Abstract
The acyl transfer activity of the amidase of Alcaligenes sp. MTCC 10674 has been applied to the conversion of benzamide and hydroxylamine to benzohydroxamic acid. The unique features of the acyl transfer activity of this organism include its optimal activity at 50 °C and very high substrate (100 mM benzamide) and product (90 mM benzohydroxamic acid) tolerance among the hitherto reported enzymes. The bench scale production of benzohydroxamic acid was carried out in a fed-batch reaction (final volume 1 l) by adding 50 mM benzamide and 250 mM of hydroxylamine after every 20 min for 80 min in 0.1 M potassium phosphate buffer (pH 7.0) at 50 °C, using resting cells equal to 4.0 mg dcm/ml of reaction mixture. From 1 l of reaction mixture 33 g of benzohydroxamic acid was recovered with 24.6 g l−1 h−1 productivity. The acyl transfer activity of the amidase of Alcaligenes sp. MTCC 10674 and the process developed in the present study are of industrial significance for the enzyme-mediated production of benzohydroxamic acid.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hydroxamic acids (R-CONHOH) are known for their ability to form stable chelates with metal ions. These chelates are finding applications as growth factors, food additives, antibiotics, antifungal agents, tumor inhibitors, siderophores, enzyme inhibitors, and in bioremediation [8, 10, 14, 17]. Most of the hydroxamic acids are synthesized through chemical routes either by N-alkylation of simple O-substituted hydroxylamine with a variety of alkylating agents or by the direct oxidation of lysine and ornithine using dimethyldioxirane (DMD) [12]. The synthesis of hydroxamic acids by chemical processes yields product contaminated with by-products and thus further processing is required to get pure hydroxamic acids and their derivatives. However, in the last two decades several reports on enzymatic synthesis of hydroxamic acid have been published [4, 6, 7, 18]. Enzyme-mediated synthesis of hydroxamic acid yields pure product under milder conditions of pH, temperature, and pressure. The enzyme-catalyzed synthesis of hydroxamic acids may involve (a) mono-enzymatic reaction employing nitrile or amide and hydroxylamine as substrates and nitrilase [4] or amidase [7] as biocatalysts, (b) bi-enzymatic reaction using nitrile and hydroxylamine as substrates and nitrile hydratase and amidase as enzymes [18]. Nitrilase or amidase exhibited dual activity, i.e., hydrolytic activity in aqueous reaction medium and acyl transfer activity in the presence of more nucleophilic hydroxylamine [15]. The acyl transfer activity of nitrilase or amidase is involved in the transfer of acyl group from nitriles or amides to hydroxylamine to form hydroxamic acid. The mono-enzymatic and bi-enzymatic reactions for the synthesis of hydroxamic acids are summarized below:
-
(a)
Mono-enzymatic
-
(b)
Bi-enzymatic
where R = aliphatic/aromatic, ATA = acyl transfer activity.
A number of hydroxamic acids, viz. acetohydroxamic acid, butyrohydroxamic acid, benzohydroxamic acid (BHA), and succinic hydroxamic acid, have been synthesized using biocatalytic routes. Among these, BHA finds application as anti-HIV, antimicrobial and antineoplastic agent. It is also used in the treatment of anemia and reported as potential inhibitor of leukemia and ureaplasma [1, 5, 9, 10]. BHA has been synthesized at test-tube scale using the acyl transfer activity of the amidase of Rhodococcus sp. R312 [7], the nitrilase of R. rhodochrous [4], and by the synergistic action of the nitrile hydratase and the acyl transfer activity of the amidase of R. erythropolis A4 [18]. A review of these earlier reports involving enzymatic synthesis of hydroxamic acid revealed that the acyl transfer activity of the amidase of Rhodococcus sp. R312 and the nitrilase of R. rhodochrous respectively suffer from substrate inhibition beyond 7.5 mM benzamide and 50 mM benzonitrile in the reactions. In the present communication bench scale (1 l) synthesis of BHA using the acyl transfer activity of the amidase of Alcaligenes sp. MTCC 10674 (whole cells) having high benzamide tolerance (100 mM) is reported for the first time.
Materials and methods
Chemicals
The nitrile and amides used in the present study were purchased from Lancaster Synthesis, England. The culture media ingredients were procured from Hi Media, (Mumbai, India). All other chemicals were of analytical grade and purchased from various commercial vendors.
Microorganism and culture conditions
Alcaligenes sp. MTCC 10674 (previously isolated in our laboratory and identified at the Institute of Microbial Technology, Chandigarh, India) was used as a source of amidase acyl transfer activity. This organism was grown aerobically in 250-ml Erlenmeyer flasks containing 50 ml salt medium [2] at 30 °C and 160 rpm in an incubator shaker for 12 h to prepare preculture for the production of amidase activity. This preculture 5 % (v/v) and isobutyronitrile 0.4 % (v/v) as inducer were added to 50 ml of salt medium in 250-ml Erlenmeyer flasks and incubated for 32 h at 30 °C and 160 rpm in an incubator shaker. The cells were harvested by centrifugation at 10,000×g for 10 min at 4 °C, washed twice and suspended in 0.1 M potassium phosphate buffer (pH 7.0), and stored at 4 °C until further use. The dry cell mass (dcm) of the resting cells was determined in the suspension.
Acyl transfer activity assay
The acyl transfer activity of amidase in the resting cells of Alcaligenes sp. MTCC 10674 was assayed using the method developed by Brammar and Clarke [3]. The assay mixture contained 0.1 M potassium phosphate buffer (pH 7.0), 100 mM benzamide, 500 mM hydroxylamine, and 4.0 mg dcm. The reaction mixture was incubated at 50 °C for 60 min and the reaction was stopped by adding 1 ml FeCl3 reagent containing 6 % FeCl3 and 2 % HCl. The mixture was centrifuged at 10,000×g for 5 min and the absorbance of the supernatant was then measured at 495 nm. One unit of acyl transfer activity of amidase was defined as the amount of enzyme which converted 1 μmol of benzamide to BHA in 1 min under the assay conditions.
Analytical methods
The concentration of benzamide and BHA in the reaction mixture was quantified using a Perkin Elmer HPLC system (200 LC pump, 785A) (programmable absorbance detector) equipped with a Nucleosil C18 column (25 cm × 4.6 mm, 5 μm particle size; GL Sciences, Japan). The compounds were detected at 254 nm, at a flow rate of 2 ml/min of mobile phase comprising 30 % acetonitrile and 0.2 % orthophosphoric acid in HPLC grade water.
Optimization of reaction conditions for conversion of benzamide to benzohydroxamic acid
The experimental design involving one variable at a time (OVAT) was followed for optimization of reaction conditions. The following variables were explored: various buffer systems (borate buffer, potassium phosphate buffer, sodium phosphate buffer, citrate buffer, carbonate buffer each of 0.1 M with pH ranging from 2 to 10), buffer molarity (0.05–0.2 M), different metal ions and inhibitors (1 mM), reaction temperature (30–70 °C), substrate benzamide and hydroxylamine concentration (50–500 mM and 100–1,000 mM, respectively), their kinetic parameters (K m, V max), and duration of reaction (20–120 min).
Fed-batch reaction at 50 ml scale
In order to scale up the process and to increase the product accumulation, the reaction was carried out in fed-batch mode at 50 °C in 50 ml reaction volume containing 0.1 M potassium phosphate buffer (pH 7.0) and Alcaligenes sp. MTCC 10674 cells (4.0 mg dcm/ml). The reaction was initiated by the addition of 50 mM of benzamide and 250 mM of hydroxylamine and five feedings of these substrates were made each at an interval of 20 min. The reaction mixture (10 μl) was periodically withdrawn and bioconversion of benzamide and accumulation of BHA in the reaction were analyzed by HPLC.
Bench scale (1 l) production of benzohydroxamic acid
On the basis of optimized reaction conditions (in the preceding sections), the conversion of benzamide to BHA was scaled up to 1 l using a New Brunswick Scientific (NBS) BIOFLO C-32 fermenter.
Recovery of benzohydroxamic acid
The reaction mixture was centrifuged (10,000×g, 30 min) to remove the cells. The supernatant was collected and freeze-dried to recover the BHA. The BHA was purified by solvent extraction [18] and analyzed by HPLC.
Results and discussion
Optimization of reaction conditions
Amount of biocatalyst
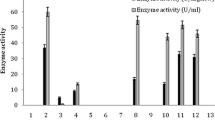
In order to find out the optimum amount of biocatalyst (resting cells) for acyl transfer activity, 1–10 mg dcm of Alcaligenes sp. MTCC 10674 was added in 1 ml reaction for conversion of benzamide to BHA. The maximum acyl transfer activity (1.16 ± 0.01 U/mg dcm) was observed when 4.0 mg dcm/ml was used in the reaction (Fig. 1). Above and below this amount of biocatalyst, a decrease in the acyl transfer activity was observed for transformation of benzamide to BHA which may be due to imbalance in the biocatalyst and substrate ratio required for the optimum activity. Earlier, 6.6 μg of biocatalyst and 0.6 U of amidase/ml of reaction were used for the conversion of 15 and 7.5 mM benzamide to BHA, respectively [7, 18], whereas the amidase of Alcaligenes sp. MTCC 10674 (4.0 mg dcm/ml) converted 100 mM of benzamide to BHA.
Buffer system and pH
For the selection of appropriate buffer and pH, five different buffers (citrate, borate, carbonate, sodium phosphate, and potassium phosphate) having 0.1 molarity and pH 2–10 were used. The acyl transfer activity was higher in potassium phosphate buffer (pH 7.0) (1.34 ± 0.02 U/mg dcm) (Fig. 2) and this buffer was used in subsequent experiments. In the remaining buffers and at pH other than 7.0, there was a decrease in the acyl transfer activity of the amidase of Alcaligenes sp. MTCC 10674. Beyond the optimum pH, i.e., pH 7.0, these buffers might not favor the ionic conditions necessary for the rapid formation of enzyme–substrate complex. Most of the reported amidases have maximum acyl transfer activity at neutral pH [7, 16].
Buffer molarity
The molarity of potassium phosphate buffer (pH 7.0) was varied from 0.05 to 0.2 M for the assay of the acyl transfer activity of Alcaligenes sp. MTCC 10674. Potassium phosphate (0.1 M) buffer (pH 7.0) was found to be suitable for the assay of the acyl transfer activity (1.42 ± 0.02 U/mg dcm) as shown in Fig. 3. A higher molarity of potassium phosphate buffers (≥0.14 M) decreased the acyl transfer activity of the amidase of Alcaligenes sp. MTCC 10674; this decrease in enzyme activity might be due to electrostatic interactions between the polar surface groups of enzymes and the buffer. In earlier studies, the optimum acyl transfer activity of amidase was reported in potassium phosphate buffer (0.1 M, pH 7.0) [4] and Tris/HCl buffer (0.05 M, pH 8.0) [18]. The amidase of Alcaligenes sp. MTCC 10674 also exhibited maximum acyl transfer activity in potassium phosphate buffer (0.1 M, pH 7.0).
Effect of metal ions and inhibitors
The effect of various metal ions and inhibitors on the acyl transfer activity of the amidase of Alcaligenes sp. MTCC 10674 is shown in Fig. 4. The presence of dithiothreitol (DTT) showed a significant increase in the acyl transfer activity (1.52 ± 0.01 U/mg dcm) as compared to control (i.e., without metal ion/inhibitor; 1.41 ± 0.01 U/mg dcm). Among the metal ions used, Cu2+ and Hg2+, which are known blockers of free thiol groups, strongly inhibited the acyl transfer activity. This indicates the presence of free thiol groups at the active site of the amidase of Alcaligenes sp. MTCC 10674. Kotlova et al. [11] also investigated the effect of metal ions and inhibitors on the amidase of R. rhodochrous M8 and reported that heavy metal ions inhibited the acyl transfer activity of amidase enzyme completely. EDTA did not affect the acyl transfer activity, whereas the presence of DTT caused a 0.7-fold increase in the amidase activity which meant that under the reducing conditions conversion of the substrate was better with whole cells. This indicates that the enzyme has free sulfhydryl groups (cysteine residue) as in other amidases and nitrilases [11, 13].
Temperature
The maximum acyl transfer activity of the amidase of Alcaligenes sp. MTCC 10674 (1.64 ± 0.02 U/mg dcm) was observed at 50 °C (Fig. 5). There was a continuous increase in activity from 25 to 50 °C; thereafter, activity decreased and it was almost negligible at 70 °C. Earlier studies have shown that 30 and 28 °C are optimum temperatures for maximum acyl transfer activity and production of BHA, respectively [7, 18]. The acyl transfer activity of the amidase of Alcaligenes sp. MTCC 10674, therefore, has higher operational stability in comparison to those in earlier reports for the production BHA.
Substrate concentration
For the production of BHA through the acyl transfer activity of the amidase, two substrates were used, i.e., benzamide and hydroxylamine. Therefore, the concentrations of both substrates were optimized for the assay of the acyl transfer activity of the amidase. The concentration of benzamide was varied from 50 to 500 mM while the concentration of hydroxylamine was kept constant (100 mM). The maximum acyl transfer activity (1.72 ± 0.03 U/mg dcm) was at 100 mM of benzamide in the reaction (Fig. 6). The kinetic constants K m and V max for both the substrates were calculated. The kinetic constant for first substrate was K m,benzamide = 100.82 mM and V max = 2.55 μmol min−1 (mg dcm)−1 of resting cells of Alcaligenes sp. MTCC 10674. Similarly the concentration of hydroxylamine was varied from 100 to 1,000 mM whereas the concentration of benzamide was kept constant, i.e., 100 mM. The acyl transfer activity was highest at 500 mM of hydroxylamine (1.86 ± 0.02 U/mg dcm) and the kinetic constants for the second substrate were K m,hydroxylamine = 587 mM and V max = 3.2 μmol min−1 (mg dcm)−1 of resting cells of Alcaligenes sp. MTCC 10674. In earlier studies the kinetic constants K m,benzamide = 0.100 mM and K m,hydroxylamine = 140 mM of purified amidase were reported [7]. From the earlier reports on the production of BHA using acyl transfer activity under similar reaction conditions it is evident that the hitherto reported enzymatic systems [7, 18] are prone to substrate and product inhibition, whereas the acyl transfer activity of Alcaligenes sp. MTCC 10674 has high tolerance for benzamide (100 mM), hydroxylamine (500 mM), and BHA (90 mM). This higher tolerance as compared to earlier reported amidases (Rhodococcus sp. R312, R. rhodochrous, R. erythropolis A4) for acyl transfer reactions makes the present enzyme more attractive for industrial applications.
Reaction time
The conversion of benzamide to BHA was followed up to 120 min under the optimized conditions using 4.0 mg dcm of Alcaligenes sp. MTCC 10674 cells/ml. The maximum acyl transfer activity was 1.92 ± 0.011 U/mg dcm after 80 min with 90 % conversion of benzamide to BHA (Fig. 7). Recently Vejvoda et al. [18] reported 40 % conversion of 15 mM benzamide to BHA using 0.6 U mg−1 amidase in 1 ml reaction in 200 min.
Fed-batch reaction at 50 ml scale
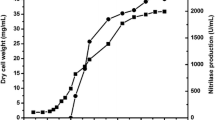
The acyl transfer activity of the amidase of Alcaligenes sp. MTCC 10674 decreased if the concentration of the benzamide was more than 100 mM in the reaction (Fig. 5). To increase the accumulation of product, the reaction was carried out in fed-batch mode at 50 ml scale and five feedings of substrate (each feeding comprising 50 mM benzamide and 250 mM of hydroxylamine at an interval of 20 min) were added. The reaction was carried out at 50 °C while maintaining biocatalyst amount at 4.0 mg dcm/ml in the reaction. There was maximum conversion of benzamide to BHA up to the fourth feeding of substrate (Fig. 8), whereas further feedings decreased the rate of production of BHA which might be due to product inhibition.
Production of benzohydroxamic acid at 1 l scale
The production of BHA at 1 l scale was carried out. A total of 200 mM of benzamide and 1 M of hydroxylamine (in four feedings, each feeding of 50 mM benzamide and 250 mM hydroxylamine, at an interval of 20 min) were added while maintaining 4.0 mg dcm/ml of Alcaligenes sp. MTCC 10674 during the reaction. Under these reaction conditions 33 g of BHA at the rate of 6.15 g h−1 (g dcm)−1 was accumulated in the reaction in 80 min. The BHA was recovered from the reaction and analyzed by HPLC. It exhibited 96 % purity.
Conclusion
Alcaligenes sp. MTCC 10674 has emerged as a novel catalyst for transformation of benzamide and hydroxylamine to BHA as it exhibited acyl transfer activity at high temperature with high substrate and product tolerance among the hitherto reported bacterial systems. The bench scale (1 l scale) production of BHA using resting cells of Alcaligenes sp. MTCC 10674 (exhibiting acyl transfer activity) showed the highest ever reported productivity (24.6 g l−1 h−1) and purity (96 %) for BHA. The bioprocess reported in the present study is potentially applicable for the industrial production of BHA.
References
Agrawal YK, Kunji PS (2005) Synthesis and dissociation constant of calix(6)arene hydroxamic acids. Iranian J Sci Technol 29:1–8
Bhalla TC, Kumar J, Kumar H, Agrawal HO (1997) Amidase production by Rhodococcus sp. NHB-2. Nat Acad Sci Lett 20:139–142
Brammar WJ, Clarke PH (1964) Induction and repression of Pseudomonas aeruginosa amidase. J Gen Microbiol 37:307–319
Dadd MR, Claridge TDW, Pettman AJ, Knowles CJ (2001) Biotransformation of benzonitrile to benzohydroxamic acid by Rhodococcus rhodochrous in the presence of hydroxylamine. Biotechnol Lett 23:221–225
Elford HL, Wampler GL, Van’t Riet B (1979) New ribonucleotide reductase inhibitors with antineoplastic activity. Cancer Res 39:844–851
Fournand D, Arnaud A (2001) Aliphatic and enantioselective amidases: from hydrolysis to acyl transfer activity. J Appl Microbiol 91:381–393
Fournand D, Bigey F, Arnaud A (1998) Acyl transfer activity of an amidase from Rhodococcus sp. Strain R312: formation of a wide range of hydroxamic acids. Appl Environ Microbiol 64:2844–2852
Holland KP, Howard L, Elford BV, Charles G, Annis SM, Schuster CD (1998) Antimalarial activities of polyhydroxyphenyl and hydroxamic acid derivatives. Antimicrob Agents Chemother 42:2456–2458
Holmes LB (1996) Hydroxamic acid: a potential human teratogen that could be recommended to treat ureaplasma. Teratology 53:227–229
Koncic MZ, Rajic Z, Petric N, Zorc B (2009) Antioxidant activity of NSAID hydroxamic acids. Acta Pharm 59:235–242
Kotlova EK, Chestukhina CG, Astaurova OB, Leonova TE, Yanenko AS, Debaboy VG (1999) Isolation and primary characterization of an amidase from Rhodococcus rhodochrous. Biochem Mosc 64:384–390
Miller MJ (1989) Syntheses and therapeutic potential of hydroxamic acid based siderophores and analogues. Chem Rev 89:1563–1579
Novo C, Farnaud S, Tata R, Clemente A, Brown PR (2002) Support for a three dimensional structure predicting a Cys-Glu-Lys catalytic triad for Pseudomonas aeruginosa amidase comes from site-directed mutagenesis and mutations altering substrate specificity. J Biochem 365:731–738
Rao MA, Scelza R, Scotti R, Gianfreda L (2010) Role of enzymes in the remediation of polluted environment. J Soil Sci Plant Nutr 10(3):333–353
Sharma NN, Sharma M, Bhalla TC (2009) Amidases: versatile enzymes in nature. Rev Environ Sci Biotechnol 8:343–366
Theiry A, Maestracci M, Arnaud A, Glazy P (1986) Acyl transfer activity of the wide spectrum amidase of Brevibacterium sp. R312. J Gen Microbiol 132:2205–2208
Valle NR, Gao S, Miller CP, Fulbright J, Gonzales C, Sirisawad M, Steggerda S, Wheler J, Balasubramanian S, Chandra J (2010) PCI-24781, a novel hydroxamic acid HDAC inhibitor, exerts cytotoxicity and histone alterations via caspase-8 and FADD in leukemia cells. Inter J Cell Biol 10:1155–1165
Vejvoda V, Martinkova L, Vesela AB, Kaplan O, Wahl SL, Fischerb L, Uhnakova B (2011) Biotransformation of nitriles to hydroxamic acids via a nitrile hydratase-amidase cascade reaction. J Mol Catal B Enzym 71:51–55
Acknowledgments
The authors are highly grateful to the New Delhi University Grant Commission and Department of Biotechnology (DBT) Ministry of Science and Technology New Delhi, India for providing financial assistance in the form of Junior Research Fellowship to Mr. Ravi Kant Bhatia and Senior Research Fellowship to Mr. Shashi Kant Bhatia and Mr. Praveen Kumar Mehta. The use of the computational facility at the Sub-Distributed Information Centre (SDIC), Himachal Pradesh University, Shimla is also duly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bhatia, R.K., Bhatia, S.K., Mehta, P.K. et al. Bench scale production of benzohydroxamic acid using acyl transfer activity of amidase from Alcaligenes sp. MTCC 10674. J Ind Microbiol Biotechnol 40, 21–27 (2013). https://doi.org/10.1007/s10295-012-1206-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-012-1206-x