial cells. DNA extraction was performed with Fast DNA SPIN kit for soil (MP Biochemicals, USA), and it was preserved at −20 °C. The polymerase chain reaction (PCR) of bacterial 16S rRNA gene was performed using the following primers: 338F (forward primer: 5′-GCclamp-ACTC CTAC GGGA GGCA G-3′) and 805R (reverse primer: 5′-GACT ACCA GGGT ATCT AATC C-3′). The PCR conditions of the bacterial 16S rRNA gene were as follows: initial denaturation at 94 °C for 10 min; 19 cycles of 94 °C (45 s), 65 °C (45 s, reducing 0.5 °C per cycle) and 72 °C(1 min); 14 cycles of 94 °C(45 s), 55 °C (45 s), and 72 °C (1 min); a final extension at 72 °C for 10 min [12]. The primers for amplifying the archaeal 16S rRNA gene were 787F (forward primer: 5′-GCclamp-ATTA GATA CCCS BGTA GTCC-3′) and 1059R (reverse primer: 5′-GCCA TGCA CCWC CTCT-3′). The PCR conditions of the archaeal 16S rRNA gene were as follows: initial denaturation at 94 °C for 10 min; 34 cycles of 94 °C (45 s), 52 °C (45 s), and 72 °C (1 min); a final extension at 72 °C for 10 min [22, 27]. The PCRs of the bacterial and archaeal 16S rRNA gene were performed using 25-μl reaction volumes containing 2-μl dNTPs mixtures (2.5 mM) (Takara, Japan), 2.5 μl 10× PCR buffer (containing 15 mM magnesium ions) (Takara, Japan), 1 μl of each of the primers (10 mM) (Takara, Japan), 2 μl of the extracted DNA, 0.2 μl rTaq DNA polymerase (Takara, Japan) and 16.3 μl deionized and distilled water (DDW). The amplified products were checked on 1 % (m/v) agarose TAE gels and finally viewed under UV light.
Denaturing gradient gel electrophoresis (DGGE) was used to separate the microbial community. DGGE of the PCR products were performed in a polyacrylamide gel (8 % w/v) with a linear denaturing gradient from 25 to 55 % using a D-code DGGE system (Bio-Rad, Hercules, CA, USA). Electrophoresis was performed in 1× TAE buffer at 200 V and 60 °C for 5 h. Polyacrylamide gels were stained by silver staining and scanned with a UV transilluminator (Bio-Rad) to acquire the DGGE band image.
Bands of interest were excised with a sterile blade, eluted into DDW, and incubated overnight at 4 °C. Eluted bands into DDW were re-amplified using DGGE primer set without GC clamp. PCR products were ligated into moderate PMD 19-T vectors (Takara, Japan) and these vectors were transformed into Escherichia coli-competent cells. After cultivating, E. coli clones were grown on Luria–Bertani medium plates that were supplemented with 100 mg/l ampicillin, 40 mg/l 5-bromo-4-chloro-indolyl-β-d-galactopyranoside (X-Gal), and 24 mg/l isopropyl β-d-1-thiogalactopyranoside (IPTG). White clones were selected randomly for further analysis. The obtained sequences were compared with sequences in GenBank using the BLAST algorithm (http://www.ncbi.nlm.nih.gov/BLAST) to ascertain the predominant microbial population.
Analytical methods
The samples were determined immediately, as the chemical property of Fe was unstable. The concentrations of nitrate, nitrite, Fe, and total iron ions were analyzed according to standard methods [2]. The pH values were determined by a S20K pH meter (Mettler Toledo, Switzerland).
Results
Utilization of methanol and nitrate
To confirm the heterotrophic denitrification activity of DEC, control groups CK01 and CK02 were cultivated for 48 h, and the results are shown in Fig. 1. Basically, methanol and nitrate concentrations remained constant in the CK01 reaction system and the removal ratios were nearly zero. In the CK02 reaction system, methanol and nitrate concentrations decreased simultaneously and the removal ratios of methanol and nitrate were 71.92 and 99.89 %, respectively.
Utilization of ferrous and nitrate
To prove the NAFO activity of DEC, control group CK11 and experimental group NF11 were cultivated for 48 h and the results are depicted in Figs. 2 and 3. In reaction system CK11 (Fig. 2a), the solution was clear but some yellow flocs appeared at the bottom. Nitrate-N concentration was not significantly changed, while Fe concentration decreased by 12.55 %. That means there is almost no chemical reaction between ferrous salt and nitrate under anaerobic conditions. In reaction system NF11 (Fig. 2b), the solution became yellow after cultivation, the surface of DEC showed dark gray, and the bottle wall was adhered with red-brown sediment. The concentrations of Fe and N decreased simultaneously. The removal ratios of Fe and N in reaction system NF11 were 87.41 and 98.74 %, respectively.
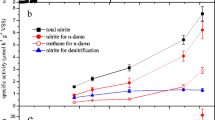
NAFO kinetics of DEC
Gradient substrate concentrations were set to investigate the NAFO kinetics of DEC. Since the Monod [24] model was popular in environmental engineering, it was applied to the fitting of the substrate utilization rate. The NAFO kinetics curve is shown in Fig. 4. According to the NAFO kinetics curve, the maximum reaction velocity and half saturation constant of Fe were 412.54 mg/(l h) and 8,276.44 mg/l, and the counterparts of N were 20.87 mg/(l h) and 322.58 mg/l, respectively.
Predominant microbial population of DEC
The 16S rRNA gene sequences of three brightest bacterial bands in DGGE profiles were compared with their affiliation in GenBank by the BLAST algorithm (Table 2). Clone B1 shared 99 % sequence similarity to Hyphomicrobium zavarzinii strain ZV-580 (GenBank accession no. Y14306.1), clone B2 shared 97 % sequence similarity to Thauera sp. ‘WSPY4 (T-III)’ (GenBank accession no. EF205257.1), and clone B3 shared 96 % sequence similarity to Flavobacterium glaciei strain R6S-5-7 (GenBank accession no. JQ692100.1). According to this, the predominant bacteria of DEC could be placed in clades within Alphaproteobacteria Hyphomicrobium, Betaproteobacteria Thauera, and Bacteroidetes Flavobacterium.
Also, the 16s rRNA gene sequences of the three brightest archaeal bands in DGGE profiles were compared with their affiliation in GenBank by the BLAST algorithm, and the results were shown in Table 2. Clone A1 was closely related to Methanomethylovorans thermophila strain L2FAW (GenBank accession no. NR_043089.1) with maximum identification of 99 %, clone A2 was closely related to Methanohalophilus strain DSM 5219 (GenBank accession no. NR_076739.1) with maximum identification of 98 %, and clone A3 was closely related to Methanolobus sp. St545Mb (GenBank accession no. EU293796.1) with maximum identification of 99 %. In conclusion, the predominant archaea of DEC could be clustered into Methanomicrobia Methanomethylovorans, Methanomicrobia Methanohalophilus, and Methanomicrobia Methanolobus.
Discussion
Heterotrophic denitrification activity
In the experiment, the DEC utilized methanol and N by 71.92 and 99.89 %. It was reported that DEC could respire anaerobically and synthesize cell materials with organic matters as electron donor [23]. The consumption ratio of methanol to nitrate in the experiment was 1.03:1 (mol/mol), which agreed with the denitrification stoichiometric ratio of organics to nitrate [23] (Eq. 1). In other words, the DEC had heterotrophic denitrification activity.
NAFO activity
Generally, a level of at least one micromolar iron is needed for microorganisms to grow in optimum condition [26]. In the experiment, the DEC utilized Fe and N by 87.41 and 98.74 %. The decreased concentration of Fe was 21.85 mM, which was much higher than the essential need of iron for microorganisms growth. The Fe/N consumption ratio was 2.3:1 (mol/mol), which was in agreement with the NAFO stoichiometric ratio from 2:1 to 5:1 (Eqs. 2.A, 2.B). This means that the DEC was capable of autotrophic NAFO as well as heterotrophic denitrification.
Kinetic characteristics of NAFO
The Monod model here was used to calculate the maximum conversion rate and the half saturation constant of substrate. The maximum conversion rate represents the maximum rate achieved by the system at maximum substrate concentrations. The half saturation constant is the substrate concentration at which the reaction rate is half of the maximum conversion rate. It represents the affinity between substrate and microbiology.
In this study, the maximum conversion rate of N by DEC was 20.87 mg/(l h) and the half the saturation constant of N by DEC was 322.58 mg/l, which was higher than the reported values of 35–43.80 mg/l [1, 18]. The maximum conversion rate of Fe by DEC was 412.54 mg/(l h) and the half saturation constant was 8,276.44 mg/l, which was higher than the reported values of 187.9–479 mg/l [8, 28, 29]. This means the DEC had high conversion rates of both ferrous and nitrate, and the DEC showed significant substrate affinity to both ferrous and nitrate.
Function of predominant microbial population
The predominant bacteria of DEC were identified as Hyphomicrobium, Thauera, and Flavobacterium. H. zavarzinii strain ZV-580 was reported to possess cytochrome cd 1 containing nitrite reductase (nirS) and Cu-containing nitrite reductase (nirK) [11], and Hyphomicrobium sp. was reported to dominate the methanol-utilizing bacterial consortium in a denitrification reactor [16]. Thauera sp. ‘WSPY4 (T-III)’ was reported to possess the gene nirS [3], and Thauera was demonstrated to dominate the two denitrification systems with starch/polylactic acid and ethanol as electron donors [31]. F. glaciei strain R6S-5-7 and Flavobacterium daejeonense were shown to have the activity for nitrate reduction [4, 20], and Flavobacterium denitrificans was proved to convert NO3 − to N2 with glucose as electron donor [15]. Above all, Hyphomicrobium, Thauera, and Flavobacterium have shown the activity for heterotrophic denitrification, but no information about NAFO activities of them is available so far.
The predominant archaea of DEC were identified as Methanomethylovorans, Methanohalophilus, and Methanolobus. Methanomethylovorans thermophila strain L2FAW was reported to utilize methanol as the carbon and energy source [17], Methanohalophilus strain DSM 5219 was shown to produce methane from methanol [30], while Methanolobus sp. St545Mb was proven to be a methylotrophic methanoarchaea with methanol as a substrate [36]. Taxonomically, Methanomethylovorans, Methanohalophilus, and Methanolobus belonged to the same family Methanosarcinaceae, which are strictly anaerobic and can produce methane from methanol or methyl amines [35]. Since the seed sludge of the denitrification reactor was taken from the anaerobic reactor to treat papermaking wastewater, it is not strange for methanogen to appear in the DEC as dominant archaea. Up till now, however, no one knows whether the methanogens can carry out NAFO.
References
An S, Stone H, Nemati M (2011) Biological removal of nitrate by an oil reservoir culture capable of autotrophic and heterotrophic activities: kinetic evaluation and modeling of heterotrophic process. J Hazard Mater 190(1):686–693
Association APH, Federation WPC, Federation WE (1915) Standard methods for the examination of water and wastewater, vol. 2. American Public Health Association, Washington, D.C.
Bae H-S, Im W-T, Suwa Y, Lee JM, Lee S-T, Chang Y-K (2009) Characterization of diverse heterocyclic amine-degrading denitrifying bacteria from various environments. Arch Microbiol 191(4):329–340
Bernardet J-F, Segers P, Vancanneyt M, Berthe F, Kersters K, Vandamme P (1996) Cutting a Gordian knot: emended classification and description of the genus Flavobacterium, emended description of the family Flavobacteriaceae, and proposal of Flavobacterium hydatis nom. nov. (basonym, Cytophaga aquatilis Strohl and Tait 1978). Int J Syst Bacteriol 46(1):128–148
Blöthe M, Roden EE (2009) Composition and activity of an autotrophic Fe(II)-oxidizing, nitrate-reducing enrichment culture. Appl Environ Microbiol 75(21):6937–6940
Chakraborty A, Picardal F (2013) Induction of nitrate-dependent Fe(II) oxidation by Fe(II) in Dechloromonas sp. strain UWNR4 and Acidovorax sp. strain 2AN. Appl Environ Microbiol 79(2):748–752
Chakraborty A, Roden EE, Schieber J, Picardal F (2011) Enhanced growth of Acidovorax sp. strain 2AN during nitrate-dependent Fe(II) oxidation in batch and continuous-flow systems. Appl Environ Microbiol 77(24):8548–8556
Chavarie C, Karamanev D, Godard F, Garnier A, Andre G (1993) Comparison of the kinetics of ferrous iron oxidation by three different strains of Thiobacillus ferrooxidans. Geomicrobiol J 11(1):57–63
Emmerich M, Bhansali A, Lösekann-Behrens T, Schröder C, Kappler A, Behrens S (2012) Abundance, distribution, and activity of Fe(II)-oxidizing and Fe(III)-reducing microorganisms in hypersaline sediments of Lake Kasin, southern Russia. Appl Environ Microbiol 78(12):4386–4399
Feinberg LF, Srikanth R, Vachet RW, Holden JF (2008) Constraints on anaerobic respiration in the hyperthermophilic archaea Pyrobaculum islandicum and Pyrobaculum aerophilum. Appl Environ Microbiol 74(2):396–402
Fesefeldt A, Kloos K, Bothe H, Lemmer H, Gliesche C (1998) Distribution of denitrification and nitrogen fixation genes in Hyphomicrobium spp. and other budding bacteria. Can J Microbiol 44(2):181–186
Fredricks DN, Schubert MM, Myerson D (2005) Molecular identification of an invasive gingival bacterial community. Clin Infect Dis 41(1):e1–e4
Hafenbradl D, Keller M, Dirmeier R, Rachel R, Roßnagel P, Burggraf S, Huber H, Stetter KO (1996) Ferroglobus placidus gen. nov., sp. nov., a novel hyperthermophilic archaeum that oxidizes Fe2+ at neutral pH under anoxic conditions. Arch Microbiol 166(5):308–314
Hedrich S, Schlomann M, Johnson DB (2011) The iron-oxidizing Proteobacteria. Microbiology 157(Pt 6):1551–1564. doi:10.1099/mic.0.045344-0
Horn MA, Ihssen J, Matthies C, Schramm A, Acker G, Drake HL (2005) Dechloromonas denitrificans sp. nov., Flavobacterium denitrificans sp. nov., Paenibacillus anaericanus sp. nov. and Paenibacillus terrae strain MH72, N2O-producing bacteria isolated from the gut of the earthworm Aporrectodea caliginosa. Int J Syst Evol Microbiol 55(3):1255–1265
Isaka K, Kimura Y, Osaka T, Tsuneda S (2012) High-rate denitrification using polyethylene glycol gel carriers entrapping heterotrophic denitrifying bacteria. Water Res 46(16):4941–4948
Jiang B, Parshina S, Van Doesburg W, Lomans B, Stams A (2005) Methanomethylovorans thermophila sp. nov., a thermophilic, methylotrophic methanogen from an anaerobic reactor fed with methanol. Int J Syst Evol Microbiol 55(6):2465–2470
Karpuzcu ME, Stringfellow WT (2012) Kinetics of nitrate removal in wetlands receiving agricultural drainage. Ecol Eng 42:295–303
Keller M, Braun F-J, Dirmeier R, Hafenbradl D, Burggraf S, Rachel R, Stetter KO (1995) Thermococcus alcaliphilus sp. nov., a new hyperthermophilic archaeum growing on polysulfide at alkaline pH. Arch Microbiol 164(6):390–395
Kim B-Y, Weon H-Y, Cousin S, Yoo S-H, Kwon S-W, Go S-J, Stackebrandt E (2006) Flavobacterium daejeonense sp. nov. and Flavobacterium suncheonense sp. nov., isolated from greenhouse soils in Korea. Int J Syst Evol Microbiol 56(7):1645–1649
Li W, Zheng P, Wang L, Zhang M, Lu H, Xing Y, Zhang J, Wang R, Song J, Ghulam A (2013) Physical characteristics and formation mechanism of denitrifying granular sludge in high-load reactor. Bioresource Technol 142:683–687
Lins P, Schwarzenauer T, Reitschuler C, Wagner AO, Illmer P (2012) Methanogenic potential of formate in thermophilic anaerobic digestion. Waste Manag Res 30(10):1031–1040
McCarty PL, Smith DP (1986) Anaerobic wastewater treatment. Environ Sci Technol 20(12):1200–1206
Monod J (1949) The growth of bacterial cultures. Annu Rev Microbiol 3(1):371–394
Neilands JB (1981) Iron absorption and transport in microorganisms. Annu Rev Nutr 1:27–46
Neilands JB (1995) Siderophores: structure and function of microbial iron transport compounds. J Biol Chem 270:26723–26726
O’Reilly J, Lee C, Collins G, Chinalia F, Mahony T, O’Flaherty V (2009) Quantitative and qualitative analysis of methanogenic communities in mesophilically and psychrophilically cultivated anaerobic granular biofilms. Water Res 43(14):3365–3374
Özkaya B, Sahinkaya E, Nurmi P, Kaksonen AH, Puhakka JA (2007) Kinetics of iron oxidation by Leptospirillum ferriphilum dominated culture at pH below one. Biotechnol Bioeng 97(5):1121–1127
Pandey R, Malhotra S, Rajvaidya A, Sharma S, Peshwe S, Raman V, Bal A (2004) Chemo-biochemical desulphurization of various gaseous streams on bench scale. Water Air Soil Pollut 154(1–4):295–311
Paterek JR, Smith PH (1988) Methanohalophilus mahii gen. nov., sp. nov., a methylotrophic halophilic methanogen†. Int J Syst Bacteriol 38(1):122–123
Shen Z, Zhou Y, Wang J (2013) Comparison of denitrification performance and microbial diversity using starch/polylactic acid blends and ethanol as electron donor for nitrate removal. Bioresource Technol 131:33–39
Straub KL, Benz M, Schink B, Widdel F (1996) Anaerobic, nitrate-dependent microbial oxidation of ferrous iron. Appl Environ Microbiol 62(4):1458–1460
Völkl P, Huber R, Drobner E, Rachel R, Burggraf S, Trincone A, Stetter KO (1993) Pyrobaculum aerophilum sp. nov., a novel nitrate-reducing hyperthermophilic archaeum. Appl Environ Microbiol 59(9):2918–2926
Weber KA, Pollock J, Cole KA, O’Connor SM, Achenbach LA, Coates JD (2006) Anaerobic nitrate-dependent iron (II) bio-oxidation by a novel lithoautotrophic betaproteobacterium, strain 2002. Appl Environ Microbiol 72(1):686–694
Whitman WB, Goodfellow M, Kämpfer P, Busse H-J, Trujillo ME, Ludwig W, Suzuki K-i, Parte A (2012) Bergey’s manual® of systematic bacteriology, vol. 5. Springer, Berlin Heidelberg New York
Wu S-Y, Lai M-C (2011) Methanogenic archaea isolated from Taiwan’s Chelungpu fault. Appl Environ Microbiol 77(3):830–838
Acknowledgments
Financial support for this work by the National Natural Science Foundation of China (51278457) and Zhejiang Provincial National Science Foundation (Z5110094) are greatly appreciated.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, R., Zheng, P., Xing, YJ. et al. Anaerobic ferrous oxidation by heterotrophic denitrifying enriched culture. J Ind Microbiol Biotechnol 41, 803–809 (2014). https://doi.org/10.1007/s10295-014-1424-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-014-1424-5