Abstract
Aspergillus unguis NII-08123, a filamentous fungus isolated from soil, was found to produce β-glucosidase (BGL) activity with high glucose tolerance. Cultivation of the fungus in different carbon sources resulted in the secretion of different isoforms of the enzyme. A low molecular weight isoform, which retained ~60 % activity in the presence of 1.5 M glucose, was purified to homogeneity and the purified enzyme exhibited a temperature and pH optima of 60 °C and 6, respectively. The K m and V max of the enzyme were 4.85 mM and 2.95 U/mg, respectively, for 4-nitrophenyl β-d-glucopyranoside. The glucose inhibition constant of the enzyme was 0.8 M, indicating high glucose tolerance, and this is the second-highest glucose tolerance ever reported from the Aspergillus nidulans group. The glucose-tolerant BGL from A. unguis, when supplemented to cellulase preparation from Penicillium, could improve biomass hydrolysis efficiency by 20 % in 12 h compared to the enzyme without additional beta glucosidase supplementation. The beta glucosidase from A. unguis is proposed as a highly potent “blend-in” for biomass saccharifying enzyme preparations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
β-Glucosidases (EC 3.2.1.21; β-d-glucoside glucohydrolase) are key enzymes in cellulose hydrolysis, being the rate-limiting enzyme that is regulated by feedback inhibition from its own product—glucose. These enzymes are therefore of considerable interest as constituents of cellulose-degrading systems to be used for biomass conversion applications [19]. Aspergilli are known to be potent sources of β-glucosidase (BGL) and the most common industrial source of BGL is Aspergillus niger [3]. Inhibition by its product and substrate limits its use, especially in the context of biomass hydrolysis. β-glucosidase insensitive to glucose and cellobiose will significantly improve enzymatic conversion of cellulosic biomass to glucose for the subsequent production of fuel ethanol [16]. Glucose-tolerant β-glucosidases (GT-BGLs) have been reported from a few filamentous fungi, and differences in their properties were studied including molecular mass, isoelectric points, pH optima, and glucose inhibition constants [6, 9, 10, 15]. From the limited number of reports about GT-BGLs, it may be speculated that such enzymes are rather uncommon or understudied. However, the impact of the addition of GT-BGLs in the cellulase cocktails for biomass hydrolysis can be significant, and improvements in the yield and concentration of sugars may be achieved by using such blends.
As a part of our screening program, 200 fungal strains from soil samples were screened for the production of extracellular β-glucosidase and a significant number of them exhibited β-glucosidase activity (unpublished results). Further analyses proved that one of the isolates coded as BTC-F58 secreted a BGL that was active even in 1 M glucose. The fungal isolate was identified by morphological as well as molecular identification techniques to be of a strain of Aspergillus unguis. Detailed studies on the GT-BGL production indicated that multiple isoforms of the enzyme were secreted by the fungus and the expression of different isoforms showed variation in response to the inducer/carbon source. SDS polyacrylamide gel electrophoresis/activity staining experiments showed that crude extract of A. unguis contained more than five isoforms of the enzyme. Zymogram analyses indicated that a low molecular weight isoform retained its catalytic activity even at very high glucose concentrations. This caused us to speculate that this isoform might be responsible for the glucose tolerance of the enzyme preparation from A. unguis. The low molecular weight isoform was isolated, purified, and the properties were studied to characterize the suspected glucose-tolerant isoform. Results showed that the enzyme of interest represented only a minor fraction of the total β-glucosidase activity and the other isoforms that formed the major fraction were highly sensitive to glucose inhibition. The enzyme, when supplemented with Penicillium janthinellum cellulase, could improve the hydrolysis performance of the enzyme cocktail.
Materials and methods
Organisms and culture conditions
Aspergillus unguis, used in this study, was obtained from the soil samples and maintained on potato dextrose agar. For enzyme production, the culture was grown on minimal medium with the composition in g/l (NH4)2SO4: 1.4, KH2PO4: 2.0, CaCl2·2H2O: 0.3, MgSO4·7H2O: 0.3, FeSO4·7H2O: 0.005, MnSO4·H2O: 0.0016, ZnSO4·7H2O: 0.002, peptone: 2.5, yeast extract: 1.0, supplemented with an appropriate carbon source at a concentration of 1 % (w/v). The initial pH of the medium was adjusted to 5.0. A spore suspension in 0.05 % Tween 80 solution containing 1 × 106 spores/ml was used to inoculate 100ml of medium taken in 250ml Elenmeyer flasks. The culture was incubated at 30 ± 1 °C for 86 h on a shaker incubator at 200 rpm and was then harvested by centrifugation (8,000 rpm, 20 min at 4 °C) followed by filtration through Whatman GF/A glass microfiber filters. These crude enzyme samples were assayed for BGL activity and were stored at 4 °C for further studies.
Identification of microorganism
The fungal isolate BTC-F58 was grown in potato dextrose agar (PDA) medium and the conidial characteristics were observed under a phase contrast microscope (Leica, Mannheim, Germany). Further, the conidia were coated with gold palladium particles using a JEOL JFC-1200 fine coater and their structures were observed using scanning electron microscope (SEM, JEOL Model JSM-5600, Japan) at 15 kV and 500–5,000× magnification. In addition to recording morphological characteristics of the fungal isolate, molecular systematic approach was also employed for identification using 18S rRNA gene sequence of the fungal isolate. A portion of the 18S rRNA gene was amplified from the genomic DNA by polymerase chain reaction (PCR) using the universal primers NS1, NS4, NS3, and NS8 [22]. Nucleotide sequences of the PCR amplicons were determined by dye terminator sequencing. Identity of the sequence assembly was established by BLAST analysis [1]. Later, a homology search was performed and based on the results; a phylogenetic tree was constructed using the neighbor-joining method implemented in PHYLIP [7].
Differential induction of BGL in response to carbon source
The carbon sources—cellulose, xylose, lactose, glucose, maltose, and sucrose—were supplied in the medium at 1 % w/v level and fermentation was carried out as outlined above. Culture filtrates were assayed for BGL activity and the samples were concentrated using vacuum centrifugation (Eppendorf, Germany). Secretion of BGL isoforms by the fungus in response to the inducer carbon source was studied by analyzing the zymogram generated by performing native PAGE followed by activity staining of the gels.
Native PAGE and zymogram analysis
Crude enzyme preparations were fractionated by native PAGE using 12 % gel with 5 % stacking gel. Sample loadings were duplicated in the same order in the same gel so as to perform the activity staining in presence and absence of glucose, respectively. Activity staining of the gels was done by incubating them with a 10 mM methyl umbelliferyl β-d-glucopyranoside (MUG) solution in citrate buffer (0.05 M, pH 4.8). While staining, identical conditions were maintained for both the sets except for presence of glucose (0.5–1.5 M) in one of the sets. Activity bands were visualized as blue green fluorescence (methyl umbelliferone) by illumination with long-wavelength UV trans-illumination and photographs were acquired using an imaging system (Syngene-GBox, UK). Both the sets of gels were pictured simultaneously to ensure even conditions of illumination and exposure. Band intensities were measured using ImageJ software [17]. The fluorescence intensities of the bands in presence and absence of glucose was compared to calculate the percentage activity retention as follows
where I g and I 0 are the fluorescence intensities in presence and absence of glucose, respectively, for each band.
Enzyme assay
β-Glucosidase activity was assayed using p-nitro phenyl-β-d-glucopyranoside (pNPG) as substrate. The reaction mixture, which consisted of 1 ml of citrate buffer (0.05 M, pH 4.8), 0.5 ml of enzyme sample and 0.5 ml of 10 mM pNPG was incubated at 40 °C for 15 min. Reaction was terminated by adding 2 ml of 0.2 M Na2CO3. The absorbance of p-nitro phenol (pNP) released was measured at 400 nm. One unit of enzyme activity was defined as the amount of enzyme required releasing 1 μM of pNP per minute and was expressed as IU/ml.
Enzyme purification and characterization
The culture broth was concentrated using acetone fractionation. Different isoforms were precipitated using varying concentrations of chilled acetone. Among the β-glucosidase active fractions, the fraction that showed glucose tolerance was further purified using chromatography. The samples were loaded onto a Q Sepharose Fast Flow ion exchange column (Bio-Rad, Hercules, CA, USA) pre-equilibrated with 0.05 M Tris–HCl Buffer (pH 7). The column was eluted using a linear gradient of 0–1.0 M NaCl in 50 mM Tris–HCl buffer (pH 7) at a flow rate of 0.75 ml/min when two activity peaks were detected. All fractions were analyzed for β-glucosidase activity and fractions corresponding to the activity peaks were pooled and concentrated. Further gel elution was carried on Sephadex G75 columns using a 50 mM, pH 4.8 citrate buffer to separate the low molecular isoform, and the fractions corresponding to the peak were pooled and concentrated. Homogeneity of the purified enzyme was tested using SDS PAGE [11]. Enzyme kinetic studies were performed and the Michaelis–Menten curve fitting and the determination of V max and K m were performed using GraphPad Prism software (GraphPad Software Inc., La Jolla, CA, USA). Inhibition kinetics was also studied using varying concentrations of inhibitors and glucose inhibition constant K i for BGL was determined using the software. Temperature optimum of the enzyme was determined by assaying the enzyme at different temperatures (40–70 °C with 10 °C intervals) as outlined above. The temperature at which maximum activity was obtained was considered as optimum. Similarly, pH optimum for activity of the GT-BGL was determined by analyzing the activity of the enzyme at various pH (4–7) achieved by use of citrate and Tris buffers.
Hydrolysis performance of enzyme cocktail containing A. unguis BGL
The cellulase preparation used for the experiments was from P. janthinellum and the substrate used was steam exploded bagasse (SEB). The P. janthinellum strain and SEB were kind gifts from Dr. DV Gokhale and Dr. AJ Verma, respectively, from the CSIR-National Chemical Laboratory, Pune, India. The P. janthinellum cellulase was produced following the protocol provided by Dr. Gokhale. The hydrolysis was carried out in 100-ml screw-capped conical flasks with a working volume of 30 ml. The biomass loading was 10 % w/w, and the cellulase and BGL loadings were 15 FPUs/g and 20 or 60 IU/g, respectively. Incubation was performed at 50 °C for 60 h with sampling at every 12 h. Controls without BGL supplementation and with supplementation of 20 IU/g commercial BGL preparations were run in parallel. Samples were analyzed for glucose released by the NREL Laboratory Analytical Protocol for sugars using HPLC [12].
Results
Identification of the fungus
Fungal isolate BTC-F58 formed dark green colonies on PDA, and the microscopic features resembled that of Aspergillus sp. Morphological examination of the fungus under microscope revealed long, roughened and thick-walled spicular hyphae, hemispherical vesicles, biseriate phialides supported by metulae, and globose conidia dull green in color—the features characteristic of A. unguis [20] (Fig. 1a–d). PCR amplification of the 18S rRNA region yielded a 1,678-bp sequence, which was submitted to GenBank with the accession JQ726491 (gi|387966750). The BLAST analyses of the partial DNA sequence showed 99 % similarity with 18S rRNA sequence of A. unguis and progressively lesser similarity with those from other Aspergilli, confirming that the isolate is a strain of A. unguis. The isolate was deposited in the NII culture collection with accession NII 08123. Pair-wise alignments of the sequence with the closest matches in the sequence depositories identified by BLASTN (http://www.ncbi.nlm.nih.gov/BLAST/) searches were performed, and the phylogenetic relationships were derived using neighbor-joining methods and represented as a phylogenetic tree (Fig. 2).
Aspergillus unguis strain morphology and BGL screening A. unguis. a Colony on PDA agar. b Conidiophore showing thick wall and biseriate phialides. c Screening of β-glucosidase production using methyl umbelliferyl β-d glucopyranoside (MUG) soft agar method. d Electron micrograph of the conidiophores showing biseriate phialides with metuale
Differential expression of BGL in response to C sources and the existence of multiple BGL isoforms
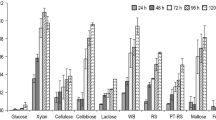
Since during the fermentation for BGL production by A. unguis NII 08123 there was a large amount of fluctuation in the glucose tolerance of BGL, it was speculated that there could be differences in the expression of enzymes depending on the immediate environment, especially the type of C source. To evaluate this, the isolate was cultured in the same basal medium under identical conditions but with different C sources, and the BGL activity and glucose tolerance was monitored. Effect of carbon source/inducer on β-glucosidase production by A. unguis and the expression of different isoforms are shown in Figs. 3 and 4. Maximum glucose tolerance (32.75 % activity retained) was observed with lactose as the C source, and the glucose tolerance and levels of BGL yield varied with C sources (Fig. 3).
Differential expression of BGL in response to C source. Enzyme samples were subjected to native PAGE and incubated with MUG for detection of activity. Lane information: carbon source followed by T g in parenthesis: lane 1 wheat bran (23 %), rice straw (1.9 %), cellulose (20.2 %), glucose (0.0 %). Image inverted for clarity
Multiplicity of BGLs has been reported earlier by several authors [5, 9, 12, 15, 18]. Difference in expression of the BGL isoforms could be the reason for the differences in glucose tolerance of the crude enzyme preparation, since the actual amount of the glucose-tolerant isoform could vary according to the carbon source. The existence of multiple isoforms of BGL in A. unguis was studied by growing the fungus in different C sources and performing zymogram analyses. Since it was noted that the crude carbon source wheat bran induced most of the BGL isoforms in A. unguis, BGL production was also conducted in media supplemented with 1 % w/v of either wheat bran or rice straw. Results shown in Figs. 4 and 5 indicate the existence of multiple isoforms (five detected here) of the enzyme. While wheat bran induced the production of five BGL isoforms (Fig. 5, lanes 1–3), rice straw and cellulose could induce only the high molecular weight and low molecular weight BGL isoforms. There was also a direct correlation between the signal intensity of the low molecular weight band and the glucose tolerance (as detected by pNPG assay) indicating that this could be the glucose-tolerant isoform (GT-BGL).
Evaluation of glucose tolerance by pixel density analysis. Glucose tolerance of the BGL proteins secreted by A. unguis when grown in 1 % w/v wheat bran as C source. Enzyme samples were subjected to native PAGE and incubated with MUG for activity detection. Arrowheads indicate the isoforms. Lane information: (normalized fluorescence intensity and T g of GT-BGL in parentheses): lanes 1, 2, 3 BGL activity signals in gel incubated without glucose (0.148, 100 %). Lanes 4, 5 BGL activity signals in presence of 0.5 M glucose (0.118, 80 %), lanes 6, 7 BGL activity signals in presence of 1.0 M glucose (0.117, 79 %), lane 9 BGL activity signal in presence of 1.5 M glucose (0.086, 58 %). Image inverted for clarity
This was further proven by fluorescence intensity analysis of the BGL activity bands observed at varying glucose concentrations. BGL produced using wheat bran as C source was run on native PAGE and activity stains were performed in buffers containing varying concentrations of glucose (0.5–1.5 M). Only the lower band was detected when assayed in the presence of glucose and the intensity of this band was negatively correlated to the glucose concentration confirming that this band itself is the glucose-tolerant isoform (Fig. 5).
Purification and characterization of β-glucosidase
To study further the properties of the glucose-tolerant BGL isoform, the low molecular weight BGL protein was purified to homogeneity from the culture filtrate of A. unguis grown on 1 % w/v lactose as C source. Crude BGL sample (500 ml) was precipitated using 2 volumes of chilled acetone and the precipitate was re-dissolved in 15 ml of 0.05 M, pH 4.8 citrate buffer. The concentrated sample contained 75 % of the initial activity. Anion exchange chromatography on Q-Sepharose Fast Flow column resulted in sample separation into a major and a minor protein peak (PI and PII respectively), both having BGL activity. Assay of the fractions corresponding to the peaks, as well as SDS-PAGE analyses using the fractions indicated that the GT-BGL protein has partitioned into the minor peak (Fig. 6). Fractions corresponding to the two peaks were pooled separately and PII was run on a Sephadex G75 column when one peak corresponding to the GT BGL was obtained. The purification results are summarized in Table 1.
Activity staining of BGL fractions separated by chormatography. a Activity staining of the pooled fractions corresponding to the major and minor protein peaks obtained after ion exchange purification of BGL. Lanes 1, 2 peaks PI and PII, respectively, run on native PAGE and activity stained. Lanes 3, 4 peaks PI and PII, respectively, run on native PAGE and activity stained in presence of 1.0 M glucose. The high molecular weight major isoform was not completely separated using the Q sepharose ion exchange chromatography, as can be gauged from the activity stain obtained for this protein (lanes 2, 4). b SDS-PAGE analysis of he purified GT-BGL. Lane 1 molecular weight marker, lane 2 purified GT-BGL—arrowhead indicates the ~10-kDa GT-BGL protein band
The purified GT-BGL was optimally active at 60 °C and at pH 6.0. An increase in temperature beyond 60 °C (pH 6.00) resulted in a rapid decline in activity (Fig. 7). At pH 7, the activity was almost the same as that obtained at pH 6.00 (Fig. 8). The relative activity of GT-BGL was more than 60 % of the activity at optimal pH over the entire range tested.
The standard assay mixture with pH 6.0 was used for the determination of reaction velocities of GT-BGL. K m and V max for the hydrolysis of pNPG by the enzyme was determined using 10–80 mM substrate. Michaelis–Menten kinetics was fitted and the determination of K m and V max was performed using the non-linear regression implemented in GraphPad Prism. K m and V max were determined to be 4.85 mM and 2.95 U/mg, respectively, for pNPG. End-product inhibition was evaluated using pNPG as substrate and in presence of 0–1.0 M glucose as the inhibitor. GT-BGL was found to be highly glucose-tolerant with a K i of 800 mM. Enzyme properties determined by the study are listed in Table 2.
Hydrolysis performance of cellulase blends containing A. unguis BGL
Penicillium janthinellum cellulase preparation containing ~5.5 IU/ml BGL was blended with additional BGL from A. unguis so that the final BGL concentrations were either 20 or 60 IU/g SEB to be hydrolyzed. Hydrolysis experiments showed that there was a considerable improvement in hydrolysis performance when A. unguis BGL was blended with the cellulase. With 60 IUs/g additional BGL loading, there was a 20 % improvement in glucose yield even within 12 h of hydrolysis. There was a linear improvement in hydrolysis performance for blends containing higher BGL loading an efficiency of ~60 % of the theoretical maximum was achieved in 48 h in blends containing 60 IU/g additional BGL loading (Table 3). Hydrolysis performance of blends containing 20 IU/g of commercial BGL was significantly better (71 % efficiency attained in 48 h). This could be due to the presence of cellulase and hemicellulase activities in the commercial BGL sample that we had detected. A hydrolysis study done with one of the best biomass hydrolyzing enzyme blends available in the market could yield >75 % efficiency with this biomass (data not shown).
Discussion
β-Glucosidase plays a major role in the conversion of cellulosic biomass to ethanol, being the rate-limiting enzyme that determines the action of all cellulase components. A large number of these enzymes from bacteria and plants have been purified and studied, but most microbial β-glucosidases are very sensitive to glucose inhibition [2, 8]. There are only limited reports in publications on glucose-tolerant BGLs and most of the reports on filamentous fungi describes Aspergilli as the producers of GT-BGLs. To the best of our knowledge, this is the first report on production of glucose-tolerant β-glucosidase from A. unguis, which belongs to the Aspergillus nidulans group. Glucose-tolerant β-glucosidases have been reported previously in Aspergillus oryzae [15] and in A. niger [21]. However, the growth characteristics of A. unguis under submerged fermentation make it attractive for large-scale production of the enzyme. Under our culture conditions, the fungus grew as pellets in an even suspension and did not form large clumps/aggregates, allowing better aeration and mixing. Other Aspergilli that we had used for BGL production (e.g., A. niger [18]) either formed a mat or ball-like structures in shake flasks and larger non-uniform aggregates in fermenter. This fact and the ability to use substrates like wheat bran for production makes A. unguis the preferred choice for large-scale BGL production for biomass hydrolysis.
The level of production of glucose-tolerant isoforms was greatly influenced by the carbon source supplied. It is interesting to note that the highest tolerance (32.75 %) was observed in cultures grown with lactose as the carbon source. Lactose is a known inducer of cellulase and could have caused a selective induction of GT-BGL. From the zymogram analysis, we identified five active isoforms of β-glucosidase, even though their expression level varies in the presence of different carbon sources. Staining the native as well as SDS PAGE gels using MUG in the presence of different concentrations of glucose indicated that the low molecular weight isoform plays a key role in activity retention, even in the presence of a considerable amount of glucose. It has previously been reported that the glucose-tolerant beta glucosidases are low molecular weight proteins expressed at lower levels compared to the highly expressed, high molecular weight glucose-sensitive major BGLs in Candida peltata [16], A. oryzae [15], Aspergillus foetidus [5], Aspergillus tubingensis [6], and in A. niger [18]. BGL multiplicity can be attributed to the presence of multiple genes or due to differential post-transcriptional modifications [4]. Differential expression of the various BGL proteins is reported in response to the carbon sources supplied in the medium or the conditions of culture [14, 23] and could be a probable adaptation of the fungi to respond to the changing immediate environments. This property, however, could be exploited for selective expression of a desired isoform from a fungus by manipulating the culture conditions/carbon source carefully. This is apparently useful not only in the context of producing BGL for biomass hydrolysis but also for flavor enhancement in wine and fruit juices, provided that the BGL in question has the suitable substrate specificity [21].
The high resistance of A. unguis BGL to glucose inhibition and the advantages due to growth properties of the fungus underlines its importance in biomass hydrolysis. With few exceptions, most microbial β-glucosidases show competitive inhibition kinetics in the presence of glucose [16]. Extracellular β-glucosidases are highly sensitive to glucose and normally have glucose inhibition constants ranging from 0.5 to 100 mM. Noticeable exceptions like A. oryzae [15] and C. peltata [16], where the K i values have been reported to be 1.36 and 1.4 M, respectively, do exist, though this is very rare. The K i (0.8 M) obtained for A. unguis GT-BGL reported here may be considered as the second-highest tolerance ever reported from the A. nidulans group. The inhibition kinetics graph was explained based on the Cornish–Bowden plot where the inhibition constant (K i) is greater than dissociation constant (K i). GT-BGL from A. unguis exhibited a type of mixed inhibition with a predominating un-competitive inhibition. Previously, it has been reported that β-glucosidase from Trichoderma viride showed a type of mixed inhibition with competitive character [13].
Additional BGL supplementation in biomass hydrolyzing enzyme preparation is a common practice, since typically the commercial cellulase-producing microbial cultures contain much less BGL activity. In all the current commercial enzyme preparations for biomass hydrolysis, the BGL is also highly sensitive to glucose, which limits their potential to achieve high sugar concentrations. In our preliminary studies conducted with steam-exploded bagasse, supplementation of the GT-BGL-containing BGL preparation from A. unguis could enhance the total sugar yield by ~20 % in 12 h. Further optimizations of enzyme loadings and ratios are expected to enhance the sugar yield further. The results indicate the potential for further development of this enzyme as a potential “blend-in” for biomass hydrolyzing enzyme preparations. In conclusion, the short duration needed for production, possibility of modulation of the expression by varying carbon sources, as well as the high glucose tolerance of this enzyme are particularly favorable for the application of this BGL in the enzymatic hydrolysis of cellulose to glucose.
References
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Bothast RJ, Saha BC (1997) Ethanol production from agricultural biomass substrates. Adv Appl Microbiol 44:261–286
Chauve M, Mathis H, Huc D, Casanave D, Monot F, Ferreira NL (2010) Comparative kinetic analysis of two fungal β-glucosidases. Biotechnol Biofuels 3(1):3. doi:10.1186/1754-6834-3-3
Collins CM, Murray PG, Denman S, Morrissey JP, Byrnes L, Teeri TT, Tuohy MG (2007) Molecular cloning and expression analysis of two distinct beta-glucosidase genes—bg1 and aven1, with very different biological roles from the thermophilic, saprophytic fungus Talaromyces emersonii. Mycol Res 111(7):840–849
Decker CH, Visser J, Schreier P (2001) β-Glucosidase multiplicity from Aspergillus tubingensis CBS 643.92: purification and characterization of four β-glucosidases and their differentiation with respect to substrate specificity, glucose inhibition and acid tolerance. Appl Microbiol Biotechnol 55:157–163
Decker CH, Visser J, Schreier P (2000) β-Glucosidases from five black Aspergillus species: study of their physico-chemical and biocatalytic properties. J Agric Food Chem 48:4929–4936
Felsenstein J (1989) PHYLIP—Phylogeny Inference Package (Version 3.2). Cladistics 5:164–166
Gueguen Y, Chemardin P, Arnaud A, Galzy P (1995) Purification and characterization of an intracellular β-glucosidase from Botrytis cinerea. Enzyme Microb Technol 78:900–906
Günata Z, Vallier MJ (1999) Production of a highly glucose tolerant extracellular β-glucosidase by three Aspergillus strains. Biotechnol Lett 21:219–223
Job J, Sukumaran RK, Jayachandran K (2010) Production of a highly glucose-tolerant b-glucosidase by Paecilomyces variotii MG3: optimization of fermentation conditions using Plackett–Burman and Box–Behnken experimental designs. World J Microbiol Biotechnol 26:1385–1391
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J, Templeton D (2008) Determination of sugars byproducts, and degradation products in liquid fraction process samples, NREL/TP-510-42623
Montero M, Romeu A (1992) Kinetic study on the β-glucosidase catalyzed reaction of Trichoderma viride cellulase. Appl Microbiol Biotechnol 38:350–353
Nazir A, Soni R, Saini HS, Kaur A, Chadha BS (2010) Profiling differential expression of cellulases and metabolite footprints in Aspergillus terreus. Appl Biochem Biotechnol 162:538–547
Riou C, Salmon J, Vallier M, Gunata Z, Barre P (1998) Purification, characterization, and substrate specificity of a novel highly glucose-tolerant β-glucosidase from Aspergillus oryzae. Appl Environ Microbiol 64:3607–3614
Saha BC, Bothast RJ (1996) Production, purification and characterization of a highly glucose tolerant novel β-glucosidase from Candida peltata. Appl Environ Microbiol 62:3165–3170
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675
Singhania RR, Sukumaran RK, Rajasree KP, Mathew A, Gottumukkala L, Pandey A (2011) Properties of a major β-glucosidase-BGL1 from Aspergillus niger NII-08121 expressed differentially in response to carbon sources. Process Biochem 46:1521–1524
Sørensen A, Lübeck PS, Lübeck M, Teller PJ, Ahring BK (2011) β-Glucosidases from a new Aspergillus species can substitute commercial β-glucosidases for saccharification of lignocellulosic biomass. Can J Microbiol 57(8):638–650
Thom C, Raper KB (1939) The Aspergillus nidulans group. Mycologia 31:653–669
Watanabe T, Sato T, Yoshioka S, Koshijima T, Kuwahara M (1992) Purification and properties of Aspergillus niger β-glucosidase. Eur J Biochem 209:651–659
White TJ, Bruns T, Lee S, Taylor JW (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and applications. Academic Press, New York, pp 315–322
Willick GE, Seligy VL (1985) Multiplicity in cellulases of Schizophyllum commune derivation partly from heterogeneity in transcription and glycosylation. Eur J Biochem 151:89–96
Acknowledgments
This work was supported by the research grant SR/FT/L-54/2006 to RKS from the Department of Science and Technology, Govt. of India and the grants from TIFAC, Department of Science and Technology, Govt. of India. RKP is thankful for financial support from CSIR and MNRE, Govt. of India. The authors are thankful to the Department of Forests, Kerala State, for permission to carry out sampling in Silent Valley National Park. We thank Dr. AJ Varma and Dr. DV Gokhale from CSIR-NCL for providing SEB and the P. janthinellum culture, respectively. We acknowledge the Electron microscopy section of CSIR-NIIST for SEM analysis of the fungus and Dr. Ramesh Kumar N, Biotechnology Division, CSIR-NIIST for DNA sequencing service.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rajasree, K.P., Mathew, G.M., Pandey, A. et al. Highly glucose tolerant β-glucosidase from Aspergillus unguis: NII 08123 for enhanced hydrolysis of biomass. J Ind Microbiol Biotechnol 40, 967–975 (2013). https://doi.org/10.1007/s10295-013-1291-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-013-1291-5