Abstract
FK506 is a clinically important macrocyclic polyketide with immunosuppressive activity produced by Streptomyces tsukubaensis. However, the low titer at which it is produced is a bottleneck to its application and use in industrial processes. We have overexpressed five potential targets associated with FK506 production (fkbO, fkbL, fkbP, fkbM, fkbD) which were identified in our previous study, with the aim to improve FK506 production. The results of the analysis showed that the constructed strains with an additional copy of each gene increased FK506 production by approximately 10–40 % compared with the wild-type strain D852. The results of the gene expression analysis indicated that each gene was upregulated. Combinatorial overexpression of the five genes resulted in a 146 % increase in the FK506 titer to 353.2 mg/L, in comparison with the titer produced by D852. To further improve the production of FK506 by the engineered strain HT-FKBOPLMD, we supplemented the medium with various nutrients, including soybean oil, lactate, succinate, shikimate, chorismate, lysine, pipecolate, isoleucine and valine. Optimization of feeding concentrations and times resulted in HT-FKBOPLMD being able to produce approximately 70 % more FK506, thereby reaching the maximal titer of 457.5 mg/L, with lower amounts of by-products (FK520 and 37,38-dihydro-FK506). These results demonstrate that the combination of the metabolically engineered secondary pathways and the exogenous feeding strategies developed here was able to be successfully applied to improve the production of industrially and clinically important compounds.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
FK506 (tacrolimus) is a 23-membered polyketide macrolide with immunosuppressant activity that was discovered in the culture broth of the soil bacterium Streptomyces tsukubaensis in 1984 [21]. It has become a clinically important drug and is used to prevent rejection of transplanted organs and treat autoimmune diseases such as atopic dermatitis [37]. FK506 also shows promising therapeutic potential in other clinical applications due to, among others, its neuroprotective and neuroregenerative activities [42]. As a result of its pharmaceutical importance and broad applicability, both researchers and pharmaceutical companies have shown a great deal of interest in FK506 and its analogues.
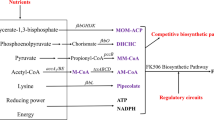
FK506 is generally biosynthesized through polyketide synthase (PKS)-catalyzed condensation of an unusual chorismate-derived starter unit [4,5-dihydroxycyclohex-1-enecarboxylic acid (DHCHC)], and ten extender units (5 methylmalonyl-CoA, 2 malonyl-CoA, 2 methoxymalonyl-ACP and an unusual allylmalonyl-CoA extender unit) are incorporated into a polyketide chain [30]. As illustrated in Fig. 1, the biosynthesis of the linear polyketide chain is followed by the incorporation of lysine-derived pipecolate and a cyclization step, mediated by FkbP peptide synthetase, resulting in the initial macrolactone intermediate of FK506 (preFK506; Fig. 1). Post-PKS processing reactions are catalyzed by a specific methyl transferase and oxidoreductase, resulting in FK506 [12, 31, 32]. Owing to the many precursors in relation to the biosynthetic pathway, which in turn is directly related to the production, many attempts have been made to engineer strains with improved levels of FK506. For example, Mo et al. [29] improved the intracellular pool of methylmalonyl-CoA in Streptomyces clavuligerus CKD1119 by introducing the methylmalonyl-CoA mutase pathway to participate in the biosynthesis of methylmalonyl-CoA from succinyl-CoA; this led to an approximately 300 % improvement in the FK506 titer. Chen et al. [4] reported an approximately 30 and 100 % improvement of FK506 yield in the FK506-producing strain S. tsukubaensis through the overexpression of two groups of pathway-specific genes encoding the biosynthesis of methoxymalonyl-ACP and allylmalonyl-CoA extender unit, respectively.
The biosynthetic pathways of FK506 (tacrolimus) and by-products (FK520, FK506D) in Streptomyces tsukubaensis. Shaded boxes Precursors of FK506 biosynthesis, thick red arrows increased flux or activity due to direct overexpression of the corresponding genes. Blue and purple structural formulas involved in the pathway indicate the corresponding units of FK506 and by-products
Directing carbon flux towards the desired antibiotic products has proven to be an effective metabolic engineering approach in strain optimization. However, this methodology is always time-consuming and subjected to laborious experiments for target validation. In addition, due to the strict control of precursor concentration inside cells and/or the remote effects of the genetic modifications or unknown regulatory interactions, a simple overexpression or knockout of the corresponding gene may not benefit final product levels [44]. Therefore, it is necessary to take the entire metabolic pathway as well as its precursors into consideration in order to predict promising targets for product yield improvement by analyzing highly interconnected metabolic networks models [1, 6]. Based on such network analysis, many engineering targets have been identified, thereby enabling prioritization of the implementation of genetic modifications [3, 16]. Recently, FK506 production has been enhanced by applying the genome-scale metabolic network model-guided metabolic engineering approach in our laboratory (unpublished article). Based on the model predictions, we focused on a number of targets to improve FK506 production. Among these targets, fkbO, fkbL, fkbP, fkbM and fkbD were screened as overexpression targets.
In addition to the engineering of structural genes coding for enzymes involved in the biosynthesis of the metabolite, nutrient feeding is also an important factor to consider in terms of improving secondary metabolite production [5, 38, 39]. In the present study, the metabolic engineering approach was used to investigate the effects of pathway enhancement on the production profile of FK506 by S. tsukubaensis. To achieve this goal, we first overexpressed each of five genes (fkbO, fkbL, fkbP, fkbM and fkbD) predicted by the model to be responsible for the starter unit, unusual incorporated unit and post-PKS processing reactions to improve FK506 production. In a second step, these genes were combinatorially overexpressed to assess the impact of FK506 biosynthesis. Based on the results using the engineered strain, we propose exogenous precursor addition strategies in fed-batch culture to further enhance FK506 production.
Materials and methods
Bacterial strains and plasmids
All strains and plasmids used in this study are listed in Electronic Supplementary Material (ESM) Table S1. The parent strain S. tsukubaensis D852, which can produce FK506, is a stock of our laboratory and is deposited in China General Microbiological Culture Collection Center under accession number CGMCC 7180. All Streptomyces mutants used in this work are derivatives of D852. Escherichia coli JM109 was used for gene cloning according to standard molecular biology procedures [40], whereas E. coli JM110 was used for preparing plasmid DNA free of Dam or Dcm methylation. E. coli ET12567/pUZ8002 was used as the nonmethylating plasmid donor strain [20] for intergeneric conjugation with S. tsukubaensis D852. The integrative E. coli–Streptomyces vector pIB139 containing the ermE* promoter [46] was used for gene overexpression in S. tsukubaensis.
Gene cloning, plasmid construction and transformation
DNA extraction, manipulation and transformation of Streptomyces were performed according to standard protocols [20]. The FkbO gene (fkbO) (accession number AFD22865), FkbP gene (fkbP) (accession number AFD22867), FkbL gene (fkbL) (accession number ZP_10073077), FkbM gene (fkbM) (accession number ZP_10073068.1) and FkbD gene (fkbD) (accession number ZP_10073069) were amplified from S. tsukubaensis genomic DNA by PCR amplification using primer pairs (ESM Table S2). The PCR products were cloned into pUC18 and sequenced to confirm the fidelity, then the NdeI–XbaI fragments were inserted into the same site of pIB139 (possessed integrase to integrate in chromosome) to generate pFKBO, pFKBP, pFKBL, pFKBM, pFKBD, respectively.
For the multiple gene overexpression study, the pUC18 plasmid was first modified to eliminate the BamHI restriction site by excision with BamHI, followed by linearization and the generation of blunt ends with the Klenow fragment, generating pUC18M with a ClaI methylation restriction site. The PCR products were cloned into pUC18M to form pUC18M-FKBO, pUC18M-FKBP, pUC18M-FKBL, pUC18M-FKBM and pUC18M-FKBD, respectively. All of these plasmids were transferred into E. coli JM110 to obtain the demethylated plasmids. For the construction of pFKBOP carrying double genes, fkbP was excised with NheI–ClaI from pUC18M-FKBP and transferred to the SpeI–ClaI sites of pUC18M-FKBO, generating pUC18M-FKBOP. The NdeI–XbaI fragment of the fkbO and fkbP genes were excised from the pUC18M-FKBOP and ligated into pIB139 to yield pFKBOP. The other plasmids harboring two target genes were constructed using a similar method. For the construction of plasmids harboring three, four or five genes for overexpression, the plasmids were based on the double-gene construction. Once the plasmids were constructed, they were separately introduced into S. tsukubaensis D852 through conjugal transfer from E. coli ET12567/pUZ8002, following the established protocol [20]. The positive exconjugants were confirmed by PCR amplification–coupled sequencing using the primer pair pIB-F/pIB-R.
To avoid any unexpected effect caused by the existence of multiple copies of the plasmid itself, the parent strain harboring pIB139 was used as the control strain (named HT-PIB139). There were no differences in FK506 production, cell growth and morphology between HT-PIB139 and the wild-type strain when grown in fermentation medium, indicating that the biosynthetic genes were unaffected by the integration.
Medium and cultivation conditions
Spores of S. tsukubaensis D852 were obtained from ISP4 agar plates [41]. Seed culture was prepared in R2YE broth [20]. Batch cultivation for FK506 production was carried out by inoculating 1 mL of a seed culture suspension into a baffled 500-mL flask containing 100 mL of fermentation medium at pH 7.0 and then incubating the suspension in an orbital shaker (220 rpm) for 6 days at 28 °C. The fermentation medium contained 60 g/L starch, 2 g/L yeast extract, 2.5 g/L peptone, 5 g/L soybean meal, 0.5 g/L K2HPO4, 0.5 g/L MgSO4 and 0.5 g/L CaCO3; pH 7.0. Antibiotics were supplemented as needed: ampicillin (100 μg/mL), apramycin (50 μg/mL), kanamycin (25 μg/mL) and chloromycin (25 μg/mL).
The fed-batch cultivation with exogenous precursor addition was carried out at 28 °C in a 7.5-L BIOFLO 110 bioreactor (New Brunswick Scientific Co., Edison, NJ) with a 4.5-L working volume. The medium contained 60 g/L starch, 2 g/L yeast extract, 2.5 g/L peptone, 5 g/L soybean meal, 0.5 g/L K2HPO4, 0.5 g/L MgSO4, 5 g/L CaCO3 and 1 g/L (NH4)2SO4. During cultivation, pH was controlled at 6.8 by 2 M HCl or 2 M NaOH. A constant airflow of 1 vvm was achieved by a flow meter, and the agitation speed was set to between 200 and 1,000 rpm. Foam was prevented by the automatic addition of 10 % (v/v) antifoam agent (Sigma 204; Sigma, St. Louis, MO). The nutrient feeding solution used for the fed-batch culture contained lactate, shikimate, lysine, soybean oil, succinate, chorismate, pipecolate, isoleucine and valine.
All fermentation experiments were performed in triplicate.
Analytical methods
The biomass was determined by dry weight. In detail, 10-mL samples (in triplicate) of fermentation broth were collected through a pre-weighed 0.45-μm pore size filter (Satorius AG, Göttingen, Germany), washed twice with a 0.9 % NaCl solution and dried at 80 °C to a constant weight. The residual total sugar in fermentation broth was quantified using the phenol–sulfuric acid method with glucose as a standard [8]. To detect FK506 as well as the by-products FK520 and 37,38-dihydro-FK506 (FK506D), the broth was mixed with an equal volume of methanol (1:1) and shaken intermittently in a water bath at 50 °C for 2 h. After centrifugation at 6,000 g for 10 min, the supernatant was determined by high-performance liquid chromatography (HPLC) using a Venusil XDB-C18 column (5 μm, 250 mm × 4.6 mm). The mobile phase was 0.1 % phosphoric acid in water and acetonitrile (35:65, v/v). The flow rate was 1 mL/min, the column temperature was 60 °C and the detection wavelength was 210 nm.
Real-time PCR analysis
The gene transcription profile was determined by real time-quantitative PCR (RT-qPCR; Bio-Rad, Hercules, CA) using the TransScriptTM II Green Two-Step qRT-PCR SuperMix (Trans). In general, the samples were isolated from the wild-type and mutant strains of S. tsukubaensis in mid-exponential phase, as described previously [16]. Total RNA was extracted by TRIzol reagent (Qiagen, Hilden, Germany) according to the manufacturer’s instructions and then treated with DNase I (Fermentas, Thermo Fisher Scientific, Waltham, MA). The primers were designed to generate PCR products of approximately 500 bp using the primer pairs listed in ESM Table S1. The RNA polymerase sigma factor hrdB was used as an internal control. Data analysis was performed according to the comparative CT method. Change of the gene expression of the engineered strains was obtained by normalizing the value of the control D852. The RT-PCR experiments were done in triplicate using RNA samples from three independent cultures. The results were analyzed using a standard Student’s t test (p < 0.05 or p < 0.01).
Results
Increasing the chorismate-derived starter unit DHCHC pool by fkbO overexpression
The first step of FK506 biosynthesis is the conversion of chorismate to 4,5-dihydroxycyclohexa-1,5-dienecarboxylic acid (DCDC); this is followed by conversion to DHCHC (Fig. 1). To investigate the effect of an elevated pool of DHCHC on FK506 production, we introduced plasmid pFKBO into the parent strain. The integration of the corresponding plasmid into the chromosome of S. tsukubaensis D852 via the attB-site was verified by PCR. Overexpression of fkbO was also confirmed by RT-PCR analysis. Taking RNA polymerase sigma factor hrdB as the internal standard, we observed that the transcription level of fkbO in the recombinant strain HT-FKBO was significantly higher (p = 0.0022) (Fig. 2), corresponding to an 800 % change compared with the control strain D852. Fermentation characterization of the genetically engineered HT-FKBO strain and its parent strain was performed in batch culture to investigate the effects of fkbO overexpression on FK506 production and cell growth. As shown in Fig. 3, the HT-FKBO strain produced 200.1 ± 9.3 mg/L of FK506, with an average volumetric productivity of 1.35 mg/L/h, consistent with the expression data. As chorismate could be converted to DHCHC (FK506 precursors) by the special pathway [2], any improvement in the production of precursor(s) might lead to an obvious enhancement of FK506 production. In addition, the fkbO overexpression strain showed almost no change in biomass [9.2 ± 0.4 vs. 8.8 ± 0.6 g/L (control strain D852)] (Fig. 3), demonstrating that the improved FK506 productivity of HT-FKBO was not caused by increased biomass. These findings clearly show that the ployketide starter unit plays a key role in FK506 biosynthesis. Furthermore, in order to test the conversion efficiency, we added chorismate (0.5 g/L) to the culture system to study its effect on FK506 accumulation. Here, for convenience, the final concentration of chorismate (shikimate, lysine or pipecolate—discussed below) was set as 0.5 g/L; optimization would be based on the exogenous feeding strategies study. The result showed that supplementation of chorismate to the HT-FKBO and D852 systems led to an additional improvement in FK506 titer, from 26.3 and 34.3 mg/L up to 226.4 ± 8.8 and 177.8 ± 5.7 mg/L, respectively (Fig. 3). This result supports the hypothesis that exogenous chorismate can be catabolized to DHCHC by FkbO in S. tsukubaensis [2]. Correspondingly, the addition of 0.5 g/L shikimate also resulted in a similar increment of the FK506 titer in HT-FKBO and D852 (26.9 and 23.6 mg/L, respectively; Fig. 3), suggesting that the exogenous feeding of shikimate enhanced the intracellular precursor to fulfill the requirement of FK506 biosynthesis and that shikimate was an important precursor in the FK506 synthetic pathway in S. tsukubaensis.
Comparison of gene expression by real time-PCR during the late-exponential phase in batch fermentation. Data are represented as the change in gene expression relative to the control (Streptomyces tsukubaensis strain D852). Values are presented as averages ± standard deviations (SD) from three independent measurements. Asterisks represent a statistically significant difference (*p < 0.05; **p < 0.01) from the D852 control, as determined by Student’s t test. See section Gene cloning, plasmid construction and transformation for detailed description of plasmids, genes and engineered strains
FK506 production and cell-growth profiles of engineered strain HT-FKBO (parent strain with inserted plasmid pFKBO) and the wild-type strain, with and without 0.5 g/L chorismate or 0.5 g/L shikimate. Plus symbol Medium was supplemented with 0.5 g/L chorismate or 0.5 g/L shikimate. Values are presented as averages ± SD from three independent measurements
Improving the pipecolate precursor supply by overexpressing the fkbP and fkbL
After ten successive polyketide chain elongation cycles, the linear chain is then condensed with nonproteinogenic amino acid pipecolate by peptide synthetase FkbP, followed by cyclization to form the macrolide ring [12, 18]. The products of these two genes are located at important branch points in the lysine (Fig. 1), with fkbL and fkbP drawing carbon flux away from the lysine degradation pathway. That lysine is used as the source of the pipecolate moiety was verified in an earlier study in which the rapamycin (a kind of polyketide) producer strain was incubated with labeled metabolites [36]. Based on the homology analysis with the ornithine cyclodeaminase and RapL (a lysine cyclodeaminase encoded in the rapamycin gene cluster), FkbL was predicted to catalyze the direct formation of pipecolate from lysine [31]. Additional evidence for this gene role comes from the observation that chromosomal disruption of the rapL gene in Streptomyces hygroscopicus required the exogenous addition of pipecolate for rapamycin production [19].
In order to increase the expression of pipecolate biosynthesis genes, we introduced and overexpressed fkbL encoding lysine cyclodeaminase and fkbP encoding pipecolate-specific peptide synthetase in the wild-type strain S. tsukubaensis D852. As shown in Fig. 4, the HT-FKBL strain showed an increase in FK506 titer (186.3 ± 7.2 mg/L) which was approximately 30 % higher than that in the wild-type strain after 6 days cultivation. RT-PCR analysis confirmed that the transcriptional level of fkbL in HT-FKBL was significantly improved by 630 % compared with the control strain D852 (p = 0.0018) (Fig. 2). In order to demonstrate the role of pipecolate on FK506 production, we cultivated wild-type strain D852 and engineered strain HT-FKBL in fermentation medium supplemented with 0.5 g/L pipecolate. As expected, both strains increased FK506 production by approximately 25 mg/L compared with the strains cultivated without the supplementation (Fig. 4), indicating that supplementation with exogenous pipecolate could improve FK506 biosynthesis.
FK506 production and cell growth profiles of engineered strains HT-FKBL, HT-FKBP and HT-FKBPL and the wild-type strain, with and without 0.5 g/L pipecolate or 0.5 g/L lysine. Plus symbol Medium was supplemented with 0.5 g/L pipecolate or 0.5 g/L lysine. Values are presented as averages ± SD from three independent measurements
In contrast, the overexpression of fkbP was less effective in increasing FK506 production and resulted in only a 12.8 % improvement to 161.9 ± 4.1 mg/L. It was noted that the change in fkbP expression level in the HT-FKBP strain was only 150 % relative to the control, and the difference between these two strains was statistically insignificant (p = 0.132), which could be one of the many limiting factors (Fig. 2). To the contrary, there were indications that insufficient intracellular pipecolate precursor might block further improvement in FK506 productivity in HT-FKBP. To verify this possibility, we supplemented the fermentation medium with 0.5 g/L pipecolate and found that strain HT-FKBP produced an increased titer of FK506 (189.9 ± 7.3 mg/L), as expected. Furthermore, when fkbP and fkbL were overexpressed in combination, the resulting strain (HT-FKBPL) showed an approximately 48.3 % improvement in the titer of FK506 in comparison with wild-type strain D852 and, surprisingly, the transcription level of both fkbP and fkbL were significantly enhanced (p < 0.05) by 750 and 400 %, respectively, compared with D852 (Fig. 2). These results suggest that FkbL plays a key role in the availability of pipecolate and that FkbP improves FK506 biosynthesis only under the condition of the high pipecolate conversion flux. In contrast to the HT-FKBO recombinant which maintained a similar cell growth to the wild-type strain, strains HT-FKBL and HT-FKBP had reduced biomass (7.0 ± 0.3 and 7.5 ± 0.4 g/L, respectively) at 6 days, respectively (Fig. 4). This finding suggests that intracellular fluxes in HT-FKBL and HT-FKBP might be altered through the biomass precursors towards pipecolate biosynthesis and incorporate into the FK506 structure, thus resulting in increased FK506 biosynthesis at the expense of decreased biomass. Since pipecolate is derived from lysine (Fig. 1), 0.5 g/L lysine was fed into the fermentation culture to test the efficiency of the conversion. Exogenous supplementation of lysine further increased FK506 production by approximately 45 mg/L, up to 256.3 ± 13.9 and 230.7 ± 10.2 mg/L for HT-FKBPL and HT-FKBL, respectively (Fig. 4). The FK506 level in the control D852 strain was increased by only 12 mg/L with lysine addition, which is almost one-third of the percentage increment. Based on these results, we conclude that the exogenous feeding of lysine in HT-FKBPL may be the better choice for FK506 improvement.
Enhancing FK506 production by regulating post-PKS processing reactions
The ring released from the PKS complex is further modified via C9 hydroxylation by FkbD and methylation of the hydroxyl group at C31 by FkbM [32]. It is worthy of attention that unlike the aforementioned strategies aimed at direct improvement of the precursor pool, the overexpression of fkbD and fkbM could accomplish the post-PKS tailoring effect. Plasmids pFKBD and pFKBM were constructed and subsequently introduced into the parent strain to yield the recombinant strain HT-FKBD and HT-FKBM, respectively. When fkbD was overexpressed, FK506 concentration reached 173.1 ± 10.8 mg/L (Fig. 5). Meanwhile, fkbD in HT-FKBD was also confirmed by the transcriptional analysis (Fig. 2). The results show that the fkbD transcription level in HT-FKBD increased by about 400 % compared with the value of the control strain D852 (p = 0.011). However, cell growth was somewhat retarded during the whole fermentation, most likely due to the metabolic burden. Similarly, the fkbM gene was overexpressed in the parent strain. The results shown in Fig. 5 indicate that efficient post-PKS modification of the macrolide ring via an elevation of FkbM activity also improved FK506 production (by up to 178.3 ± 9.9 mg/L). The relative expression level of fkbM in HT-FKBM was found to be 460 % of the value detected in the wild-type (p = 0.022) (Fig. 2), thereby confirming that FkbM was well expressed in the recombinant. It was noted that overexpression of FkbM did not exert profound effects on cell growth (Fig. 5), indicating that the effect of the expression of an additional enzyme in the strain was marginal. In addition, the intracellular preFK506 level of the recombinant strain was decreased by an elevation in FkbM activity (data not shown), demonstrating that an improvement in FK506 production resulted from an elevated methyltransferase activity available for the methylation of the macrolactone intermediate of FK506. These results clearly suggest that enzymes responsible for post-PKS processing reactions are the key factors for improving FK506 production.
Construction of FK506-overproducing strain by combinatorial overexpression of multiple genes
Inspired by the success of the respective five genes in FK506 overproduction at different levels, we investigated whether the simultaneous enhancement of several pathways would result in a greater degree of production improvement. Therefore, we constructed the plasmid based on the strategy that pUC18M could be inserted with the target genes to generate the new conjugative vectors containing multiple genes without introducing the extra restriction enzyme site. The results of these genes in terms of combinatorial overexpression are shown in Fig. 6. The first two overexpression genes of FK506 production, fkbO, for enhancement of carbon flux to DHCHC production, and fkbL, for promotion of competing lysine towards pipecolate formation, were directed at improving the carbon flux towards FK506. For this double gene overexpression strain we took two paths towards improving the FK506 precursor production. The combinatorial overexpression of these two genes resulted in a large increase in FK506 production, up to 273.9 ± 11.1 mg/L (Fig. 6). Based on this metric, nearly 0.34 mM chorismate and lysine were converted to DHCHC and pipecolate in HT-FKBOL, whereas only 0.18 mM chorismate and lysine were converted to DHCHC in the wild-type. Unexpectedly, different from the positive effect of combinatorial overexpression of fkbO and fkbL genes, the strain HT-FKBOP harboring the fkbO and fkbP genes produced only a small amount of FK506 (215.3 ± 7.9 mg/L), which was the accumulation of the increment in the HT-FKBO and HT-FKBP strains (Fig. 6), indicating that in terms of FK506 production these two genes had additive effects and thereby affected two entirely unrelated pathways. Moreover, the strain further engineered by overexpressing the above three genes in the hope of increasing the amount of related precursors gave rise to a considerable amount of FK506, up to 298.6 ± 13.3 mg/L, which is 108 % higher than the value obtained by D852 (Fig. 6). Based on the increased production of FK506 by engineered strains HT-FKBO, HT-FKBL, HT-FKBP, HT-FKBPL, HT-FKBOL and HT-FKOP compared to strain D852 (39.3, 29.8, 12.8, 48.3, 90.9, 50 %, respectively), the impact of the three genes on FK506 production was not only an additive effect but also a positively synergistic effect.
As for additional genes and combinatorial overexpression, strain HT-FKBMD, with overexpression of fkbM and fkbD, seemed to produce an FK506 titer (approx. 5 % increase) close to that obtained with individual gene overexpression, almost weakening the sole gene contribution to FK506 production. While cell growth was reduced by only 6 %, total sugar consumption was also slightly reduced by about 10 % by these additional enzymes. These results suggest that instead of repressing strain growth, the limited increase in FK506 might ascribe to the restricted chain elongation which had saturated the FK506 production capability of the strain. Therefore, in order to completely test the effect of the five gene operon on FK506 production, we engineered the final strain, HT-FKBOPLMD, by conjugation and selection for apramycin resistance. This recombinant strain was able to produce 353.2 ± 8.5 mg/L of FK506 with a highest volumetric productivity of 2.45 mg/L/h during batch fermentation (Fig. 6). The titer and productivity of FK506 were increased by approximately 150 %, compared with the parent strain D852. In contrast, the maximal biomass for the engineered strain was reduced to 6.5 ± 0.2 g/L, which might be due to the heavy metabolic burden.
To understand the reason for the additive and synergistic effect of combinatorial overexpression on the FK506 production, we investigated the expression levels of the key enzymes in different combinatorial overexpression engineered strains (Fig. 2). In HT-FKBOL, the transcriptional level of the fkbO and fkbL was significantly increased by 1,240 % and 950 %, respectively, compared with D852 (p < 0.01), which was consistent with the cultivation data. For HT-FKBOP, fkbO and fkbP were only enhanced by 850 % (p < 0.01) and 150 % (p < 0.05), respectively, the same level as with individual modification, indicating these two genes did not upregulate or downregulate each other. However, the transcriptional levels of fkbO, fkbL and fkbP in HT-FKBOPL were increased by 1,150 %, 1,020 % (p < 0.01) and 330 % (p < 0.05), respectively, indicating the three genes were also positively synergistic in terms of benefitting FK506 production. In addition, there was no change in the expression of fkbM and fkbD between HT-FKBMD and individual modification. A similar change between HT-FKBOPLMD and HT-FKBOL (HT-FKBOPL) further validates the effect of combinatorial overexpression on FK506 production. These results demonstrate that FK506 production could be further improved by overexpressing in combination the biosynthesis of the chorismate-derived starter unit route, pipecolate precursor pathways and post-PKS processing reactions in S. tsukubaensis.
Efforts to boost FK506 production based on the exogenous addition strategies
To further exploit the positive role of the engineered strain HT-FKBOPLMD, exogenous addition strategies were adopted. Based on previous reports [29, 43, 49] and the results of our intracellular metabolite analysis (unpublished data), the nutrients lactate, succinate, chorismate, shikimate, lysine, pipecolate, isoleucine, valine and soybean oil, which are involved in central carbon metabolism, amino acid metabolism and fatty acid metabolism, were added at different stages and final concentrations (Fig. 7). In fact, these supplementations could provide primary pathway precursors (such as malonyl-CoA and methylmalonyl-CoA) [29, 39] or secondary pathway precursors (such as pipecolate and DHCHC) for FK506 biosynthesis [9, 45].
The effects of exogenous nutrients on FK506 production by engineered strain HT-FKBOPLMD. Optimization included addition times and final concentrations of lactate (a), succinate (b), soybean oil (c), shikimate (d), chorismate (e), pipecolate (f), lysine (g), valine (h) and isoleucine (i). Values are presented as the averages ± SD from three independent measurements
At the optimum addition time and final concentration, 1.5 g/L of succinate supplemented at 96 h resulted in the maximal FK506 concentration, up to 388.7 mg/L (Fig. 7b), indicating that succinate absorbed by strain HT-FKBOPLMD enhanced carbon flow from succinyl-CoA to methylmalonyl-CoA, further boosting the conversion of methylmalonyl-CoA to FK506 to some extent. As a metabolite of the glycolytic pathway, lactate could be fed into the acetyl-CoA pool to be further catalyzed into malonyl-CoA, another important precursor of FK506 synthesis. Therefore, various concentrations of lactate were added at different stages. It can be seen in Fig. 7a that 15 g/L of lactate added at 36 h resulted in the highest FK506 production (382.3 mg/L).
According to the above analysis, the addition of chorismate and shikimate could improve FK506 production by both D852 and engineered strain HT-FKBO. Therefore, in order to further strengthen the intracellular level of the FK506 precursor (DHCHC), the levels of both exogenous shikimate and chorismate were optimized. As shown in Fig. 7d, shikimate added at 48 h at a final concentration of 0.5 g/L led to the highest production of FK506, which reached 402.8 mg/L (14 % improvement). Similarly, when adding 0.25 g/L of chorismate at 48 h, the FK506 concentration increased by 15.2 %, from 353.2 to 406.9 mg/L, compared with the control group (Fig. 7e).
Based on the previous analysis of pipecolate biosynthetic pathway for both D852 and engineered strains HT-FKBP and HT-FKBL, we added lysine and pipecolate to further enhance another important precursor level for FK506 biosynthesis. Here, the concentrations of lysine and pipecolate were optimized, respectively, as well as the addition time. The results showed that lysine and pipecolate could accelerate FK506 production, consistent with rapamycin [5]. In particular, 1.0 g/L of lysine and 0.25 g/L of pipecolate supplemented at 48 h resulted in the highest production of FK506, at 398.8 and 394.9 mg/L, respectively (Fig. 7f, g).
Valine and isoleucine were also added to the medium since they can be transformed to methylmalonyl-CoA via a multi-step reaction. For the engineered strain HT-FKBOPLMD, 1.5 g/L of valine feeding at 96 h had the greatest effect on FK506 yield, with an increase of 42.4 mg/L, compared with the same strain without exogenous addition (Fig. 7h). When isoleucine was supplemented at a final concentration of 1.0 g/L at 96 h, FK506 production was enhanced to 389.9 mg/L (Fig. 7I), representing the highest improvement among all the supplementation stages and final concentrations.
Taking into account that the metabolic pathway of fatty acids was related with FK506 precursors malonyl-CoA and methylmalonyl-CoA, we added soybean oil to improve the availability of intracellular methylmalonyl-CoA and malonyl-CoA for FK506 biosynthesis. As shown in Fig. 7c, when 5 g/L soybean oil was added at 24 h, the production of FK506 was enhanced to 391.1 mg/L, which exhibited a larger increment on FK506 biosynthesis compared with the effect of soybean oil. In contrast, a final concentration of soybean oil of >20 g/L inhibited cell growth and reduced FK506 production.
In view of the enhancement measures described above, we tested combinatorial feeding strategies based on the initial fermentation medium used during fed-batch fermentations. These nutrients were fed into the bioreactor as follows: soybean oil and lactate were added at 24 and 36 h at a final concentration of 5 and 15 g/L, respectively; shikimate, chorismate, lysine and pipecolate were added at 48 h at 0.5, 0.25, 1.0 and 0.25 g/L, respectively; succinate, isoleucine and valine were added at 96 h at 1.5, 1.0 and 1.5 g/L, respectively.
As shown in Fig. 8a, both the total sugar consumption rate and cell growth rate were affected. After feeding various nutrients into the culture under the optimized conditions, the total sugar consumption rate and biomass growth rate during the exponential phase by parent strain D852 was reduced. In contrast, in the engineered strain HT-FKBLOPLMD the total sugar consumption rate was elevated, with unchanged biomass. Under both conditions, the maximal biomass for HT-FKBLOPLMD was always lower than that for D852. Moreover, FK506 production was greatly improved by feeding various nutrients at different phases (Fig. 8b). The recombinant strain accumulated as much as 457.5 ± 10.3 mg/L of FK506, which was a 29.5 % increase compared with the same strain without nutrient feeding (Fig. 8b). S. tsukubaensis can produce two major by-products, FK520 and FK506D, which differ from FK506 in structure only by the C-21 substitution with an ethyl and propyl group, respectively, instead of an allyl group (Fig. 1). This difference is dependent on the corresponding AT domain of the PKS system in substrate utilization, with the selection of ethylmalonyl-CoA benefitting the extender unit for generating FK520 versus the choice of allylmalonyl-CoA for promoting FK506 as well as FK506D [23]. To evaluate the relevance of the production of the FK506 and by-products FK520/FK506D, we sampled the recombinant strain HT-FKBLOPLMD and subjected it to HPLC quantification. For the engineered strain HT-FKBLOPLMD, when no precursors were added, the yield of FK520 (53.1 ± 6.6 vs. 33.1 ± 3.6 mg/L for parent strain) increased accordingly with FK506, indicating a ratio of FK520 to FK506 similar to that in D852 (Fig. 8b). In the case of FK506D, a similar observation was made, with FK506D increasing at the same ratio as the improvement in FK506 production. This finding is consistent with the conclusion that the duplication of secondary metabolic pathways and precursor supplementation not only enhanced the flux towards the formation of polyketide chains but also concomitantly boosted the formation of the extender unit shared by the biosynthetic pathway of FK520, FK506D and FK506 [13, 23]. However, surprisingly, following the exogenous addition, the final concentration of FK520 and FK506D by HT-FKBOPLMD fell by 44 % and 35 % compared with the values of no exogenous addition (Fig. 8b).
The production performance profiles of wild-type strain S. tsukubaensis D852 and engineered strain HT-FKBOPLMD in fed-batch fermentation with various exogenously feeding nutrients. a Total sugar and biomass profiles, b FK506 and by-products (FK520, FK506D) profiles. Values are presented as the averages ± SD from three independent measurements
Discussion
Metabolic engineering has become a rational alternative to classical strain improvement for the optimization of metabolite production. The introduction of directed genetic modifications through recombinant DNA technology can be visualized as a means by which to improve the cellular properties of production strains and result in substantial increases in existing polyketide antibiotic fermentation processes [4, 26, 39, 48]. Immunosuppressants, such as FK506, FK520 and rapamycin, have been available for clinical use for many decades, and their high effectiveness, safety and tolerance profiles continue to make them important agents in the treatment of autoimmune diseases, such as atopic dermatitis, and the prevention of transplanted organ rejection. Hence, the medical significance of these compounds requires higher industrial production. Increasing the pool size of the rate-limiting precursor is a fundamental approach to strain improvement. This can be achieved by increasing the gene dosage of key enzymes involved in precursor biosynthesis [7, 44], regulating the expression of secondary metabolic pathways [25, 28, 47], overexpressing structural genes coding for enzymes involved in the biosynthesis of the metabolite [34], as well as by supplementing the desired precursors or closely related derivatives [15]. In this study, we report an approach to improve the intracellular secondary metabolic pathway precursor pool based on a prior model prediction to enhance the upstream portion of the FK506 biosynthesis pathway in S. tsukubaensis.
FkbO and FkbL are known to provide or incorporate direct precursors for FK506 production, independently of the polyketide biosynthetic elongation units (including methylmalonyl-CoA, malonyl-CoA, methoxymalonyl-ACP, allylmalonyl-CoA) in microorganisms. Moreover, FK506 biosynthesis requires two unusual pathway-specific building blocks, namely, the chorismate-derived starter unit DHCHC and lysine-derived pipecolate, raising the question of whether their intracellular concentrations are a limiting factor in production improvement. Therefore, we partially diverted the carbon flux that would have normally gone through central metabolic pathways during the growth phase into the FK506 biosynthesis structure unit instead, resulting in an overall increase in FK506 yield in the mutant strain. Indeed, the k cat (17.6 ± 0.7 s−1; catalytic rate) and the K m value (0.2 ± 0.03 mM; concentration of substrate that gives half-maximal activity) of the FkbO enzyme for chorismate compares favorably to the k cat and the K m previously reported for the chorismate mutase activity [2, 10]. In addition, steady-state kinetic analysis of the pipecolate biosynthetic reaction encoded by FkbL yielded a K m for lysine of 46 ± 4 μM and a k cat of 0.61 ± 0.02 min−1, resulting in a k cat/K m of 13 ± 1 mM−1 min−1 [12]. FkbO and FkbL both out-competed other enzymes for the corresponding substrates chorismate and lysine. Our findings clearly demonstrate that FK506 production in the fkbO-overexpression mutant was greatly improved without any change in the components of the batch cultures (Fig. 3). A higher yield of 226.4 mg/L (158 % of wild-type strain) was achieved in batch cultures into which chorismate was fed to the mutant strain at low concentration. On the other hand, although individual fkbP overexpression resulted in a slight increase in FK506 production, strains (HT-FKBL and HT-FKBPL) with an extra fkbL copy produced a considerable yield. These data indicate that the intracellular formation of the PKS starter and extender units seemed to be the rate-limiting factors. It was noteworthy that only one molecule of DHCHC and pipecolate was incorporated into the FK506 scaffold (Fig. 1), yet the production in HT-FKBO following duplication of the DHCHC biosynthetic gene and in HT-FKBPL upon amplification of the pipecolate biosynthetic gene increased by approximately 40 % compared to that in parent strain D852. It is likely that in the original strain the above two biosynthetic pathways have a lower turnover number than that of PKS and thus are unable to provide sufficient precursors for FK506 biosynthesis.
Another two post-PKS modification genes are fkbM and fkbD, which are involved in C31 methylation and C9 hydroxylation, respectively, and which were also overexpressed in an effort to further understand the effect of these pathways on polyketide formation as well as to increase the target product titers. The biochemical activities and functions of these two enzymes have been studied in detail in a previous study [32]. Sequence analysis from various species has shown that they resemble the rapamycin biosynthetic genes, with little or no change in most of the amino acid residues. In addition, the relatively low homology between FkbM and S-adenosylmethionine-dependent methyltransferase indicates that some evolutionary distance exists between the primary and secondary pathways. The genes fkbM and fkbD have been found to be linked to the post-PKS modification genes in many organisms [14, 24], suggesting that fkbM and fkbD could be also clustered as a tricistronic operon in S. tsukubaensis. Importantly, inactivation of fkbD has been found to result in the production of 9-deoxo-31-O-demethyl-FK506 instead of 9-deoxo-FK506, which demonstrates a polar effect of the fkbD gene on the expression of fkbM [32]. In our study, when fkbM or fkbD were overexpressed, gene expression level increased by roughly 400 % and FK506 production increased by 24 % and 20 %, respectively (Fig. 5), indicating that fkbM and fkbD could influence FK506 biosynthesis.
In general, the overexpression of an individual gene caused more carbon flux to FK506. However, the impact of individual genetic modifications on cellular phenotype can be accumulative due to the interactions among the interconnected pathways [17]. The synergistic effect could be evaluated by determining to which extent the investigated genes coupled together to affect the phenotype. Based on our analysis, the impact of multiple genetic modifications on FK506 production can be divided into three patterns: additive effects, which essentially reflect two entirely unrelated pathways, positive synergistic effects, which reflect positively related pathways, and negative synergistic effects, which reflect negatively related pathways.
Our results indicate that the overexpression of fkbP had an additve effect on FK506 production when combined with fkbO gene overexpression (Fig. 6). Moreover, overexpression of the above three genes simultaneously exerted a distinctly positive synergistic effect, which resulted in a 108 % improvement of FK506 production (Fig. 6). We therefore concluded that both positive synergistic and additive existed among the three overexpressed genes, which was validated by gene expression data (Fig. 2). Moreover, it could be noted that the overexpression of the fkbM and fkbD genes in combination caused only a limited increase in production compared with that by the corresponding control strains HT-FKBM and HT-FKBD, indicating that these genes influenced FK506 production only to a limited extent. This might be due to the fact that the increased intracellular activity of methylation and hydroxylation saturated preFK506 (unmodified FK506), the last two dedicated biosynthetic enzymes of the pathway. Additionally, directing the carbon flow towards the secondary precursor pathways might be superior to post-PKS modification pathways since the former specifically increased the level of polyketide starter and elongation precursors for FK506 and influenced antibiotic synthesis more directly, whereas the latter only elevated the expression level of modification in general [35]. Furthermore, the five genes overexpressed in the parent strain gave rise to an increased FK506 titer by 18.4 % compared with the HT-FKBOPL strain, demonstrating that the impact of overexpressing fkbM and fkbD depended upon the level of the other structural genes.
Overexpressing five genes relieved the limitation in the starter and incorporation of precursors. However, the cellular levels of some precursors, such as malonyl-CoA and methylmalonyl-CoA, were too low for chain elongation [29, 38]. Thus, we hypothesized that the other precursors might influence FK506 biosynthesis, suggesting that feeding the mutant with optimized nutrients may be necessary (Fig. 7). The increased pool of CoA-esters precursor via exogenous supplementation was so significant that even the very effective CoA-esters precursor biosynthetic pathway in the gene cluster could not provide sufficient precursors to the mutant. On the other hand, lysine, pipecolate, chorismate or shikimate precursor addition could improve production for the engineered strain (Figs. 3, 4). Further improvement in the production of FK506 was achieved by feeding the direct precursors in combination to allow extended production, although cell growth slowed down to a normal rate after 96 h (Fig. 8), possibly due to precursor availability, since methylmalonyl-CoA or malonyl-CoA was available for FK506 synthesis [31]. There has been considerable interest in enhancing the yield of polyketides by increasing the supply of precursors because intracellular availability of biosynthetic precursors is a key factor determining the productivity of secondary metabolites [39]. Nutrients such as the branched-chain amino acids isoleucine and valine, odd-numbered fatty acids, and succinate all enter secondary metabolism through related pathways. In fact, these nutrients have also been previously shown to boost the production of some macrolide antibiotics when supplemented [22, 33, 39]. We therefore concluded that a promotion in precursor supply led to an increase in FK506 in S. tsukubaensis HT-FKBOPLMD since methylmalonyl-CoA was located at a metabolic branch point [29]. Hence, the exogenous feeding strategies switched the metabolic flow of methylmalonyl-CoA away from the central metabolic branch towards the FK506 biosynthetic branch. It should also be noted that although the amount of shikimate added was low, shikimate (or chorismate, or pipecolate) is an expensive material and therefore not suitable for use in large-scale fermentation systems [50]. Therefore, the amplification of carbon flux towards shikimate or chorismate using metabolic engineering strategies, as well as the elimination of feedback inhibition of aromatic amino acids on shikimate pathway could be performed; both approaches are very significant in terms of improving FK506 production in large-scale fermentation systems.
FK506 and FK506-related compounds use the same primary metabolic precursors and share the early part of the biosynthetic pathway up to the common intermediate compound [23]. Thus, some of the increased precursor pools likely flow into the biosynthetic pathway and thereby could be expected to generate an increased yield. The metabolic engineering approach for decreasing the by-products would eliminate contamination. However, it has been shown that disruption of ethylmalonyl-CoA biosynthesis also exerts a negative effect on FK506 production due to the common encoding genes [23]. Therefore, the yield of FK506 could not be improved further if either FK520 and FK506D biosynthesis were to be blocked in S. tsukubaensis under the initial fermentation condition (Fig. 8b). Nevertheless, the levels of the by-products (FK520 and FK506D) were reduced by applying the exogenous addition stragies (Fig. 8b). Such an approach is advantageous to industrial production strategies since these by-products complicate the subsequent extraction and purification of FK506 from the fermentation broth.
In fact, improved production of secondary metabolites involves significant changes in precursor, energy and cofactors from primary to secondary metabolism [35]. Based on an understanding of the biosynthetic pathway characterized so far, we suggest that the increased FK506 productivity in the engineered strain increases the competition for DHCHC, methylmalonyl-CoA, malonyl-CoA, methoxylmalonyl-CoA, allylmalonyl-CoA, pipecolate, ATP and NADPH between primary and secondary metabolism and introduces new metabolic bottlenecks. These new key steps could be identified by metabolic flux analysis and modified by further rational genetic engineering approaches, which is the focus of our future research in the search for improved FK506 production. On the other hand, existing synthetic biology technology can also be applied to increase productivity and yield [11, 27], as has been demonstrated for the production of a number of valuable metabolites.
In the study reported here, we employed an effective strategy to increase the precursor pool pathway in order to upregulate the upstream portion of the FK506 biosynthesis pathway in S. tsukubaensis. This approach opens the door to the possibility of using more effective metabolic engineering strategies and more appropriate exogenous addition strategies for improved FK506 production. In addition, the industrial production of FK506 uses a similar fermentation medium that also contains starch and dextrin, suggesting that our technology could be used in an industrial setting. Importantly, our improved method for the industrial production of macrocyclic polyketide (FK506) is a successful example which can be applied to guide the manufacture of high-quality clinical compounds or novel production systems in industrial processes.
References
Alper H, Jin YS, Moxley JF, Stephanopoulos G (2005) Identifying gene targets for the metabolic engineering of lycopene biosynthesis in Escherichia coli. Metab Eng 7(3):155–164. doi:10.1016/j.ymben.2004.12.003
Andexer JN, Kendrew SG, Nur-e-Alam M, Lazos O, Foster TA, Zimmermann AS, Warneck TD, Suthar D, Coates NJ, Koehn FE, Skotnicki JS, Carter GT, Gregory MA, Martin CJ, Moss SJ, Leadlay PF, Wilkinson B (2011) Biosynthesis of the immunosuppressants FK506, FK520, and rapamycin involves a previously undescribed family of enzymes acting on chorismate. Proc Natl Acad Sci USA 108(12):4776–4781. doi:10.1073/pnas.1015773108
Asadollahi MA, Maury J, Patil KR, Schalk M, Clark A, Nielsen J (2009) Enhancing sesquiterpene production in Saccharomyces cerevisiae through in silico driven metabolic engineering. Metab Eng 11(6):328–334. doi:10.1016/j.ymben.2009.07.001
Chen D, Zhang Q, Zhang Q, Cen P, Xu Z, Liu W (2012) Improvement of FK506 production in Streptomyces tsukubaensis by genetic enhancement of the supply of unusual polyketide extender units via utilization of two distinct site-specific recombination systems. Appl Environ Microbiol 78(15):5093–5103. doi:10.1128/aem.00450-12
Cheng YR, Fang A, Demain AL (1995) Effect of amino acids on rapamycin biosynthesis by Streptomyces hygroscopicus. Appl Microbiol Biotechnol 43(6):1096–1098
Choi HS, Lee SY, Kim TY, Woo HM (2010) In silico identification of gene amplification targets for improvement of lycopene production. Appl Environ Microbiol 76(10):3097–3105. doi:10.1128/aem.00115-10
Duan Y, Chen T, Chen X, Zhao X (2010) Overexpression of glucose-6-phosphate dehydrogenase enhances riboflavin production in Bacillus subtilis. Appl Microbiol Biotechnol 85(6):1907–1914. doi:10.1007/s00253-009-2247-6
DuBois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28(3):350–356. doi:10.1021/ac60111a017
Fang A, Demain AL (1995) Exogenous shikimic acid stimulates rapamycin biosynthesis in Streptomyces hygroscopicus. Folia Microbiol 40(6):607–610
Görisch H, Lingens F (1973) Chorismate mutase from Streptomyces aureofaciens: a heat-stable enzyme. J Bacteriol 114(2):645–651
Gao H, Zhuo Y, Ashforth E, Zhang L (2010) Engineering of a genome-reduced host: practical application of synthetic biology in the overproduction of desired secondary metabolites. Protein Cell 1(7):621–626. doi:10.1007/s13238-010-0073-3
Gatto GJ, Boyne MT, Kelleher NL, Walsh CT (2006) Biosynthesis of pipecolic acid by RapL, a lysine cyclodeaminase encoded in the rapamycin gene cluster. J Am Chem Soc 128(11):3838–3847. doi:10.1021/ja0587603
Goranovic D, Kosec G, Mrak P, Fujs S, Horvat J, Kuscer E, Kopitar G, Petkovic H (2010) Origin of the allyl group in FK506 biosynthesis. J Biol Chem 285:14292–14300. doi:10.1074/jbc.M109.059600
Healy FG, Krasnoff SB, Wach M, Gibson DM, Loria R (2002) Involvement of a cytochrome P450 monooxygenase in thaxtomin A biosynthesis by Streptomyces acidiscabies. J Bacteriol 184(7):2019–2029. doi:10.1128/jb.184.7.2019-2029.2002
Huang D, Jia X, Wen J, Wang G, Yu G, Caiyin Q, Chen Y (2011) Metabolic flux analysis and principal nodes identification for daptomycin production improvement by Streptomyces roseosporus. Appl Biochem Biotechnol 165(7):1725–1739. doi:10.1007/s12010-011-9390-0
Huang D, Wen J, Wang G, Yu G, Jia X, Chen Y (2012) In silico aided metabolic engineering of Streptomyces roseosporus for daptomycin yield improvement. Appl Microbiol Biotechnol 94(3):637–649. doi:10.1007/s00253-011-3773-6
Jin YS, Stephanopoulos G (2007) Multi-dimensional gene target search for improving lycopene biosynthesis in Escherichia coli. Metab Eng 9(4):337–347. doi:10.1016/j.ymben.2007.03.003
König A, Schwecke T, Molnár I, Böhm GA, Lowden PAS, Staunton J, Leadlay PF (1997) The pipecolate-incorporating enzyme for the biosynthesis of the immunosuppressant rapamycin—nucleotide sequence analysis, disruption and heterologous expression of rapP from Streptomyces Hygroscopicus. Eur J Biochem 247(2):526–534. doi:10.1111/j.1432-1033.1997.00526.x
Khaw LE, Böhm GA, Metcalfe S, Staunton J, Leadlay PF (1998) Mutational biosynthesis of novel rapamycins by a strain of Streptomyces hygroscopicus NRRL 5491 disrupted in rapL, encoding a putative lysine cyclodeaminase. J Bacteriol 180(4):809–814
Kieser T, Bibb MJ, Buttner MJ, Chater KF, Hopwood DA (2000) Practical Streptomyces genetics. John Innes Foundation, Norwich
Kino T, Hatanaka H, Hashimoto M, Nishiyama M, Goto T, Okuhara M, Kohsaka M, Aoki H, Imanaka H (1987) FK-506, a novel immunosuppressant isolated from a Streptomyces. I. Fermentation, isolation, and physico-chemical and biological characteristics. J Antibiot 40(9):1249–1255
Koju D, Maharjan S, Dhakal D, Yoo JC, Sohng JK (2012) Effect of different biosynthetic precursors on the production of nargenicin A1 from metabolically engineered Nocardia sp. CS682. J Microbiol Biotechnol 22(8):1127–1132. doi:10.4014/jmb.1202.02027
Kosec G, Goranovič D, Mrak P, Fujs Š, Kuščer E, Horvat J, Kopitar G, Petković H (2012) Novel chemobiosynthetic approach for exclusive production of FK506. Metab Eng 14(1):39–46. doi:10.1016/j.ymben.2011.11.003
Kudo F, Yonezawa T, Komatsubara A, Mizoue K, Eguchi T (2011) Cloning of the biosynthetic gene cluster for naphthoxanthene antibiotic FD-594 from Streptomyces sp. TA-0256. J Antibiot 64(1):123–132. doi:10.1038/ja.2010.145
Kuščer E, Coates N, Challis I, Gregory M, Wilkinson B, Sheridan R, Petković H (2007) Roles of rapH and rapG in positive regulation of rapamycin biosynthesis in Streptomyces hygroscopicus. J Bacteriol 189(13):4756–4763. doi:10.1128/jb.00129-07
Maharjan S, Park JW, Yoon YJ, Lee HC, Sohng JK (2010) Metabolic engineering of Streptomyces venezuelae for malonyl-CoA biosynthesis to enhance heterologous production of polyketides. Biotechnol Lett 32(2):277–282. doi:10.1007/s10529-009-0152-9
Medema MH, Alam MT, Breitling R, Takano E (2011) The future of industrial antibiotic production: from random mutagenesis to synthetic biology. Bioeng Bugs 2(4):230–233. doi:10.1111/j.1751-7915.2010.00226.x
Mendes MV, Tunca S, Antón N, Recio E, Sola-Landa A, Aparicio JF, Martín JF (2007) The two-component phoR-phoP system of Streptomyces natalensis: inactivation or deletion of phoP reduces the negative phosphate regulation of pimaricin biosynthesis. Metab Eng 9(2):217–227. doi:10.1016/j.ymben.2006.10.003
Mo S, Ban YH, Park JW, Yoo YJ, Yoon YJ (2009) Enhanced FK506 production in Streptomyces clavuligerus CKD1119 by engineering the supply of methylmalonyl-CoA precursor. J Ind Microbiol Biotechnol 36(12):1473–1482. doi:10.1007/s10295-009-0635-7
Mo S, Kim DH, Lee JH, Park JW, Basnet DB, Ban YH, Yoo YJ, Chen SW, Park SR, Choi EA, Kim E, Jin YY, Lee SK, Park JY, Liu Y, Lee MO, Lee KS, Kim SJ, Kim D, Park BC, Lee SG, Kwon HJ, Suh JW, Moore BS, Lim SK, Yoon YJ (2011) Biosynthesis of the allylmalonyl-CoA extender unit for the FK506 polyketide synthase proceeds through a dedicated polyketide synthase and facilitates the mutasynthesis of analogues. J Am Chem Soc 133(4):976–985. doi:10.1021/ja108399b
Motamedi H, Shafiee A (1998) The biosynthetic gene cluster for the macrolactone ring of the immunosuppressant FK506. Eur J Biochem 256(3):528–534. doi:10.1046/j.1432-1327.1998.2560528.x
Motamedi H, Shafiee A, Cai SJ, Streicher SL, Arison BH, Miller RR (1996) Characterization of methyltransferase and hydroxylase genes involved in the biosynthesis of the immunosuppressants FK506 and FK520. J Bacteriol 178(17):5243–5248
Mouslim J, David L, Pétel G, Gendraud M (1993) Effect of exogeneous methyl oleate on the time course of some parameters of Streptomyces hygroscopicus NRRL B-1865 culture. Appl Microbiol Biotechnol 39(4–5):585–588. doi:10.1007/bf00205056
Murrell JM, Liu W, Shen B (2004) Biochemical characterization of the SgcA1 α-d-glucopyranosyl-1-phosphate thymidylyltransferase from the enediyne antitumor antibiotic C-1027 biosynthetic pathway and overexpression of sgcA1 in Streptomyces globisporus to improve C-1027 production. J Nat Prod 67(2):206–213. doi:10.1021/np0340403
Olano C, Lombó F, Méndez C, Salas JA (2008) Improving production of bioactive secondary metabolites in actinomycetes by metabolic engineering. Metab Eng 10(5):281–292. doi:10.1016/j.ymben.2008.07.001
Paiva NL, Demain AL, Roberts MF (1993) The immediate precursor of the nitrogen-containing ring of rapamycin is free pipecolic acid. Enzyme Microb Technol 15(7):581–585. doi:10.1016/0141-0229(93)90020-3
Parsons WH, Sigal NH, Wyvratt MJ (1993) FK-506–a novel immunosuppressant. Ann N Y Acad Sci 685(1):22–36. doi:10.1111/j.1749-6632.1993.tb35847.x
Reeves AR, Brikun IA, Cernota WH, Leach BI, Gonzalez MC, Weber JM (2006) Effects of methylmalonyl-CoA mutase gene knockouts on erythromycin production in carbohydrate-based and oil-based fermentations of Saccharopolyspora erythraea. J Ind Microbiol Biotechnol 33(7):600–609. doi:10.1007/s10295-006-0094-3
Reeves AR, Cernota WH, Brikun IA, Wesley RK, Weber JM (2004) Engineering precursor flow for increased erythromycin production in Aeromicrobium erythreum. Metab Eng 6(4):300–312. doi:10.1016/j.ymben.2004.03.003
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual, 3rd edn. Cold Spring Harbor Laboratory Press, New York
Shirling EB, Gottlieb D (1966) Methods for characterization of Streptomyces species. Int J Syst Bacteriol 16(3):313–340. doi:10.1099/00207713-16-3-313
Sierra-Paredes G, Sierra-Marcuño G (2008) Ascomycin and FK506: pharmacology and therapeutic potential as anticonvulsants and neuroprotectants. CNS Neurosci Ther 14(1):36–46. doi:10.1111/j.1527-3458.2008.00036.x
Singh BP, Behera BK (2009) Regulation of tacrolimus production by altering primary source of carbons and amino acids. Lett Appl Microbiol 49(2):254–259. doi:10.1111/j.1472-765X.2009.02652.x
Thykaer J, Nielsen J, Wohlleben W, Weber T, Gutknecht M, Lantz AE, Stegmann E (2010) Increased glycopeptide production after overexpression of shikimate pathway genes being part of the balhimycin biosynthetic gene cluster. Metab Eng 12(5):455–461. doi:10.1016/j.ymben.2010.05.001
Turło J, Gajzlerska W, Klimaszewska M, Król M, Dawidowski M, Gutkowska B (2012) Enhancement of tacrolimus productivity in Streptomyces tsukubaensis by the use of novel precursors for biosynthesis. Enzyme Microb Technol 51(6–7):388–395. doi:10.1016/j.enzmictec.2012.08.008
Wilkinson CJ, Hughes-Thomas ZA, Martin CJ, Bohm I, Mironenko T, Deacon M, Wheatcroft M, Wirtz G, Staunton J, Leadlay PF (2002) Increasing the efficiency of heterologous promoters in actinomycetes. J Mol Microbiol Biotechnol 4(4):417–426
Yepes A, Rico S, Rodriguez-Garcia A, Santamaria RI, Diaz M (2011) Novel two-component systems implied in antibiotic production in Streptomyces coelicolor. PLoS ONE 6(5):e19980. doi:10.1371/journal.pone.0019980
Yin H, Xiang S, Zheng J, Fan K, Yu T, Yang X, Peng Y, Wang H, Feng D, Luo Y, Bai H, Yang K (2012) Induction of holomycin production and complex metabolic changes by the argR mutation in Streptomyces clavuligerus NP1. Appl Environ Microbiol 78(9):3431–3441. doi:10.1128/aem.07699-11
Yoon YJ, Choi CY (1997) Nutrient effects on FK-506, a new immunosuppressant, production by Streptomyces sp. in a defined medium. J Ferment Bioeng 83(6):599–603. doi:10.1016/s0922-338x(97)81145-2
Zhu X, Zhang W, Chen X, Wu H, Duan Y, Xu Z (2010) Generation of high rapamycin producing strain via rational metabolic pathway-based mutagenesis and further titer improvement with fed-batch bioprocess optimization. Biotechnol Bioeng 107(3):506–515. doi:10.1002/bit.22819
Acknowledgments
This work was financially supported by the National 973 Project of China (No. 2013CB733600), the Key Program of National Natural Science Foundation of China (No. 21236005) and the Program of Introducing Talents of Discipline to Universities (No. B06006). The authors especially thank Professor A.L. Demain in Drew University for valuable suggestion on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Huang, D., Xia, M., Li, S. et al. Enhancement of FK506 production by engineering secondary pathways of Streptomyces tsukubaensis and exogenous feeding strategies. J Ind Microbiol Biotechnol 40, 1023–1037 (2013). https://doi.org/10.1007/s10295-013-1301-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-013-1301-7