Abstract
The dextranase added in current commercial dextranase-containing mouthwashes is largely from fungi. However, fungal dextranase has shown much higher optimum temperature than bacterial dextranase and relatively low activity when used in human oral cavities. Bacterial dextranase has been considered to be more effective and suitable for dental caries prevention. In this study, a dextranase (Dex410) from marine Arthrobacter sp. was purified and characterized. Dex410 is a 64-kDa endoglycosidase. The specific activity of Dex410 was 11.9 U/mg at optimum pH 5.5 and 45 °C. The main end-product of Dex410 was isomaltotriose, isomaltoteraose, and isomaltopentaose by hydrolyzing dextran T2000. In vitro studies showed that Dex410 effectively inhibited the Streptococcus mutans biofilm growth in coverage, biomass, and water-soluble glucan (WSG) by more than 80, 90, and 95 %, respectively. The animal experiment revealed that for short-term use (1.5 months), both Dex410 and the commercial mouthwash Biotene (Laclede Professional Products, Gardena, CA, USA) had a significant inhibitory effect on caries (p = 0.0008 and 0.0001, respectively), while for long-term use (3 months), only Dex410 showed significant inhibitory effect on dental caries (p = 0.005). The dextranase Dex410 from a marine-derived Arthrobacter sp. strain possessed the enzyme properties suitable to human oral environment and applicable to oral hygiene products.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dextrans are high molecular weight exopolysaccharides composed of α-d-glucopyranose units, of which at least 50 % form linear α-1,6 linkages, and the rest are linked by α-1,2, α-1,3 and α-1,4 branching bonds [11]. Dextrans are synthesized from sucrose mainly by Streptococcus mutans, constituting the glucan matrix of dental plaque where dextrans are essential for adhesion and cariogenicity of the dental plaque. Dextrans play a crucial role in the development, proliferation, and structural integrity of the dental plaque [8, 19].
Solutions to remove or prevent dental plaque mainly rely on mechanical cleaning, antimicrobial agents, and degradation of the plaque matrix that contains protein, dextran, and lipids [24, 27]. Degradation of the dextran with dextranase (EC 3.2.1.11) has been identified as a safe and effective way to prevent dental plaque and further caries [2, 4]. Microbial dextranase is produced mainly by bacteria and fungi and has been proven to be effective in preventing dental caries [13]. Fungal dextranase showed higher optimum temperature that ranges between 50 and 60 °C compared to bacterial dextranase that averages 40 °C [11]. Bacterial dextranase was considered to be more stable and effective at the oral temperature (around 35.5 °C) and suitable for industrial application as a dental caries-preventing agent [13, 22]. Thus more new bacterial dextranases are desired to be explored for preventing dental caries.
The dextranase added in current commercial dextranase-containing mouthwashes is largely from fungi, such as Penicillium lilacinum and Chaetomium erraticum [6, 19]. Due to the fact that marine enzymes have properties like high salt tolerance, hyperthermostability, and low optimum temperature, the marine bacterial dextranase should be suited for the human oral environment. Thus, the effectiveness of current commercial dextranase mouthwashes in preventing dental caries could be enhanced by replacing the fungal dextranases with marine bacterial ones. In future development of efficient dextranase-containing anticariogenic products, the marine bacterial dextranase must be greatly emphasized.
In this study, a dextranase (Dex410) from marine Arthrobacter sp. was purified and characterized. The effectiveness of Dex410 on prevention and reduction of S. mutans biofilm was assessed. The inhibitory effect of a Dex410 solution (Dex410S) at the mean MBIC90 (6 U/ml) on dental caries developed with multiple oral strains was evaluated by rat experiment. The time course of S. mutans biofilm formation in the presence of Dex410 was examined by light and scanning electron microscopy. Biofilm coverage, biomass, water-soluble and insoluble glucans of the biofilm grown for different lengths of time within 24 h were also determined.
Materials and methods
Isolation of dextranase-producing marine bacterial strains
The screening was carried out with samples of beach mud, fishes, and seaweeds from the lower part of the intertidal zone of Lianyungang port, China. Gills and intestines of the fishes and the seaweeds were sheared and triturated separately in a mortar with sea sand to homogenate. The beach mud and the homogenates from seaweeds and fishes were diluted in appropriate volumes of sterile distilled water. The dilutions were spread evenly over the screening medium plates containing (g/l): peptone 5, yeast extract 1, blue dextran 2, dextran T2000 8, agar 20, dissolved in filtered seawater through a 0.45-μm-pore-size mixed cellulose ester filter (Millipore, Bedford, MA, USA), pH 8.0. After incubation of the plates at 25 °C for 2 days, the microorganisms showing an extracellular dextranase activity were screened by observing the transparent circles. Colonies with a clear halo were further isolated and identified. The producing strains in the same inoculum size were then cultured in 250-ml Erlenmeyer flasks containing 100 ml of the screening medium (without agar) with shaking at 25 °C for 24 h at 180 rpm. Dextranase productivity of the strains was evaluated by measuring the enzyme activity of the supernatant of the cultures.
Identification of a dextranase-producing marine bacterial strain Arth410
A dextranase-producing marine bacterial strain Arth410 was first identified by colonial morphology and microscopy. A series of biochemical reactions were then performed according to Bergey’s Manual of Determinative Bacteriology (9th Edition, Baltimore: Williams & Wilkins), such as methyl red (MR) test, Voges-Proskauer (VP) test, indole test, and catalase test. The genomic DNA of the strain Arth410 was extracted using a SK1201 DNA extraction kit (Sangon, China). PCR amplification of 16S rRNA gene was performed using the primers 27F (5′-AGAGTTTGATCCTGGCTCAG-3′) and 1492R (5′-GGTTACCTTGTTACGACTT-3′) [26]. The PCR reaction mixture contained 1× Ex Taq buffer (Takara Bio Inc., Japan), 0.2 mM each dNTP, 0.02 U/μl Ex Taq polymerase, and 0.1 μM each primer. The PCR condition was one cycle of pre-denaturation at 94 °C for 2 min, 34 cycles of denaturation at 94 °C for 30 s, annealing at 60 °C for 45 s, and elongation at 72 °C for 45 s, and one cycle of post-elongation at 72 °C for 7 min. The 16-s rRNA gene amplicon was sequenced and compared with the reported sequences in GenBank using BLASTN with default setting.
Determination of enzyme properties of Dex410
Enzyme preparation and in-gel activity assay
One-day culture of the strain Arth410 in a 250-ml flask was centrifuged at 10,000 rpm for 10 min. The supernatant was collected and filtered through a hollow-fiber cross flow filtration cartridge (30-kDa nominal molecular weight cut-off) with a Quixstand Benchtop System (GE Healthcare, Waukesha, WI, USA). The filtrate was further purified by column chromatography on DEAE-Sepharose (Pharmacia) pre-equilibrated with 10 mM sodium phosphate buffer, pH 7.0. Dextranase was eluted using a linear gradient with an increasing concentration of sodium phosphate. A 5-μl aliquot of the eluate was applied to 10 % SDS–polyacrylamide gel electrophoresis (PAGE) to estimate purity and apparent molecular mass of the dextranase. The purified protein was desalted, concentrated by filtration as described above, and then lyophilized to powder and stored at −20 °C until use. For in-gel activity assay, the dextranase preparation was applied to 10 % native PAGE gel containing 0.5 % blue dextran and the dextranase activity was detected as a clear band on blue background.
Enzyme properties and activity assay
Dextranase activity was measured by incubation of 20 μg/ml of Dex410 in 50 mM sodium acetate buffer (pH 5.5) with 1 % (w/v) dextran T2000 (Pharmacia) at 45 °C for 15 min. The amount of reducing sugars liberated was measured by the 3,5-dinitrosalicylic acid method [20]. One unit of dextranase enzyme activity was defined as enzyme that released 1 μM of isomaltose per minute under the assay conditions.
The effect of pH on enzyme activity was determined at a pH of 4–9 and temperature of 45 °C. The pH of 4.0–5.5, 6.0–7.5, and 8.0–9.0 were maintained by sodium acetate buffer (50 mM), sodium phosphate buffer (50 mM) and Tris–HCl buffer (50 mM), respectively. The effect of temperature on enzyme activity was determined by incubating the reaction mixture at 0–60 °C for 15 min in 50 mM NaAc buffer (pH 5.5).
End-product analysis
Six units of Dex410 per milliliter was incubated with 1 % dextran T2000 in 50 mM NaAc buffer (pH 5.5) at 25 °C for 24 h. The hydrolysis product of Dex410 were analyzed by thin layer chromatography (TLC) using a silica gel 60 plate (Merck, Darmstadt, Germany) developed in a solvent system of chloroform/methanol/water, 3/3/1 (v/v/v) with glucose and isomaltooligosaccharides (from isomaltose to isomaltoheptaose, purchased from Seikagaku Co., Japan) as the standards. A mixture of dextran T2000 and heat-inactivated Dex410 was used as a control. The sugars were visualized by spraying onto the plate a diphenylamine/aniline/phosphate reagent and followed by heating at 85 °C for 10 min [1].
Assessment of inhibition and reduction effects of Dex410 on S. mutans biofilm
Streptococcus mutans ATCC 25175 was inoculated into brain heart infusion (BHI) medium in test tubes and grown for 24 h at 37 °C to OD600 of 1.0. The 24-h culture was then used as an inoculum, and 20 μl of which was inoculated in wells of a 96-well polystyrene microtiter plate (Falcon 3072, Becton–Dickinson, San Jose, CA, USA), each well containing 180 μl of BHI medium supplemented with 1 % (w/v) sucrose (BHIS). Dex410 was sterilized by filtration (Amicon 10-kDa molecular weight cut-off membrane; Millipore Co., MA, USA) and added to the wells to final concentrations ranging from 1 to 10 U/ml. Biofilms of S. mutans ATCC 25175 (n = 3 for each the concentration of Dex410) were formed on inner walls of each well by culturing at 37 °C and 5 % CO2 for 24 h without agitation [25]. After incubation, the medium was decanted and the biofilm in wells was rinsed three times with stroke-physiological saline solution to remove loosely attached cells. The biofilms were then fixed with methanol for 15 min and then stained with 200 μl of 0.1 % (w/v) crystal violet for 10 min [25]. After staining, the biofilms were rinsed with distilled water to remove excess dye, and followed by adding 200 μl of 95 % ethanol to each well. The plate was then shaken horizontally at room temperature for 30 min. The inhibitory effect of Dex410 on S. mutans biofilm was quantified by measuring the absorbance at 595 nm with an enzyme-linked immunosorbent assay microplate reader (model 3550; Bio-Rad Laboratories, Richmond, CA, USA). Each assay was performed in triplicate. Wells containing 200 μl BHIS without the inoculum and Dex410 were used as blank controls after staining. Wells containing 90 % BHIS, 20 % inoculum, and no Dex410 were negative controls. Wells containing 0.4, 0.8, 1.2, 1.6, and 2.0 mg/l chlorhexidine (Sigma Chemical Co., St. Louis, MO, USA) were used as positive controls. The percentage of inhibition or reduction was calculated using the equation (1–A595 of the test/A595 of non-treated control) 100 [25]. The minimum biofilm inhibition concentration (MBIC50) was defined as the lowest Dex410 concentration that showed 50 % or more inhibition on the formation of biofilm [25].
To assess the reduction effect of Dex410, the 24-h S. mutans biofilm was formed in the wells of a 96-well polystyrene microtiter plate containing 200 μl TSB medium per well in the absence of Dex410. The developed biofilms were rinsed three times with stroke-physiological saline solution. Fresh prepared BHIS media containing gradient Dex410 at concentrations of 1–10 U/ml were added to the biofilms. The plate was then incubated at 37 °C for 24 h. The biofilms were then quantified as described above. Minimum biofilm reduction concentration (MBRC50) was defined as the lowest Dex410 concentration that showed reduction of the biofilm by 50 % or more [25].
Microphotography of formation of S. mutans biofilm treated with Dex410 at the concentration of MBIC90
Twenty-microliter aliquots of the overnight culture (OD600 = 1.0) were dispensed to each well of a 24-well polystyrene microtiter plate, each seeded with an 8 × 8-mm coverslip in a horizontal position, containing 180 μl of BHIS and 6 U/ml Dex410. Biofilms of S. mutans ATCC 25175 (n = 4 for each time point) were formed on the coverslips by incubation without disturbance at 37 °C and 5 % CO2 for 3, 6, 9, 15, 18, 21, and 24 h. Biofilms grown on the surface of the coverslips for different times were rinsed three times with distilled water, fixed in 2.5 % glutaraldehyde for 2 h, and followed by post-fixation in 1 % osmic acid dissolved in 0.1 M cacodylate buffer for 1.5 h. The biofilms were then dehydrated through an ethanol gradient from 50 to 90 %, air dried, and sputter coated with aurum. The samples were photographed by SEM (Hitachi S-4000; Hitachi Instruments Inc., San Jose, CA, USA).
Assay for biomass, biofilm coverage, water-soluble glucan (WSG), and water-insoluble glucan (WIG) of the biofilms
Biofilms formed at the different time points (as described above) were gently rinsed with distilled water to remove the unattached cells. The biofilms were each placed in 2 ml of distilled water, and the biofilm mass was harvested by scraping with a sterile spatula and aspiration. The biofilm mass was then freeze-dried. The dry weight (μg/mm2) was determined in triplicate for each experiment. Coverslips not inoculated with S. mutans ATCC 25175 (n = 3 for each of the time points above) were used as blank controls.
Biofilms were observed under a Nikon 90i microscope and photographed with a DXM1200F camera at a magnification of 10×. Images from five random microscopic fields per coverslip (n = 3) were captured. Biofilm coverage at the different time points was analyzed using the Image Pro-Plus image processing and analysis software, v. 6.0 (Media Cybernetics, Bethesda, MD, USA) and represented as the mean of coverage (biofilm area per image area, %) per microscopic field. The data represent mean ± SD of three experiments performed in triplicate. For measuring water-soluble glucan (WSG) and water-insoluble glucan (WIG), biofilms were grown in BHIS supplemented with [14C-glucose] sucrose (0.02 μCi/ml; PerkinElmer) according to Koo et al. [16]. WSG and WIG of the biofilms (n = 3) at each of the time points were quantified. The biofilms were sonicated in distilled water for homogeneous dispersion. One milliliter of the cell suspension was centrifuged at 12,000 rpm for 10 min at 4 °C. The supernatant was collected. The cell pellet was resuspended in 1 ml of distilled water, centrifuged, and the supernatant was collected, and this procedure was repeated twice. All the supernatants were pooled and three volumes of cold ethanol were added. The mixture was placed at 4 °C overnight. The precipitate (containing WSG) was recovered by centrifugation and washed three times with cold ethanol and lyophilized. The WIG in the washed cell pellet was extracted using 0.5 mol/l NaOH as detailed by Koo et al. [5]. The extract (containing WIG) was precipitated with three volumes of cold ethanol and lyophilized. Both the WSG and WIG were determined by scintillation counting as described by Koo et al. [17].
Animal experiments
Streptococcus mutans ATCC 25175, Streptococcus sanguis ATCC10556, Streptococcus salivarius ATCC 13419, Actinomyces viscosus ATCC 15987, and Latobacillus casei ATCC 393 were separately cultured in BHI medium at 37 °C and 5 % CO2 until the OD600 of each culture reached 1.0. Then the five cultures were pooled in equal volume to generate a mixed-strain inoculum for the rat experiment. A 6 U/ml Dex410 solution (Dex410S) in 50 mM sodium acetate buffer (pH 5.5) was prepared for treating rats that were fed a cariogenic diet.
The animal study was approved by the local animal ethics committee and conformed to the animal protection law of the People’s Republic of China. Female Wistar rats, 32 for the 1.5-month (M) experiment and 32 for the 3-month experiment were all weanling rats (21 days of age). The inoculation of all 64 rats was performed once a day for three consecutive days by adding 100 μl of the multiple-strain cell suspension to their oral cavity with a micropipettor (from 24–28 days of age). All 64 rats were fed with a normal pellet cereal-based diet LAD1000G (TROPHIC Animal Feed High-tech Co Ltd, Nantong, China) and distilled water during the first 3 days of inoculation and then fed a cariogenic diet LAD1000G supplemented with 50 % (w/w) sucrose. Both for the 1.5- and 3-month treatment, the rats at the age of 24 days were randomly divided into four groups (8 for each of the groups) and treated once a day with Dex410S, Biotene PBF mouthwash (Laclede Professional Products, Gardena, CA, USA), deionized water, and the 50 mM sodium acetate buffer, respectively. The treatment was performed by adding 200 μl of each of the four liquids to the oral cavity of rats with a micropipettor.
The rats (at the age of 66 days in 1.5-month treatment and 81 days in 3-month treatment) were killed by decapitation. The jaws were removed and autoclaved at a pressure of 10 Ib/in2 for 10 min to facilitate removal of the muscle and connective tissue. The teeth were cleaned by removing the periodontium and immersed in 2 % ammonium hydroxide for 30 min, and finally stained with 0.4 % murexide for 12 h. Dental caries in the crown and cervical region of maxillary and mandibular molars were observed in a Nikon SMZ 1500 stereomicroscope. Scoring of caries was performed according to Keyes’ method [12]. The statistical significance of the difference between the groups was evaluated by Student’s t test. Statistically significant tests were set at a p value of <0.05.
Results and discussion
Identification of the strain Arth410
The strain Arth410 was a non-sporeforming, Gram-positive bacterium without flagella. The strain fermented glucose and no acid or gas was produced. The strain Arth410 was catalase positive, MR test negative, VP test negative, and indole test negative. The strain could not survive incubation with 5 % skimmed milk at 65 °C for 30 min. The detailed physiological properties were consistent with the Arthrobacter genus (Table 1). The 16S rRNA gene sequence (1, 485 bp) was submitted to GenBank under an accession number of JX481352 and showed 99 % identity to that of the most Arthrobacter sp. deposited in GenBank. Thus, the strain was preliminary identified as an Arthrobacter sp.
Arthrobacter sp. has begun to be a main source of dextran hydrolases that act mainly on the α-1,6 glycosidic bond, such as dextranase from A. oxydans [14, 18], glucodextranase (EC 3.2.1.70) from A. globiformis T-3044 and I42 [21, 23], and isomaltodextranase (EC 3.2.1.94) from A. globiformis T6 and A. dextranolyticus [9, 10]. All dextranase-producing Arthrobacter sp. strains that have been reported so far are from terrestrial environments. To our knowledge, this is the first report on a dextranase produced by marine Arthrobacter sp.
Enzyme properties of Dex410
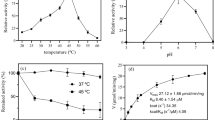
The calculated molecular weight of the Dex410 was 64 kDa on a 10 % SDS-PAGE gel and the Dex410 protein appeared homogeneous by Coomassie stained SDS-PAGE (Fig. 1). TLC analysis showed that the major end-products of dextran T2000 hydrolysis by Dex410 were isomaltotriose, isomaltotetraose, and isomaltopentose (Fig. 2). Thus, Dex410 was identified as an endo-hydrolyzing dextranase that had been shown to have higher efficiency in degrading plaque matrix than exo-type dextranase [13]. The specific activity of Dex410 was assayed as 11.9 U/g. The optimum pH and temperature of Dex410 were 5.5 and 45 °C, respectively (Fig. 3).
a A Coomassie-stained 10 % SDS–PAGE gel showing the purity and size of the dextranase Dex410. M Molecular weight standard (Fermentas, SM0431); L1 Dex410 preparation; L2 crude fermentation broth of Arthrobacter sp. strain Arth410. b Clear band showing the dextranase activity of Dex410 on a 10 % native PAGE gel containing 0.5 % (w/v) blue dextran. The numbers on the left indicate the size of the markers
TLC of dextran hydrolysis with the dextranase Dex410. The reaction consisting of 1 % dextran T2000, 6 U/ml of Dex410, and 50 mM NaAc buffer (pH5.5) was incubated at 25 °C for 24 h. Lanes 1–7, the standards glucose, isomaltose, isomaltotriose, isomaltotetrose, isomaltopentose, isomaltohexaose, isomaltoheptaose, respectively; lane 8, the heat-inactivated control; lanes 9–11, the enzymatic digest for 6, 12, and 24 h, respectively. Development was done with a solvent system of chloroform/methanol/water, 3/3/1 (v/v/v)
Effect of pH (a) and temperature (b) on the enzyme activity of Dex410. The reactions consisting of 1 % dextran T2000, 6 U/ml of Dex410, and buffers were performed by incubation for 15 min. The effect of pH on enzyme activity was determined at 45 °C in sodium acetate buffer (50 mM), sodium phosphate buffer (50 mM), and Tris–HCl buffer (50 mM). The effect of temperature was determined at 0–60 °C in 50 mM NaAc buffer (pH 5.5)
The commercial dextranase dextranase 50 l from P. lilacinum showed optimum temperature of 55 °C and pH 4.5, and retained <50 % of its maximum activity at 37 °C and pH 6 [19]. However, Dex410 retained more than 85 % of its maximum activity at 37 °C and pH 5.5. Compared with Dex410 50 l, Dex410 should be more suitable to be used in the oral environment in terms of the fact that dental caries usually form at a pH in plaque lower than 5.5 [3].
Though with exactly the same nucleotide sequence registered in GenBank (Accession no. DQ497801), Arthrobacter sp. dextranase Dex2 reported by Kim and AODex reported by Lee showed different optimum pH, temperature, and specific activity when subjected to different conditions such as reaction buffers and the enzyme/substrate ratios [14, 18]. It is therefore impractical to compare Dex410 with Dex2 in enzyme properties despite the fact that they are both endo-type dextranases and have similar end-products.
Effect of different concentrations of Dex410 on the inhibition and reduction of S. mutans biofilm
The inhibition on formation of S. mutans biofilm and the reduction on developed S. mutans biofilm in vitro are shown in Fig. 4. Dex410 of different concentrations was incubated with S. mutans in vitro for a non-physiologically relevant incubation time (24 h), and the data gained might not reflect the practical time course of inhibition or reduction by in vivo treatment, since agents in oral-care products always contact the biofilms in very short times.
Inhibition and reduction of S. mutans biofilm by Dex410 at different concentrations. The closed diamonds and triangles refer to inhibition of biofilm formation and reduction of developed biofilm, respectively. For assessment of growth inhibition effect of Dex410, the S. mutans biofilms were cultured in 96-well microtiter plates at 37 °C for 24 h in the presence of Dex410 at different initial concentrations. For assessment of reduction effect of Dex410, 24-h S. mutans biofilms were developed as described in the text. BHIS medium with different concentrations of Dex410 was added to the biofilms, followed by a further incubation at 37 °C for 24 h. Cultures without Dex410 were control. The biofilm assay was performed as described in the text. Mean values from the Dex410-treated cultures were expressed as a percentage of control values. Data represent the mean and SD of two independent tests with duplicates for each
The inhibition enhanced gradually with increasing concentrations of Dex410. MBIC50 of Dex410 was in the range of 1–5 U/ml (1.27–6.35 μM) and the mean 2 U/ml (2.54 μM). MBIC90 of Dex410 was 4–8 U/ml and the mean 6 U/ml. The reduction of 24-h S. mutans biofilms by 50 % or above was achieved in the presence of Dex410 at MBRC50 of 3–7 U/ml (3.81–8.89 μM). The MBRC50 was lower than that of chlorhexidine (>20 μM), an effective antibacterial widely used in oral hygiene products. The mean MBIC50 of Dex410 (2.54 μM) was similar to that of chlorhexidine (2.6 μM).
In the presence of 6 U/ml Dex410, the formation of biofilm by planktonic S. mutans cells was significantly inhibited by about 90 % (p < 0.05). In contrast, at the same concentration, Dex410 reduced by only about 60 % the formed S. mutans biofilm (Fig. 4). The results suggested that Dex410 was more effective in inhibiting biofilm formation than removing developed biofilm.
Inhibitory effect of 6 U/ml Dex410 on the formation of S. mutans biofilm
For the control group, microscopical analysis showed that a conditioning film was formed on the coverslip surface at 3 h (Fig. 5). No apparent aggregation of the S. mutans cell observed during 3–6 h and the surface was largely unattached. The cells begun to adhere to the pellicle and aggregate quickly to form patches at 6 h. Then the cells spread the entire field of the surface and formed an integrate biofilm at 9 h. The coverage at 9 h reached approximately 100 %, and the fluid channel of a biofilm could be observed. The biofilm became dense and more complex from 9 to 24 h with the coverage stabilized around 100 %. For the treatment group, the time-course coverage of biofilm on coverslips from 0 to 24 h was suppressed to the range of 10–21 % (Fig. 6a). It was not until at 9 h that patches formed leaving more than 95 % of the surface unattached from 9 to 24 h. The biofilm coverage increased to a maximum about 21 % at 9 h but thereafter decreased from 18 h to a minimum about 10 % at 24 h.
SEM micrographs of the S. mutans biofilm formation on coverslip surface for 0–24 h. Biofilms were grown in BHIS in the presence or absence of Dex410 (6 U/ml). All images shown were taken at a magnification of 3,000×. The selected images were chosen as the best representatives of the amount of biofilms on the coverslip surfaces. Each image is marked with experimental group and biofilm age in the top-left corner where “T” and “C” refer to the treated and the control biofilms, respectively. The images were acquired at a gray-scale resolution of 8 bits and an image size of 1,280 × 960 pixels. Biofilm patches smaller than 10 pixels were omitted from our analysis
In the presence of Dex410, the WSG content of the 24-h biofilms was inhibited by more than 95 % compared with the control group (Fig. 6b). The WSG content of the Dex410-treated biofilms was suppressed to no more than 10 μg/ml during 24 h of growth and a minimum of 1.131 ± 0.5 μg/ml at 24 h. The average WIG content (76.787 ± 3.8) of the Dex410-treated biofilms grown for 24-h was close to that of the control group (78.606 ± 3.9).
Biofilm mass of the control group increased steeply from 6 to 9 h and thereafter slowly to a maximum value of 12.313 ± 1.719 μg/mm2 at 24 h (Fig. 6c). Biofilm mass of the Dex410-treated group reached a maximum of 1.172 ± 0.938 μg/mm2 at 9 h and then decreased modestly to 0.606 ± 1.25 μg/mm2 at 24 h. Meanwhile, the WSG and WIG only slightly increased from 9 to 15 h in the control groups. The results implied that the cells of the control biofilms increased quickly from 9 to 15 h in terms of the complete glucan matrix formed in the biofilms. However, during the same period, cells could not effectively accumulate in the Dex410-treated biofilms, as the adhesiveness of the glucan matrix was reduced due to the hydrolysis by Dex410 [19].
Assessment of inhibitory effect of Dex410 on dental caries by animal experiment
In the 1.5- and 3-month treatment groups, caries mainly occurred in the occlusal pits, fissures, and proximal regions of teeth. No caries was observed in lingual or buccal aspect of the crown of all the molars and incisors. In the 1.5-month treatment, caries affected only the crown of all the molars. No caries was observed in the cervical region of all the molars. In the 3-month treatment, both the crown and cervical region of all the molars were affected by caries.
The statistical analysis showed that in the short-term treatment (1.5 months), both Dex410S and Biotene had significant inhibitory effect on caries (p = 0.0008 and 0.0001, respectively) compared with the control. The average scores of the Dex410S and the Biotene group were 1.667 ± 1.221 and 1.333 ± 0.816, respectively (Fig. 7). However, in the long-term treatment (3 months), only Dex410S showed a significant inhibitory effect on caries (p = 0.005). Both in the 1.5- and 3-month experiments, there was no statistically significant difference between the buffer and the control groups. The results indicated that Dex410 could be suitable for long-term prevention of dental caries.
Mean caries scores from rats infected with five oral strains after treatment with Dex410 for 1.5 and 3 months. The blank and filled columns refer to the 1.5- and the 3-month experiments, respectively. All groups were fed a cariogenic diet and distilled water. Control, treated with deionized water; Dex410S, treated with Dex410 buffer solution; Biotene, treated with commercial Biotene mouthwash; Buffer, treated with the Dex410-free buffer. All four jaw quadrants from each rat were scored for caries by the method of Keyes [12]. Stars above the error bars indicate groups that are statistically different from control groups in caries scores. *p < 0.05 compared with the 3-months control; **p < 0.001 compared with the 1.5-month control. The strains used were S. mutans ATCC 25175, S. sanguis ATCC10556, S. salivarius ATCC 13419, A. viscosus ATCC 15987, and L. casei ATCC 393
Biotene is a dextranase-containing mouthwash that had shown caries prevention capability [7, 15]. The dextranase in Biotene was from C. erraticum at a concentration of 0.0013 % (w/w) according to the manufacturer’s specification. The optimum temperature of the dextranase was 50–60 °C and the specific activity of it at 40 °C and pH 4.5 was 6,356.3 U/g [6]. The specific activity was about 0.3 U/g by converting to the unit defined in this study. Thus, the dextranase activity of Biotene was about 0.004 U/ml, and much lower than that of Dex410 (6 U/ml). Despite other antiseptic enzymes added in Biotene like lactoperoxidase, glucose oxidase, and lysozyme, the low dextranase units per milliliter of the Biotene could account for its ineffectiveness in long-term prevention of dental caries.
Conclusions
Dextranase Dex410 from a marine-derived Arthrobacter sp. strain possessed the enzymatic properties suitable for the oral environment and applicable for oral hygiene products. Dex410 hydrolyzed dextran T2000 resulting in an end-product mainly as isomaltotriose by endo-type mechanism. Dex410 showed a better performance in inhibiting S. mutans biofilm formation than reducing the biofilm that had already formed. Dex410 effectively inhibited the S. mutans biofilm coverage, biomass, and WSG by more than 80, 90, and 95 %, respectively. Dex410 was more effective in inhibiting dental caries for long-term use than the commercial Biotene mouthwash.
References
Anderson K, Li SC, Li YT (2000) Diphenylamine-aniline-phosphoric acid reagent, a versatile spray reagent for revealing glycoconjugates on thin-layer chromatography plates. Anal Biochem 287:337–339
Bowen W (1971) The effect of dextranase on caries activity in monkeys (Macaca irus). Br Dent J 131:445
Bradshaw DJ, Marsh PD (1998) Analysis of pH-driven disruption of oral microbial communities in vitro. Caries Res 32:456–462
Caldwell RC, Sandham HJ, Mann WV Jr, Finn SB, Formicola AJ (1971) The effect of a dextranase mouthwash on dental plaque in young adults and children. J Am Dent Assoc 82:124–131
Cury JA, Rebelo MA, Del Bel Cury AA, Derbyshire MT, Tabchoury CP (2000) Biochemical composition and cariogenicity of dental plaque formed in the presence of sucrose or glucose and fructose. Caries Res 34:491–497
Eggleston G, Monge A (2005) Optimization of sugarcane factory application of commercial dextranases. Process Biochem 40:1881–1894
Featherstone JD, Singh S, Curtis DA (2011) Caries risk assessment and management for the prosthodontic patient. J Prosthodont 20:2–9
Gibbons RJ, Banghart SB (1967) Synthesis of extracellular dextran by cariogenic bacteria and its presence in human dental plaque. Arch Oral Biol 12:11–23
Hatada Y, Hidaka Y, Nogi Y, Uchimura K, Katayama K, Li Z, Akita M, Ohta Y, Goda S, Ito H, Matsui H, Ito S, Horikoshi K (2004) Hyper-production of an isomalto-dextranase of an Arthrobacter sp. by a proteases-deficient Bacillus subtilis: sequencing, properties, and crystallization of the recombinant enzyme. Appl Microbiol Biotechnol 65:583–592
Iwai A, Ito H, Mizuno T, Mori H, Matsui H, Honma M, Okada G, Chiba S (1994) Molecular cloning and expression of an isomalto-dextranase gene from Arthrobacter globiformis T6. J Bacteriol 176:7730–7734
Jiménez ER (2009) Dextranase in sugar industry: a review. Sugar Tech 11:124–134
Keyes PH (1958) Dental caries in the molar teeth of rats II. A method for diagnosing and scoring several types of lesions simultaneously. J Dent Res 37:1088–1099
Khalikova E, Susi P, Korpela T (2005) Microbial dextran-hydrolyzing enzymes: fundamentals and applications. Microbiol Mol Biol Rev 69:306–325
Kim YM, Ko EA, Kang HK, Kim D (2009) Construction, expression and characterization of fusion enzyme from Arthrobacter oxydans dextranase and Klebsiella pneumoniae amylase. Biotechnol Lett 31:1019–1024
Kocak MM, Ozcan S, Kocak S, Topuz O, Erten H (2009) Comparison of the efficacy of three different mouth rinse solutions in decreasing the level of Streptococcus mutans in saliva. Eur J Dent 3:57–61
Koo H, Hayacibara MF, Schobel BD, Cury JA, Rosalen PL, Park YK, Vacca-Smith AM, Bowen WH (2003) Inhibition of Streptococcus mutans biofilm accumulation and polysaccharide production by apigenin and tt-farnesol. J Antimicrob Chemother 52:782–789
Koo H, Rosalen PL, Cury JA, Park YK, Bowen WH (2002) Effects of compounds found in propolis on Streptococcus mutans growth and on glucosyltransferase activity. Antimicrob Agents Chemother 46:1302–1309
Lee JH, Nam SH, Park HJ, Kim Y-M, Kim N, Kim G, Seo E-S, Kang S–S, Kim D (2010) Biochemical characterization of dextranase from Arthrobacter oxydans and its cloning and expression in Escherichia coli. Food Sci Biotechnol 19:757–762
Marotta M, Martino A, De Rosa A, Farina E, Carten M, De Rosa M (2002) Degradation of dental plaque glucans and prevention of glucan formation using commercial enzymes. Process Biochem 38:101–108
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428
Mizuno M, Tonozuka T, Suzuki S, Uotsu-Tomita R, Kamitori S, Nishikawa A, Sakano Y (2004) Structural insights into substrate specificity and function of glucodextranase. J Biol Chem 279:10575–10583
Moore RJ, Watts JT, Hood JA, Burritt DJ (1999) Intra-oral temperature variation over 24 hours. Eur J Orthod 21:249–261
Oguma T, Kurokawa T, Tobe K, Kitao S, Kobayashi M (1999) Cloning and sequence analysis of the gene for glucodextranase from Arthrobacter globiformis T-3044 and expression in Escherichia coli cells. Biosci Biotechnol Biochem 63:2174–2182
Peterson SN, Snesrud E, Liu J, Ong AC, Kilian M, Schork NJ, Bretz W (2013) The dental plaque microbiome in health and disease. PLoS ONE 8:e58487
Wei GX, Campagna AN, Bobek LA (2006) Effect of MUC7 peptides on the growth of bacteria and on Streptococcus mutans biofilm. J Antimicrob Chemother 57:1100–1109
Weisburg WG, Barns SM, Pelletier DA, Lane DJ (1991) 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol 173:697–703
Xavier JB, Picioreanu C, Rani SA, van Loosdrecht MC, Stewart PS (2005) Biofilm-control strategies based on enzymic disruption of the extracellular polymeric substance matrix–a modelling study. Microbiology 151:3817–3832
Acknowledgments
This study was supported by the National High-tech R&D Program of China (863 Program, Grant No. 2011AA09070302), the Priority Academic Program Development of Jiangsu Higher Education Institutions, and the Industrial R&D Program of Lianyungang (Grant No. CG1003).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jiao, YL., Wang, SJ., Lv, MS. et al. Characterization of a marine-derived dextranase and its application to the prevention of dental caries. J Ind Microbiol Biotechnol 41, 17–26 (2014). https://doi.org/10.1007/s10295-013-1369-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-013-1369-0