Abstract
A Gram-negative anaerobic bacterium, Citrobacter sp. NC-1, was isolated from soil contaminated with arsenic at levels as high as 5,000 mg As kg−1. Strain NC-1 completely reduced 20 mM arsenate within 24 h and exhibited arsenate-reducing activity at concentrations as high as 60 mM. These results indicate that strain NC-1 is superior to other dissimilatory arsenate-reducing bacteria with respect to arsenate reduction, particularly at high concentrations. Strain NC-1 was also able to effectively extract arsenic from contaminated soils via the reduction of solid-phase arsenate to arsenite, which is much less adsorptive than arsenate. To characterize the reductase systems in strain NC-1, arsenate and nitrate reduction activities were investigated using washed-cell suspensions and crude cell extracts from cells grown on arsenate or nitrate. These reductase activities were induced individually by the two electron acceptors. This may be advantageous during bioremediation processes in which both contaminants are present.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Arsenic (a combination of arsenate and arsenite) is toxic to bacteria, as well as to most other forms of life. Arsenic has been identified as a major risk for human health in northeast India, Bangladesh, the northwest United States, and other parts of the world [4, 25]. Arsenic forms a very small percentage of the earth’s crust, but can become enriched in soil and aquatic environments as a result of dissolution and weathering [12]. This toxic element has a complex biogeochemical cycle that is partially mediated by microorganisms, including both oxidation and reduction reactions involving arsenite and arsenate [31, 35].
In Japan, soil contamination by arsenic from anthropogenic sources in urban areas has become a serious problem. To address this soil contamination, which is typically caused by industrial sites that use harmful substances, the Japanese Ministry of Environment enacted the Soil Contamination Countermeasure Law in 2003 [33]. This law sets a soil concentration standard for arsenic of 150 mg kg−1. Remediation methods for arsenic contamination include containment, solidification, and stabilization; however, these all require appropriate controls and long-term monitoring because the arsenic is retained in the treated soil and continues to pose a leaching risk. Soil washing techniques using chemical agents have also been developed, but these involve the risk of depleting valuable minerals from the soil [3, 36]. Consequently, a cost-effective remediation method that readily reduces the environmental risk posed by arsenic with less damage to the soil must be developed.
In the subsurface environment, arsenic primarily exists in inorganic forms as oxyanions of As(III) (arsenite) or As(V) (arsenate). Under oxidizing conditions in the surface soil, the predominant form of arsenic is As(V). Bacterial reduction of arsenic in surface soil from As(V) to As(III) can cause the transfer of arsenic from the solid to the liquid phase because As(III) is much less strongly adsorbed to soil than As(V) [20, 29, 32]. Once the As(III) is present in the liquid phase, it can easily be removed from the liquid phase through precipitation or complexation with sulfide or sulfide-containing materials or adsorption to Fe(II)-based solids [21, 27, 28].
Lovley reported that microorganisms can remove a number of metals and metalloids from the environment or waste streams by reducing them to a lower oxidation state [18]. Microbial arsenic mobilization has bioremediation potential for the removal of arsenic from contaminated soils [8, 17] because it converts the arsenic into arsenite, which is more mobile than arsenate.
Dissimilatory arsenate-reducing bacteria (DARB) are able to reduce As(V) to As(III) and can use this toxic metalloid as a terminal electron acceptor in anaerobic respiration [2]. Since the first report of an anaerobic bacterium capable of using arsenate as an electron acceptor for growth, at least 11 other phylogenetically diverse prokaryotes that can achieve growth via dissimilatory arsenate reduction (DAsR) to As(III) have been identified [11]. DARB are agents with the potential for cost-effective bioremediation [38] of As(V), but only one attempt has been made to develop a biological treatment process that uses these organisms [38]. Yamamura et al. reported that a DARB, facultatively anaerobic Bacillus sp. SF-1, effectively extracted arsenic from various arsenic-contaminated solids via the reduction of solid-phase arsenate to arsenite [38], indicating that DARB could be useful in arsenic contaminated sites as an arsenic extraction agent. However, little is currently known about the reducing reactions of other DARB on arsenic contaminated sites; thus, additional experiments using other DARB are required to further investigate their potential use.
In this study, we describe isolation of a novel arsenate-reducing bacterium, Citrobacter sp. NC-1, which was capable of using arsenate as an electron acceptor. In addition, the isolate was characterized during the reduction of arsenate. Arsenic extraction was also investigated experimentally to determine if strain NC-1 could efficiently remove arsenate from As(V)-containing soils.
Materials and methods
Media and enrichment
Bacterial enrichment cultures were set up in 50-ml serum bottles containing 20 ml of a basal salt medium. The basal salt medium used in this study contained 0.05 g of K2HPO4, 0.05 g of KH2PO4, 0.1 g of NaCl, 0.3 g of MgSO4·7H2O, 0.2 g of CaCl2·2H2O, 0.6 mg of H3BO3, 0.169 mg of CoCl2·6H2O, 0.085 mg of CuCl2·2H2O, 0.099 mg of MnCl2·4H2O, and 0.22 mg of ZnCl2, and was supplemented with 0.1 g (0.01%) of yeast extract (BSMY) in 1,000 ml of Tris–HCl buffer (pH 8.0). l-cysteine (1.5 g/l) and either 10 mM or 100 mM Na2HAsO4·7H2O were added separately from sterile, anaerobic stocks. Unless otherwise stated, 2.0 g/l of glucose (glucose medium, GM) was added as the sole carbon source.
Soil samples collected from an old industrial site located in Hyogo Prefecture, Japan, were used as the source of the inoculums for the enrichment cultures. The representative soil sample contained 5,000 mg As kg−1 soil. The enrichment cultures were maintained with a weekly subculture using the medium described above for 6 months. A yellow color indicated a positive arsenate reduction reaction [the formation of As(III)]. After approximately 20 enrichment cultures at 28°C, the arsenate-reducing bacterium was successfully isolated using the traditional serial dilution method. To isolate the colonies, a 10-fold dilution of the enrichment culture was spread on Petri plates containing glucose (2.0 g/l), BSMY, and arsenate (2 mM) with 1.5% agar. The plate was then incubated under anaerobic conditions using an Anaerobic Gas Generation Kit (Oxoid Ltd, Hants, UK). The procedure was repeated twice to ensure a pure culture. The purity of the isolated culture was confirmed using an inverted microscope (Diaphot TMD300, Nikon, Tokyo, Japan) equipped for simultaneous recording of cell length.
Growth experiments
The ability of the isolated strain to reduce and grow on arsenate and other oxyanions was investigated by several growth experiments. In liquid culture, 20 ml of medium was used in 50-ml serum bottles. Cells of the isolated strain were cultivated anaerobically in glucose-BSMY and l-cysteine (1.5 g/l) for 24 h, then harvested by centrifugation (6,000 × g, 10 min, 4°C) and washed twice with Tris–HCl buffer (pH 8.0). Next, 200 μl of cell suspension was used to inoculate the medium to give an optical density of 0.03 at 600 nm (OD600). For anaerobic cultivation, the bottles were sealed with a butyl rubber septum and aluminum crimp seals. The headspace above the liquid phase was replaced with N2 gas and cultivation was conducted by rotary shaking. The cultures were incubated in the dark at 28°C and periodically sacrificed, at which time the cell density was determined. The population of strain NC-1 was monitored using the plate-count technique with CGY medium (casitone 5.0 g/l, glycerin 5.0 g/l, yeast extract 1.0 g/l, and agar 15 g/l). The plate was then incubated under anaerobic conditions using an Anaerobic Gas Generation Kit (Oxoid Ltd, Hants, UK). Portions of the samples were filtered (0.45 μm, DISMIC-25cs; Advantec, Tokyo) and frozen until analysis. All experiments were performed in duplicate and the results shown are the mean values.
Electron donors and electron acceptors used for growth
Several electron acceptors were tested for their ability to support growth when glucose was present as the electron donor, including arsenate (5 mM), nitrate (5 mM), nitrite (5 mM), sulfate (5 mM), thiosulfate (5 mM), Fe(III) (as described by Lovley and Phillips [18]), and selenate (5 mM). The electron donors tested for their ability to support growth when arsenate was present as the electron acceptor included formate, molecular hydrogen, acetate, pyruvate, lactate, malate, fumarate, citrate, glycerol, phenol, ethanol, methanol, benzoate, fructose, sucrose, ribose, and xylose (all at 5 mM, except molecular hydrogen, for which 10 ml was added). The initial NC-1 inoculum used for these experiments was grown in minimal medium containing glucose (2.0 g/l), l-cysteine (1.5 g/l), and arsenate (5 mM). Growth with a given electron acceptor was only considered positive if a minimum of 90% of the electron acceptor was reduced after at least three subsequent subcultures. Since good growth (i.e., an increase in the number of bacteria from about 5 × 106 ml−1 to at least 108 ml−1 in non-pH controlled cultures) was only observed in cultures where arsenate was the terminal electron acceptor, the ability of NC-1 to grow with various electron donors was only determined using arsenate as the electron acceptor. Growth with a given electron donor was only considered positive if the numbers of motile organisms had increased from about 5 × 106 ml−1 to at least 108 ml−1 after at least three subsequent subcultures, and if at least 90% of the arsenate initially present in the culture was reduced to arsenite. In cultures in which the arsenate was reduced to arsenite, the total amount of arsenic in the culture remained constant throughout the experiment.
Experiments with washed-cell suspensions
The objective of these investigations was to determine whether arsenate reduction is catalyzed by an enzyme specific for arsenate or by other reductases in strain NC-1, for example nitrate reductase, which are active nonspecifically for arsenate. Log-phase cells of the isolated strain were grown anaerobically with arsenate (10 mM) or nitrate (10 mM) in glucose-BSMY and then harvested by centrifugation (6,000 × g, 10 min, 4°C). The harvested cells were washed twice in Tris–HCl buffer (pH 8.0) and then suspended in the same buffer containing glucose (2.0 g/l) and arsenate (1 mM) or nitrate (1 mM). Cell suspensions (20 ml) were incubated in 50-ml serum bottles with a headspace of N2 gas on a rotary shaker (120 rpm, 28°C). The arsenate or nitrate concentration in the suspensions was monitored to confirm which oxyanion induced the reducing activity.
Effect of pH and electron donors on arsenate reduction
To evaluate the effect of pH on arsenate reduction, cell suspensions grown on arsenate were prepared with ultrapure water (pH adjusted to 6.5 with HCl), Tris–HCl buffer (pH 7.2–9.0) or glycine–NaOH buffer (pH 9.4–10.0). To investigate the effect of the electron donors, various electron donors instead of glucose were added to cell suspensions to give final concentrations of 5 mM.
Oxygen sensitivity
Strain NC-1 was grown to the mid-log phase on 10 mM lactate and 10 mM As(V), and a 10% inoculum was used to inoculate the experimental tubes in triplicate. Sterile air was added to give final concentrations of 0, 1, 2, 5, and 10% air by volume in the Balch tube headspace, and no reductant was added to the experimental tubes. To determine if strain NC-1 could resume growth after being exposed to 10% air, cells from the 10% air treatment were subsampled after 24 h of incubation and reinoculated into the 0% air tubes. The cultures were then shaken at 120 rpm and 28°C. Growth was monitored spectrophotometrically, and the accumulation of As(III) was quantified once growth was evident. Controls consisted of autoclaved cells.
Extraction of As from forest soil
To confirm that the isolated strain could extract As from natural soil systems, we investigated the reductive extraction of As from a soil artificially contaminated with As(V), simulating soil contamination by As discharges or emissions. A forest soil was collected from the nearby countryside in Muroran (pH, 5.3; ignition loss, 13.4%) and used to make a model of contaminated soil. The soil was dried at 60°C for 2 days, after which it was sieved through a 2-mm mesh sieve. Next, 1.5 ml of 1 M As(V) solution was added to 100-g portions of the soil, followed by vigorous shaking at room temperature for 12 h. After drying, the soil was used as a model contaminated soil. The concentration of As in each model soil was calculated at approximately 1,200 mg kg−1. One gram of the model-contaminated soil was added to each 50-ml serum bottle. The bottle was then autoclaved (1 h, 121°C), and 20 ml of glucose-BSMY was added (for comparison of results, the amount of As(V) contained in the 5% [w/v] soil-medium mixture, if completely extracted, would equate to 0.76 mM dissolved As) [38]. An anaerobically grown cell suspension was then inoculated into each bottle, because As(V)-reducing activity can be readily induced under anaerobic conditions in the presence of As(V).
Analytical procedures
The arsenate and selenate concentrations in filtered samples were quantified by ion chromatography (IC, DX-300 system; Dionex, CA, USA) using a conductivity detector [9, 13]. The levels of arsenite were indirectly determined by measuring the difference in arsenate concentration between oxidized samples (oxidized by 9.1 mM H2O2) [50] and untreated samples [9, 13]. Nitrate and nitrite were determined using an ion chromatography system equipped with an IonPac AS4A-SC column, an IonPac AG4S-SC guard column (Dionex) and a SPD-10AV UV–VIS detector (Shimadzu, Kyoto, Japan) at 215 nm. The total arsenic in the filtrates was measured using a Hitachi Z6100 polarization Zeeman atomic adsorption spectrophotometer (Hitachi, Ibaraki, Japan).
An assay for dissimilatory arsenate reductase and nitrate reductase was conducted as previously described [14, 34] by measuring the oxidation of reduced benzyl viologen as an artificial electron donor, with the activity being calculated as one μmol of benzyl viologen oxidized per min using an extinction coefficient of 19.5 cm−1 mM−1.
Nucleotide sequence accession number
The sequence determined in this study for strain NC-1 has been deposited in the DNA Data Bank of Japan (DDBJ) under accession number AB602381. Strain NC-1 (NBRC 107886) has been deposited in the NITE Biological Resource Center (NBRC) in Japan.
Results
Taxonomy of the isolated organism
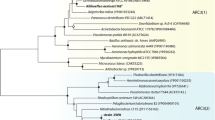
The isolated organism was named strain NC-1. The arsenate-reducing organism is an anaerobic, Gram-negative, rod-shaped bacterium. NC-1 colonies were white when cultured on glucose-BSMY agar with arsenate (2 mM). Strain NC-1 is able to produce β-galactosidase, but not indole, arginine dihydrolase, lysine decarboxylase, or ornithine decarboxylases (data not shown). The strains were positive for H2S production and citrate utilization, but did not produce urease. As shown in the phylogenetic tree (Supplementary Fig. 1), strain NC-1 was identified as a Citrobacter sp. Strain NC-1 is a member of the γ-Proteobacteria family and is most closely related to Citrobacter freundii AB210978 (99.9% sequence identity), but also shares significant identity (99.7%) with Citrobacter braakii NR02868.
Growth characteristics
When NC-1 was grown in minimal medium with arsenate (5 mM) as the terminal electron acceptor, the following electron donors and carbon sources supported its growth: glucose, fructose, sucrose, ribose, xylose, acetate, pyruvate, lactate, formate, citrate, hydrogen, fumarate, glycerol, and malate (data not shown). No growth occurred on phenol, ethanol, methanol, benzoate, hydrogen, or fumarate when arsenate was absent. Phenol, ethanol, methanol, and benzoate also did not support growth in the presence of arsenate, but slight growth (from 5 × 106 to 9 × 106 cells ml−1) was observed when hydrogen was added. When NC-1 was grown with glucose (2.0 g/l) as the electron donor and carbon source, only nitrate (5 mM) was able to replace arsenate as the terminal electron acceptor (data not shown). The electron acceptors sulfate, thiosulfate, Fe(III), selenate, and oxygen did not support its growth.
Arsenate reduction by strain NC-1
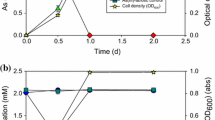
Figure 1 shows the timing of the growth of strain NC-1 during arsenate reduction. In cultures containing 5, 10, and 20 mM of arsenate, strain NC-1 began to reduce arsenate within 12 h, and the arsenate was completely reduced within 20, 24, and 48 h, respectively (Fig. 1). Cell growth occurred concurrently with arsenate reduction. However, in cultures containing 60 mM of arsenate, the growth of strain NC-1 was significantly inhibited, and the cell density decreased after about 15 mM of arsenate was reduced, although the arsenate reduction proceeded further (Fig. 1). During cell growth, lactic and pyruvic acid accumulation was observed as a result of glucose consumption (data not shown). However, yeast extract (0.1 g/l) did not serve as a carbon and energy source, as no growth or arsenate reduction occurred in the absence of glucose (data not shown).
Arsenate reduction by strain NC-1 and cell growth. Cultures were incubated with glucose (2.0 g/l) and 5, 10, 20, 60 mM arsenate. Solid symbols represent arsenate concentrations (diamonds are 5 mM; circles are 10 mM; squares are 20 mM; triangles are 60 mM); open symbols represent the number of cells in the corresponding cultures. Each value represents an average of two analyses (the difference between the data obtained in the two analyses was within 5%)
The growth of strain NC-1 in the presence and absence of arsenate under anaerobic conditions was compared (data not shown). The growth of strain NC-1 was observed under both conditions, but more significant growth was observed in the presence of arsenate, indicating that arsenate can act as the terminal electron acceptor for anaerobic respiration (dissimilatory arsenate reduction). Arsenate reduction was not observed in the control experiments without NC-1 cells (data not shown).
When about 10 mM of arsenite was present with 10 mM of arsenate, cell growth inhibition was observed, suggesting that high concentrations of arsenite are toxic to strain NC-1 (data not shown).
Effect of other electron acceptors on arsenate reduction
Strain NC-1 can use nitrate as a terminal electron acceptor for anaerobic respiration in addition to arsenate (data not shown). When nitrate was present with arsenate, arsenate reduction proceeded concomitantly with nitrate reduction, although a slight inhibitory effect was observed (data not shown). These findings indicate that nitrate did not significantly inhibit the arsenate-reducing activity of strain NC-1.
Effect of pH and electron donors on arsenate reduction
The effect of pH on arsenate reduction by strain NC-1 was studied using washed cells grown on arsenate. The NC-1 cell suspension showed arsenate reducing activity across a pH range of 7.2–9.0, with an optimal pH of approximately 8.5 (data not shown). In a previous study, the optimal growth of strain NC-1 occurred at pH 8.0 [6].
Various carbon sources that can be used for the growth of strain NC-1 promoted arsenate reduction. Lactate and glucose were particularly effective substrates, while fumarate was not very effective when compared to the other carbon sources (data not shown). Phenol, methanol, ethanol, and benzoate, which are not growth substrates for strain NC-1, did not promote arsenate reduction. Hydrogen enhanced the reduction of arsenate, but the degradation rate was much lower than when lactate or glucose was used, possibly because of poor growth of NC-1. Pyruvate and fumarate can be used for good growth substrates similar to lactate or glucose, but their reducing activity was lower than those of lactate or glucose (data not shown). These results indicate that strain NC-1 can use various carbon sources as electron donors for arsenate reduction, although a degree of substrate specificity was observed.
Oxygen sensitivity
Strain NC-1 was capable of growth and As(V) respiration when 0 or 1% air was present in the headspace of the culture tubes. However, no cell growth occurred in cultures containing 2, 5, or 10% air (data not shown), and no As(V) respiration occurred when 2, 5, or 10% air was present (data not shown).
Arsenate and nitrate reduction by washed-cell suspensions
Washed cells of strain NC-1 grown on either arsenate or nitrate as the electron acceptor were examined for their ability to reduce arsenate. Cells of strain NC-1 grown on arsenate actively reduced arsenate, with l mM being almost completely reduced within 10 h. However, cells grown on nitrate could not significantly reduce arsenate (Table 1). No activity was shown in control experiments without any electron donor (data not shown).
The nitrate-reducing activity was also investigated using washed-cell suspensions. In suspensions containing nitrate, cells grown on arsenate did not reduce nitrate, with only cells grown on nitrate being able to reduce nitrate (Table 1).
Reductase activities in crude cell extracts
To determine the dissimilatory arsenate and nitrate-reductase activities in strain NC-1, crude extracts from cells grown on arsenate or nitrate as the sole electron acceptor were tested for the ability to couple the oxidation of benzyl viologen with the reduction of each electron acceptor. Crude extracts from cells grown on arsenate exhibited the highest arsenate reductase activity. Similarly, crude cell extracts grown on nitrate showed the highest reductase activity for nitrate. The maximum reductase activity in a given crude cell extract was obtained against the substrate on which the cells were grown (data not shown).
Inhibition of arsenate and nitrate reduction by tungstate
In the absence of tungstate, strain NC-1 actively reduced l mM arsenate and nitrate, with the arsenate and nitrate being completely reduced within 12 and 8 h, respectively (data not shown). However, the addition of tungstate (1 mM) lowered the arsenate and nitrate-reduction activities. The inhibition ratios for arsenate and nitrate reduction were 55.7 and 47.3%, respectively, indicating that tungstate inhibited both reduction activities.
Extraction of As from contaminated forest soil
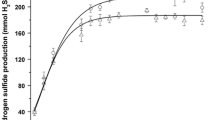
In the experiment using the model-contaminated forest soil, after 100 h in the presence of NC-1, the concentration of dissolved As increased to 80% of the total As initially added to the soil, and most of the dissolved As was present as As(III) (Fig. 2). In the control (no NC-1) experiment, although a slight increase in the dissolved As concentration was followed by a plateau, the dissolved As concentration was much lower than that observed in the experiment with NC-1, and the majority of the As was detected as As(V). These findings indicated that the dissolution of As observed in the control experiment was caused by the desorption of excess As(V) from the soil.
Extraction of As from forest soil artificially contaminated with As(V). Cultures were incubated for 100 h with 2.0 g/l glucose. The pre-cultivated cultures of strain NC-1 (about 1.2 × 108 ml−1) were added to the soil-medium mixture. The concentration of As in each model soil was calculated to be approximately 1,200 mg kg−1. Data represent the averages of two separate experiments (the difference between the data obtained in the two experiments was within 5%)
Discussion
Citrobacter sp. NC-1, which was isolated from arsenic-contaminated soil, was characterized as a DARB. Although a number of DARBs have been reported (Supplementary Table 1), only Citrobacter sp. TSA-1 is from the Citrobacter genus [11]. However, Herbel et al. assumed that Citrobacter sp. capable of reducing arsenate may also exist in nature, because Citrobacter sp. TSA-1 was isolated from the termite hindgut rather than from nature. Thus, the isolation of Citrobacter sp. NC-1 from arsenic-contaminated soils strongly support their suggestion.
Strain NC-1 could grow on glucose as an electron donor and arsenate as an electron acceptor. Arsenate reduction by strain NC-1 was significantly inhibited by aerobic conditions. Although arsenate reduction can also be catalyzed by arsenic-resistant microbes, this can occur in the presence of oxygen [19]. Thus, this inhibition by oxygen is evidence that strain NC-1 is a DARB. The toxic effect of arsenite may explain the growth inhibition of strain NC-1 at high concentrations (60 mM) of arsenate (Fig. 1). These results suggest that arsenate reduction by strain NC-1 does not occur via the arsenic resistance system, which does not appear to be involved in energy conservation [5, 15], but via dissimilatory reduction.
DARB are considered to be attractive agents for the bioremediation of arsenic contaminated soils and sediments [8, 17] because they can mobilize arsenic from the solid phase into the liquid phase [1, 39]. The experimental results reported here indicate that this strain has several properties that make it advantageous for bioremediation. The arsenate-reducing activity of strain NC-1 is comparable or superior to that of previously reported DARB, and even occurred at an extremely high concentration of arsenate (~60 mM). This report presents data that reveal, for the first time, that bacterial reduction of arsenate at high concentrations (~60 mM) may be possible. The presence of other electron acceptors, such as nitrate, did not inhibit the arsenate reduction, and various electron donors supported the arsenate reduction. Strain NC-1 has separate pathways for the dissimilatory reduction of arsenate and nitrate. Interestingly, there seem to be significantly different reductase systems between strain NC-1 and other prokaryotes that can reduce arsenate, selenate, and nitrate. Washed-cell suspensions of both selenate- and nitrate-grown cells of Sulfurospirillum barnesii had a constitutive ability to reduce arsenate, and the arsenate-grown cells catalyzed selenate reduction [16, 23]. Thus, controlling the expression of the reductases may lead to effective removal of target contaminants, even in the presence of alternative electron acceptors.
Tungstate, which is known to block a number of molybdoenzymes, including nitrate reductase, by substituting tungsten for molybdenum at the active site [7, 10, 26], had strong inhibitory effects against arsenate, selenate, and nitrate reduction under anaerobic conditions. Therefore, the dissimilatory arsenate and nitrate reductases in strain NC-1 may contain molybdenum as a cofactor as well as the dissimilatory arsenate reductase of C. arsenatis [30] and B. selenitireducens [24].
Strain NC-1 was capable of extracting As from a model soil artificially contaminated with As(V) to a greatly improved extent when compared to the abiotic control. The amount of As extracted by NC-1 considerably exceeded the levels reported in a study conducted by Yamamura et al. [38], where the extraction rate reached 56% of the total As initially added to the soil (1,124 mg kg−1) after 120 h in the presence of Bacillus sp. SF-1. The soil conditions (i.e., pH and ignition loss) were similar to those of the soil used in the experiments with NC-1. Thus, these results indicate that strain NC-1 is more effective than Bacillus sp. SF-1 for the extraction of arsenate from contaminated soils. Taken together, these results confirmed that NC-1 possesses the potential to efficiently extract As from soil via the reduction of As(V) to As(III), and demonstrated that NC-1 can be used for the extraction of As from diverse As(V)-contaminated soils.
A study to develop a soil-cleanup process using a slurry-phase bioreactor and strain NC-1 is currently underway.
References
Ahmann D, Krumholz LR, Hemond HF, Lovley DR, Morel FMM (1997) Microbial mobilization of arsenic from sediments of the Aberjona watershed. Environ Sci Technol 31:2923–2930
Ahmann D, Roberts AL, Krumholtz LR, Morel FMM (1994) Microbe grows by reducing arsenic. Nature 371:750
Alam MG, Tokunaga S, Maekawa T (2001) Extraction of arsenic in a synthetic arsenic-contaminated soil using phosphate. Chemosphere 43:1035–1041
Bagla P, Kaiser J (1996) India’s spreading health crisis draws global arsenic experts. Science 274:174–175
Chang YC, Shintaro K (2010) Isolation and characterization of arsenate-reducing bacteria from arsenic-contaminated site in Japan. In: 2nd proceedings of studies of environmental issues in the Asian mega-cities, Seoul, Korea. pp 213–221
Butcher BG, Deane SM, Rawlings DE (2000) The chromosomal arsenic resistance genes of Thiobacillus ferrooxidans have an unusual arrangement and confer increased arsenic and antimony resistance to Escherichia coli. Appl Environ Microbiol 66:1826–1833
Chauret C, Knowles R (1991) Effect of tungstate on nitrate and nitrite reductases in Azospirillum brasilense Sp7. Can J Microbiol 37:744–750
Dowdle PR, Laverman AM, Oremland RS (1996) Bacterial dissimilatory reduction of arsenic(V) to arsenic(III) in anoxic sediments. Appl Environ Microbiol 62:1664–1669
Fujita M, Ike M, Nishimoto S, Takahashi K, Kashiwa M (1997) Isolation and characterization of a novel selenate-reducing bacterium, Bacillus sp. SF-1. J Ferment Bioeng 83:517–522
Gates AJ, Hughes RO, Sharp SR, Millington PD, Nilavongse A, Cole JA, Leach ER, Jepson B, Richardson DJ, Butler CS (2003) Properties of the periplasmic nitrate reductases from Paracoccus pantotrophus and Escherichia coli after growth in tungsten supplemented media. FEMS Microbiol Lett 220:261–269
Herbel MJ, Blum JS, Hoeft SE, Cohen SM, Arnold LL, Lisak J, Stolz JF, Oremland RS (2002) Dissimilatory arsenate reductase activity and arsenate-respiring bacteria in bovine rumen fluid, hamster feces, and the termite hindgut. FEMS Microbiol Ecol 41:59–67
Jareonmit P, Kannika S, Michael JS (2010) Structure and diversity of arsenic-resistant bacteria in an old tin mine area of Thailand. J Microbiol Biotechnol 20:169–178
Kashiwa M, Nishimoto S, Takahashi K, Ike M, Fujita M (2000) Factors affecting soluble selenium removal by a selenate-reducing bacterium Bacillus sp. SF-1. J Biosci Bioeng 89:528–533
Kraft T, Macy JM (1998) Purification and characterization of the respiratory arsenate reductase of Chrysiogenes arsenatis. Eur J Biochem 255:647–653
Langner HW, Inskeep WP (2000) Microbial reduction of arsenate in the presence of ferrihydrite. Environ Sci Technol 34:3131–3136
Laverman AM, Blum JS, Schaefer JK, Phillips EJP, Lovley DR, Oremland RS (1995) Growth of strain SES-3 with arsenate and other diverse electron acceptors. Appl Environ Microbiol 61:3556–3561
Lovley DR, Coates JD (1997) Bioremediation of metal contamination. Curr Opin Biotechnol 8:285–289
Lovley DR, Phillips EJP (1986) Organic matter mineralization with reduction of ferric iron in anaerobic sediments. Appl Environ Microbiol 51:683–689
Macur RE, Wheeler JT, Mcdermott TR, Inskeep WP (2001) Microbial populations associated with the reduction and enhanced mobilization of arsenic in mine tailings. Environ Sci Technol 35:3676–3682
Manning BA, Goldberg S (1997) Arsenic(III) and arsenic(V) adsorption on three California soils. Soil Sci 162:886–895
Newman DK, Beveridge TJ, Morel FMM (1997) Precipitation of arsenic trisulfide by Desulfotomaculum auripigmentum. Appl Environ Microbiol 63:2022–2028
Newman DK, Kennedy EK, Coates JD, Ahmann D, Ellis DJ, Lovley DR, Morel FMM (1997) Dissimilatory arsenate and sulfate reduction in Desulfotomaculum auripigmentum sp. nov. Arch Microbiol 168:380–388
Oremland RS, Blum JS, Bindi AB, Dowdle PR, Herbel M, Stolz JF (1999) Simultaneous reduction of nitrate and selenate by cell suspensions of selenium respiring bacteria. Appl Environ Microbiol 65:4385–4392
Oremland RS, Stolz JF (2003) The ecology of arsenic. Science 300:939–944
Pontius F, Brown KG, Chen CJ (1994) Health implications of arsenic in drinking water. J Am Water Works Assoc 86:52–63
Prins RA, Cline-Theil W, Malestein A, Counotte GHM (1980) Inhibition of nitrate reduction in some rumen bacteria by tungstate. Appl Environ Microbiol 40:163–165
Rittle KA, Drever JI, Colbeerg PJS (1995) Precipitation of arsenic during sulfate reduction. Geomicrobiol J 13:1–12
Roberts LC, Hug SJ, Ruettimann T, Billah MM, Khan AW, Rahman MT (2004) Arsenic removal with iron(II) and iron(III) in waters with high silicate and phosphate concentrations. Environ Sci Technol 38:307–315
Sakata M (1987) Relationship between adsorption of arsenic(III) and boron by soil and soil properties. Environ Sci Technol 21:1126–1130
Saltikov CW, Cifuentes A, Venkateswaran K, Newman DK (2003) The ars detoxification system is advantageous but not required for As(V) respiration by the genetically tractable Shewanella species strain ANA-3. Appl Environ Microbiol 69:2800–2809
Saltikov CW, Olson BH (2002) Homology of Escherichia coli R773 arsA, arsB, and arsC genes in arsenic-resistant bacteria isolated from raw sewage and arsenic-enriched creek waters. Appl Environ Microbiol 68:280–288
Smith E, Naidu R, Alston AM (1999) Chemistry of arsenic in soils: I. Sorption of arsenate and arsenite by for Australian soils. J Environ Qual 28:1719–1726
Soda S, Kanzaki M, Yamamura S, Kashiwa M, Fujita M, Ike M (2009) Slurry bioreactor modeling using a dissimilatory arsenate-reducing bacterium for remediation of arsenic-contaminated soil. J Biosci Bioeng 107:130–137
Stolz JF, Gugliuzza T, Blum JS, Oremland R, Murillo FM (1997) Differential cytochrome content and reductase activity in Geospirillum barnesii strain SeS3. Arch Microbiol 167:1–5
Tamaki S, Frankenberger WT (1992) Environmental biochemistry of arsenic. Rev Environ Contam Toxicol 124:79–110
Tokunaga S, Hakuta T (2002) Acid washing and stabilization of an artificial arsenic contaminated soil. Chemosphere 46:31–38
Yamamura S, Ike M, Fujita M (2003) Dissimilatory arsenate reduction by a facultative anaerobe, Bacillus sp. strain SF-1. J Biosci Bioeng 96:454–460
Yamamura S, Yamamoto N, Ike M, Fujita M (2005) Arsenic extraction from solid phase using a dissimilatory arsenate-reducing bacterium. J Biosci Bioeng 100:219–222
Zobrist J, Dowdle PR, Davis JA, Oremland RS (2000) Mobilization of arsenite by dissimilatory reduction of adsorbed arsenate. Environ Sci Technol 34:4747–4753
Acknowledgments
We thank Dr. Michihiko Ike, University of Osaka, for his kind cooperation in collection of the samples. The authors also thank Dr. Tadashi Toyama, University of Yamanashi, for technical support with the analysis of arsenate. This work was partly supported by a grant (Adaptable and Seamless Technology Transfer Program through Target-driven R&D, AS211Z00600E) from the Japan Science and Technology Agency.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10295_2011_996_MOESM2_ESM.doc
Fig. 1. Phylogenic tree based on comparison of the 16S rRNA gene sequence. The phylogenic tree was generated using the neighbor-joining method. Bootstrap values shown are based on 100 replications. Scale bar represents 0.005% sequence difference. Supplementary material 2 (DOC 42 kb)
Rights and permissions
About this article
Cite this article
Chang, Y.C., Nawata, A., Jung, K. et al. Isolation and characterization of an arsenate-reducing bacterium and its application for arsenic extraction from contaminated soil. J Ind Microbiol Biotechnol 39, 37–44 (2012). https://doi.org/10.1007/s10295-011-0996-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-011-0996-6