Abstract
Actinomycetes, a class of filamentous bacteria, are an important source of several industrially relevant secondary metabolites. Several environmental factors including the media composition affect both biomass growth and product formation. Likewise, several studies have shown that environmental factors cause changes in cellular morphology. However, the relationship between morphology and product formation is not well understood. In this study, we first characterized the effect of varying concentrations of phosphate and ammonia in defined media on pellet morphology for an actinomycete Amycolatopsis balhimycina DSM 5908, which produces balhimycin, a glycopeptide antibiotic. Our results show that higher balhimycin productivity is correlated with the following morphological features: (1) higher pellet fraction in the biomass, (2) small elongated pellets, and (3) shorter filaments in hyphal growth in the periphery of the pellets. The correlation between morphology and product formation was also observed in industrially relevant complex media. Although balhimycin production starts after 72 h with maximum production around 168 h, the morphological changes in pellets are observed as early as 24 h after commencing of the batch. Therefore, morphology may be used as an early predictor of the end-of-batch productivity. We argue that a similar strategy can be developed for other strains where morphological indicators may be used as a batch monitoring tool.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Production of a majority of industrially important secondary metabolites is carried out using submerged fermentation in batch and fed-batch processes. Batch-to-batch variability in productivity is an intrinsic feature of such processes, possibly due to inconsistencies in raw material quality, state of seed culture, and operator skills [15, 26]. In order to maintain the productivity and product quality, monitoring and control of the fermentation process is required from an early phase of the process. Product quality and batch performance can be monitored via parameter measurements made off-line such as concentrations of the biomass, substrates and products, and those made online such as temperature, pH, concentrations of dissolved oxygen, and carbon dioxide. The objective of monitoring is to ensure a consistent end-of-batch productivity and take corrective mid-course measures. However, these measurements may not be sufficient to predict the fate of the batch during the early phase of the process. Therefore, there is a need for alternative monitoring tools that would enable early fault detection. This is especially true in the case of secondary metabolite production, which starts quite late in the batch [7].
The majority of secondary metabolites are produced by filamentous fungi and bacteria (actinomycetes), which exhibit diverse morphological forms during submerged cultivation [4, 30, 35]. Morphology is influenced by environmental conditions such as medium composition and shear stress [19, 20, 31]. Further, morphology and product formation have been observed to be closely related [9, 16, 23, 33]. To exemplify, morphology and avermectin production by Streptomyces avermitilis, were influenced by factors such as the nitrogen source, dissolved oxygen level, and inoculum volume [33]. Pellets of small size and high density were found to promote avermectin production. Likewise, nystatin production by Streptomyces noursei NG7.19 has been reported to be correlated with smaller pellet size [11]. Morphological differentiation of these filamentous microorganisms can be monitored in terms of different morphological parameters such as distribution of pellets and dispersed mycelia [13, 29, 32], size and shape of pellets [8, 10, 11, 21, 34] and branching behavior in peripheral hyphae that emanate from pellets [22].
Our preliminary study indicated that there is a strong correlation between balhimycin production and cellular morphology, both of which are influenced by media composition (data not shown). Notably, product formation starts in the late exponential phase while morphological changes are observed in the early exponential phase. Therefore, we argue that morphology can be an early indicator of end-of-batch productivity. To this end, we have systematically investigated the effects of phosphate and ammonia on productivity and morphology in defined and complex media for the balhimycin producer strain A. balhimycina. A. balhimycina belongs to a class of filamentous bacteria called actinomycetes, which account for over 45% of secondary metabolites produced commercially [3]. Balhimycin belongs to the class of glycopeptide antibiotics that are considered as the treatment of last resort against methicillin resistant Staphylococcus aureus (MSRA) strains [5, 18].
Materials and methods
Materials
Dextrose, yeast extract (YE), peptone, meat extract, agar powder, MES buffer, Bennet’s agar, and vitamins were purchased from Hi-Media Laboratories (Mumbai, India) whereas glycerol, (NH4)2SO4, NaCl, MgSO4.7H2O, FeSO4.7H2O, Na3(C6H5O7).2H2O, ZnSO4.7H2O, MnSO4.H2O, CaCl2.2H2O were purchased from Merck (Mumbai, India). Soya peptone and CaCO3 were purchased from Micro Master Laboratories Pvt. Ltd. (Thane, India) and S.D. Fine Chemicals (Mumbai, India), respectively. Defatted soy flour (DSF) and KH2PO4 were purchased from General Foods (Indore, India) and Thomas Baker, Chemical Ltd. (Mumbai, India), respectively. Balhimycin standard was a kind gift from Prof. Anna Eliasson Lantz, Denmark’s Technical University, Denmark.
Inoculum preparation and fermentation
A. balhimycina was a kind gift from Prof. Anna Eliasson Lantz, Denmark’s Technical University, Denmark. The strain was cultivated and maintained as reported previously [14, 15]. Briefly, 100-ml cultures were grown at 30°C and 150 rpm in single baffled Erlenmeyer flasks of 500-ml capacity for all the experiments. The seed medium contained 15.0 g dextrose, 15.0 g glycerol, 5.0 g YE, 15.0 g soya peptone and 3.0 g sodium chloride per liter of distilled water. The production medium was inoculated with 2.5% (v/v) of seed culture having optical density of ~12.0 at 600 nm. Four different types of production media were used. Production medium type I was a defined medium with variable phosphate concentration containing 0.2–1.0 g KH2PO4, 80.0 g dextrose, 6.6 g (NH4)2SO4, 1.0 g NaCl, 1.5 g MgSO .4 7H2O, 0.02 g FeSO4.7H2O, 0.025 g Na3(C6H5O7).2H2O, 0.02 g ZnSO4.7H2O, 0.01 g MnSO .4 H2O, 0.01 g CaCl2.2H2O, 9.762 g MES, 11.0 g CaCO3, and 1-ml vitamin solution (1000×) per liter of distilled water. Production medium type II was a complex medium with variable phosphate concentration containing 0.0–1.0 g KH2PO4, 84.0 g dextrose, 1.6 g YE, 0.4 g DSF, 6.0 g (NH4)2SO4, 1.0 g NaCl, 1.5 g MgSO .4 7H2O, 0.02 g FeSO4.7H2O, 0.025 g, Na3(C6H5O7).2H2O, 0.02 g ZnSO4.7H2O, 0.01 g MnSO .4 H2O, 0.01 g CaCl2.2H2O, 9.762 g MES, 11.0 g CaCO3, and 1-ml vitamin solution (1000×) per liter of distilled water. Production medium type III was a defined medium with variable (NH4)2SO4 concentration containing 0.0–10.0 g (NH4)2SO4, 80.0 g dextrose, 0.2 g KH2PO4, 1.0 g NaCl, 1.5 g MgSO .4 7H2O, 0.02 g FeSO4.7H2O, 0.025 g, Na3(C6H5O7).2H2O, 0.02 g ZnSO4.7H2O, 0.01 g MnSO .4 H2O, 0.01 g CaCl2.2H2O, 9.762 g MES, 11.0 g CaCO3, and 1-ml vitamin solution (1000×) per liter of distilled water. Production medium type IV was a complex media with variable (NH4)2SO4 concentration containing 0.0–12.0 g (NH4)2SO4, 84.0 g dextrose, 1.6 g YE, 0.4 g DSF, 0.17 g KH2PO4, 1.0 g NaCl, 1.5 g MgSO .4 7H2O, 0.02 g FeSO4.7H2O, 0.025 g, Na3(C6H5O7).2H2O, 0.02 g ZnSO4.7H2O, 0.01 g MnSO .4 H2O, 0.01 g CaCl2.2H2O, 9.762 g MES, 11.0 g CaCO3, and 1-ml vitamin solution (1000×) per liter of distilled water. Initial pH of the medium was adjusted to 7.00 with 2 N NaOH or 2 N H2SO4. The 1000× vitamin stock solution contained, per 100 ml of distilled water: 0.005 g biotin, 0.1 g calcium-pantothenate, 0.1 g nicotinic acid, 2.5 g myo-inositol, 0.1 g thiamin HCl, 0.1 g pyridoxine HCl, and 0.02 g para-amino benzoic acid. Samples were collected from the seed and production media at regular intervals for microscopic analysis as well as balhimycin and biomass estimation.
Estimation of Balhimycin and dry cell weight
Concentration of balhimycin was estimated from the fermentation broth using bioassay and further confirmed via HPLC as previously described [1, 14, 15]. In the bioassay, Kocuria rhizophila ATCC9341, old name Micrococcus luteus [28], was used as a test organism to measure antimicrobial activity of balhimycin. For this purpose, Petri plates were poured with agar containing the test organism. Holes 10 mm in diameter were punched in the agar and filled with 0.1 ml sample of the centrifuged fermentation broth. The plates were incubated at 30°C for 2 days and the diameter of the zone of inhibition around the holes was measured. The balhimycin concentration was estimated using a standard calibration curve. Balhimycin concentration was confirmed with HPLC by using RP-C18 column (Merck KGaA Darmstadt, Germany) and a mobile phase (1 ml/min) consisting of 18% of acetonitrile and 0.1% of TFA (trifluoro acetic acid) in water. Separated components were detected at 220-nm wavelength using an L-7420 UV detector (Merck Hitachi KGaA, Darmstadt, Germany) [18]. Estimation of biomass was done as described earlier [2, 15].
Imaging and morphological analysis
Biomass samples drawn at different times were centrifuged, washed twice, and re-suspended in saline (0.9% NaCl) from which 20 μl was taken on a clean slide for microscopy (TE-2000, Nikon, Tokyo, Japan). Images were captured with a CCD camera (Retiga-2000R, QImaging, Surrey, Canada). The images were further analyzed using the software ImageJ 1.40g (http://rsb.info.nih.gov/ij/) [6].
Morphology of A. balhimycina was characterized in terms of (1) the ratio of dispersed mycelia to pellets (2) size and shape of pellets, and (3) branching frequency and length of peripheral filaments that arise from pellets. To measure dispersed or pellet dimension, we first enclosed dispersed or pellets into a best-fit circle or ellipse, and subsequently measured the radius of the circle or major axis and minor axis of the ellipse (Fig. 1a, p4). The dispersed or pellet was assumed to be a prolate ellipsoid for estimation of volume [10]. Dispersed mycelia (Fig. 1a, d1–d4) were distinguished as those with calculated volume less than 5 × 104 μm3 while pelleted forms were those with a volume greater than 5 × 104 μm3 (Fig. 1a, p1–p4). Nearly 500 events were counted per time point per experiment with data represented as mean ± SEM (standard error of mean) of three independent experiments. Deviation from the spherical shape of pellet was estimated in terms of the ratio of ellipse major to minor axis. Distributions were generated with a population size of ~600 events per time points and per media composition, pooled from three independent experiments. The length of peripheral hypha was measured from the filament tip to its point of attachment on the pellet core (~110 events were measured). Branching frequency was measured as number of branches per μm length of peripheral filament on the pellet. Data reported as mean ± standard deviation obtained from a population size of ~110 events. Experiments were also carried out in complex media and data was reported as mean ± SEM of three independent experiments.
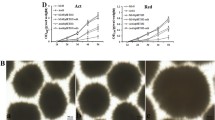
Morphological forms of Amycolatopsis balhimycina observed in batch cultivation. a Panels d1–d4 represent dispersed forms while panels p1–p4 represent pelleted morphology. Samples from different media compositions are imaged to illustrate different morphological forms. An ellipse is fitted around a dispersed and pellet and the major axis (X) and minor axis (Y) measured to quantify the number of dispersed and pellet form and shape and size of a pellet as illustrated in panel p4. b A pellet with peripheral filamentous growth is shown with a magnified portion illustrating the strategy for measurement of peripheral filament length (L). Imaging was performed by using a phase-contrast inverted microscope. Scale bar 100 μm
Results
In our previous work, it was shown that balhimycin production was significantly influenced by phosphate concentration in defined media [14]. Specifically, increasing phosphate concentration beyond the optimal value led to higher biomass growth but lower balhimycin production. Likewise, our preliminary results indicate that ammonia concentration in defined medium affected balhimycin synthesis (data not shown). Furthermore, our results suggest that phosphate and ammonia concentrations have an influence on the morphology of A. balhimycina. Therefore, our objective was to examine whether morphological changes are correlated with balhimycin productivity under varying phosphate and ammonia concentrations in defined medium. Our second objective was to verify if a similar correlation exists in complex media, which are preferred for industrial fermentation processes due to their lower cost and increased productivity.
Effect of phosphate in defined medium
In the present study, we monitored the concentrations of biomass and balhimycin and observed cellular morphology under different phosphate concentrations in defined medium (Fig. 2). While biomass concentrations increased with phosphate concentration, balhimycin production was highest in medium containing 0.2 g.l−1 of KH2PO4 (Fig. 2b). In media combinations yielding balhimycin, product formation was observable only after 72 h and reached the maximum value at 144–168 h. As a first step toward a correlation between morphology and production, we estimated the relative abundance of dispersed and pelleted forms. Although there was no significant variation in this parameter between producing and non-producing media at 24 h, pelleted form was more dominant at 48 h in producing media (Fig. 2c). However, other morphological characteristics of the pelleted forms were distinguishable in different media even at 24 h (Fig. 3). Pellets in producing media were smaller and elongated and showed substantially more branching in the peripheral region.
Effect of KH2PO4 concentration in defined medium on biomass growth (a), balhimycin production (b), and morphology (c). Symbol (○) represents seed medium whereas (◊), (∆) and (□) represent 0.2 g.l−1, 0.5 g.l−1 and 1.0 g.l−1 of KH2PO4, respectively, in defined medium. Fermentation was carried out in single baffled Erlenmeyer flasks of 500-ml capacity containing 100 ml of medium and incubated at 30°C and 150 rpm. For panels a and b, data are represented as mean ± SEM (standard error of mean) obtained from three independent experiments. For panel c, data represented as mean ± SEM obtained from three independent experiments with a population size of ~500 events per time point per experiment
We quantified the size and shape of the pellet by first circumscribing the pellet with an ellipse and then measuring its major and minor axes. The size of the pellet was estimated in terms of the volume of a prolate ellipsoid while the shape was quantified as a ratio of the major to minor axis. It is seen that pellets of smaller size are the dominant form with a narrow size distribution in producing media (containing 0.2 and 0.5 g.l−1 KH2PO4) (Fig. 4a). While this effect is visible at 24 h, it is more pronounced at 48 h where the size distribution is highly polydisperse with an increased occurrence of larger pellets in non-producing media (containing 1.0 g.l−1 KH2PO4). Concomitant to a smaller size, pellets in producing media were elongated with a significant fraction showing a high value of the major-to-minor axis ratio at 24 h (Fig. 4b). Note that the pellets in producing media do not grow in size between 24 and 48 h but undergo elongation. On the other hand, pellets in non-producing media continue to grow in size without any noticeable change in their shape (Fig. 4). In fact, we observed that the size and shape of the pellets are correlated with almost all the elongated pellets in the producing media having volume less than 1 × 106 μm3 (Fig. 5a) compared to pellets in the non-producing media, which are mostly spherical with a majority of these having volumes greater than 1 × 106 μm3(Fig. 5b).
Distribution of size (a) and shape (b) of pellets in seed medium and defined media containing 0.2–1.0 g.l−1 KH2PO4. Each box shows the lower quartile, median, upper quartile, and notches for 90% confidence interval for the median. The medians are significantly different when notches of two medians do not overlap. Distribution was generated with data pooled from three independent experiments amounting to ca. 600 events per time point for each media composition
We observed that long peripheral hyphae emanated from pellets in seed medium whereas the peripheral hyphae on pellets in defined medium were relatively shorter and highly branched (Fig. 3). Therefore, we quantified the length of peripheral hyphae and number of branches per unit length of filament as a function of phosphate concentration in defined medium. The number of branches per unit length increased with increasing KH2PO4 concentration (0.2–1.0 g.l−1) (Fig. 6a) but did not show any correlation with balhimycin productivity (Fig. 2b). In contrast, we observed that the length of peripheral filaments were relatively shorter in producing media (containing 0.2 and 0.5 g.l−1 of KH2PO4) compared to non-producing media containing 1.0 g.l−1 of KH2PO4 (Fig. 6B). Furthermore, while the length of the peripheral filaments remained unchanged in producing medium in the 24–48 h time period, filament length in non-producing medium continued to increase in the same period.
Effect of KH2PO4 concentration on branching frequency (a), and length of peripheral filament (b) in defined medium. Length of peripheral filament was measured from tip of filament to its point of attachment on the pellet core. Branching frequency was measured as number of branches per μm length of peripheral filament arising from the pellet. Data reported as mean ± standard deviation obtained from a population size of ~110 events
Effect of ammonium sulfate in defined medium
Our previous work has shown that the concentration of ammonia has a significant effect on balhimycin productivity [14]. Therefore, we wanted to investigate whether variation in ammonia affected morphology. The biomass concentration was maximum at an ammonium sulfate concentration of 2 g.l−1 and further increase led to a concentration dependent inhibition of growth (Fig. 7a). On the contrary, the balhimycin production was maximum for ammonium sulfate concentrations between 4.0 and 6.6 g.l−1 (Fig. 7b). In the ammonium sulfate concentration range of 2–8 g.l−1, the pellets were smaller and elongated while at ammonium sulfate concentration of 10 g.l−1, the pellets were larger and spherical in shape (Fig. 7c). Moreover, we observed that the mean length of peripheral hyphae of pellets in producing media was relatively smaller compared to that in non-producing media (data not shown). This is in agreement with our observation with defined media with varying phosphate concentration. Our results suggest that changes in ammonium sulfate concentration modulate balhimycin production and pellet morphology in a correlated manner.
Effect of phosphate and ammonium sulfate in complex medium
Our next objective was to verify whether the correlations observed in defined media are also observed in the presence of complex media, which are the media of choice in industrial fermentations. To that end, we chose a complex medium composition in which the concentrations of glucose, ammonium sulfate, potassium dihydrogen phosphate, YE, and DSF were optimized for balhimycin production using genetic algorithm (unpublished data). Keeping other components unchanged, we first varied the phosphate concentration around its optimal value and observed effects on growth, product formation and morphology (Fig. 8). Although the biomass concentration increased in going from 0 to 1.0 g.l−1 KH2PO4 (Fig. 8a), optimal balhimycin production was observed at a KH2PO4 concentration of 0.17 g.l−1 (Fig. 8b). At this optimal phosphate concentration, pellets were smaller in size and elongated compared to those at other phosphate concentrations tested (Fig. 8c). Likewise, we varied the concentration of ammonium sulfate around its optimal value in complex medium (Fig. 9). While the biomass concentration did not vary appreciably between ammonium sulfate concentration of 1 and 12 g.l−1 (Fig. 9a), the balhimycin production varied substantially with optimal productivity at 6 g.l−1 (Fig. 9b). Pellet morphology also correlated well with balhimycin productivity with small and elongated pellets in the high producing medium and larger and spherical pellets in other media (Fig. 9c). This shows that even in complex medium, a strong correlation exists between morphology and product formation.
Discussion
We report a strong correlation between morphology and balhimycin production in A. balhimycina. Several morphological parameters were systematically quantified as a function of phosphate and ammonia in defined as well as complex media. Concentrations of ammonium sulfate and phosphate were varied around their optimal values for balhimycin production. Higher productivity of balhimycin was correlated with (1) higher pellet fraction in the biomass, (2) small elongated pellets and (3) shorter hyphae in the periphery of the pellets. Interestingly, while the product formation started at 72 h or later, the morphological differences between producing and non-producing media were observed as early as 24 h. Although, antibiotic production in different actinomycetes and fungi has been attributed to changes in morphology [8, 11, 23, 24, 33], a rigorous study on the correlation of morphological parameters and productivity as a function of media composition is not available. Some studies have shown that mycelial forms are required for antibiotic production [25, 27], whereas several other studies show that higher productivity is associated with pelleted forms [12, 17, 33]. While the specifics of this observation may be dependent on the strain, we argue that the underlying mechanisms that affect morphological differentiation and antibiotic production might have certain commonalities leading to a correlation between morphology and productivity.
We did not find a clear correlation between biomass growth and pellet size. For example, on one hand, increased phosphate in defined media led to higher biomass and larger pellets (Fig. 2 and 3), increased ammonium sulfate in defined medium led to lesser biomass yet larger pellets (Fig. 7). In complex media, varying the ammonium sulfate or phosphate concentration away from their respective optimal values for balhimycin production led to larger and spherical pellets.
Depending on the medium composition, balhimycin production starts at 72 h or later with maximum product observed at 144–168 h. Further, the profiles of concentrations of nutrients or biomass do not provide any distinction between producing and non-producing media combinations. However, we observe that morphological changes not only correlate with balhimycin productivity, these changes occur at 24–48 h, which is much before the actual production starts. Therefore, we argue that cellular morphology can be used as a monitoring tool to predict productivity of a given batch. While the actual nature of the correlation between morphology and productivity may vary from strain to strain, we hypothesize that a similar monitoring strategy can be developed for other products.
References
Allen NE, LeTourneau DL, Hobbs JN (1997) The role of hydrophobic side chains as determinants of antibacterial activity of semisynthetic glycopeptide antibiotics. J Antibiot 50(8):677–684
Bapat PM, Bhartiya S, Venkatesh KV, Wangikar PP (2006) Structured kinetic model to represent the utilization of multiple substrates in complex media during rifamycin B fermentation. Biotech Bioeng 93(4):779–790. doi:10.1002/bit.20767
Berdy J (2005) Bioactive microbial metabolites—a personal view. J Antibiot 58(1):1–26
Braun S, Vechtlifshitz SE (1991) Mycelial morphology and metabolite production. Trends Biotechnol 9(2):63–68
Chatterjee S, Vijayakumar EKS, Nadkarni SR, Patel MV, Blumbach J, Ganguli BN, Fehlhaber HW, Kogler H, Vertesy L (1994) Balhimycin, a new glycopeptide antibiotic with an unusual hydrated 3-amino-4-oxoaldopyranose sugar moiety. J Org Chem 59(12):3480–3484
Collins TJ (2007) ImageJ for microscopy. Biotechniques 43(1 Suppl):25–30. doi:000112517[pii]
Damain AL, Fang A (1995) Emerging concepts of secondary metabolism in actinomycetes. Actinomycetologica 9:98–117
Dobson LF, O’Cleirigh CC, O’Shea DG (2008) The influence of morphology on geldanamycin production in submerged fermentations of Streptomyces hygroscopicus var. geldanus. Appl Microbiol Biotechnol 79(5):859–866. doi:10.1007/s00253-008-1493-3
Grimm LH, Kelly S, Krull R, Hempel DC (2005) Morphology and productivity of filamentous fungi. Appl Microbiol Biotechnol 69(4):375–384. doi:10.1007/s00253-005-0213-5
Hamanaka T, Higashiyama K, Fujikawa S, Park EY (2001) Mycelial pellet intrastructure and visualization of mycelia and intracellular lipid in a culture of Mortierella alpina. Appl Microbiol Biotechnol 56(1–2):233–238
Jonsbu E, McIntyre M, Nielsen J (2002) The influence of carbon sources and morphology on nystatin production by Streptomyces noursei. J Biotechnol 95(2):133–144. doi:S0168165602000032[pii]
Konig B, Schugerl K, Seewald C (1982) Strategies for penicillin fermentation in tower-loop reactors. Biotechnol Bioeng 24(2):259–280. doi:10.1002/bit.260240202
Lucatero S, Galindo E, Larralde-Corona CP (2004) Quantitative characterisation of the morphology of Trichoderma harzianum cultured in shake-flasks and containing Tween 40. Biotechnol Lett 26(1):41–44
Maiti SK, Singh KP, Lantz AE, Bhushan M, Wangikar PP (2010) Substrate uptake, phosphorus repression, and effect of seed culture on glycopeptide antibiotic production: process model development and experimental validation. Biotechnol Bioeng 105(1):109–120. doi:10.1002/bit.22505
Maiti SK, Srivastava RK, Bhushan M, Wangikar PP (2009) Real-time phase detection based online monitoring of batch fermentation processes. Process Biochem 44(8):799–811. doi:10.1016/j.procbio.2009.03.008
Manteca A, Alvarez R, Salazar N, Yague P, Sanchez J (2008) Mycelium differentiation and antibiotic production in submerged cultures of Streptomyces coelicolor. Appl Environ Microbiol 74(12):3877–3886. doi:10.1128/Aem.02715-07
Metz B, Kossen N, van Suijdam J (1979) The rheology of mould suspensions. Adv Biochem Eng 11:103–156
Nadkarni SR, Patel MV, Chatterjee S, Vijayakumar EKS, Desikan KR, Blumbach J, Ganguli BN, Limbert M (1994) Balhimycin, a new glycopeptide antibiotic produced by Amycolatopsis Sp Y-86, 21022—taxonomy, production, isolation and biological-activity. J Antibiot 47(3):334–341
O’Cleirigh C, Casey JT, Walsh PK, O’Shea DG (2005) Morphological engineering of Streptomyces hygroscopicus var. geldanus: regulation of pellet morphology through manipulation of broth viscosity. Appl Microbiol Biotechnol 68(3):305–310. doi:10.1007/s00253-004-1883-0
Papagianni M (2004) Fungal morphology and metabolite production in submerged mycelial processes. Biotechnol Adv 22(3):189–259. doi:10.1016/j.biotechadv.2003.09.005
Papagianni M, Mattey M (2006) Morphological development of Aspergillus niger in submerged citric acid fermentation as a function of the spore inoculum level. Application of neural network and cluster analysis for characterization of mycelial morphology. Microbial Cell Factories 5. doi: Artn 3 doi: 10.1186/1475-2859-5-3
Papagianni M, Mattey M, Berovic M, Kristiansen B (1999) Aspergillus niger morphology and citric acid production in submerged batch fermentation: effects of culture pH, phosphate and manganese levels. Food Technol Biotechnol 37(3):165–171
Paul GC, Priede MA, Thomas CR (1999) Relationship between morphology and citric acid production in submerged Aspergillus niger fermentations. Biochem Eng J 3(2):121–129
Reichl U, King R, Gilles ED (1992) Characterization of pellet morphology during submerged growth of Streptomyces tendae by image analysis. Biotechnol Bioeng 39(2):164–170. doi:10.1002/bit.260390207
Schatz A, Waksman SA (1945) Strain specificity and prodiction of antibiotic substances. IV. Variations among actinomycetes with special reference to, Actinomyces griseus. Proc Nat Acad Sci 31:129–137
Sonnleitner B, Locher G, Fiechter A (1991) Automatic bioprocess control. 1. A general concept. J Biotechnol 19(1):1–17
Szabó G, Barabás G, Vályi-Nagy T (1961) Comparison of Str. griseus strains which produce streptomycin and those which do not. Arch Microbiol 40(3):261–274. doi:10.1007/bf00540578
Tang JS, Gillevet PM (2003) Reclassification of ATCC 9341 from Micrococcus luteus to Kocuria rhizophila. Int J Syst Evol Microbiol 53(Pt 4):995–997
Thomas CR (1992) Image-analysis—putting filamentous microorganisms in the picture. Trends Biotechnol 10(10):343–348
Treskatis SK, Orgeldinger V, Wolf H, Gilles ED (1997) Morphological characterization of filamentous microorganisms in submerged cultures by on-line digital image analysis and pattern recognition. Biotechnol Bioeng 53(2):191–201
Whitaker A (1992) Actinomycetes in submerged culture. Appl Biochem Biotechnol 32:23–35
Yang YK, Morikawa M, Shimizu H, Shioya S, Suga K, Nihira T, Yamada Y (1996) Image analysis of mycelial morphology in virginiamycin production by batch culture of Streptomyces virginiae. J Ferment Bioeng 81(1):7–12
Yin P, Wang YH, Zhang SL, Chu J, Zhuang YP, Chen N, Li XF, Wu YB (2008) Effect of mycelial morphology on bioreactor performance and avermectin production of Streptomyces avermitilis in submerged cultivations. J Chin Inst Chem Eng 39(6):609–615. doi:10.1016/j.jcice.2008.04.008
Žnidaršič P, Komel R, Pavko A (2000) Influence of some environmental factors on Rhizopus nigricans submerged growth in the form of pellets. World J Microbiol Biotechnol 16(7):589–593
Znidarsic P, Pavko A (2001) The morphology of filamentous fungi in submerged cultivations as a bioprocess parameter. Food Technol Biotechnol 39(3):237–252
Acknowledgments
The authors gratefully acknowledge Vibha J. Arockia for technical assistance and Dr. Anna Eliasson Lantz for providing A. balhimycina DSM 5908 and the balhimycin standard. This work was partially funded by a grant awarded to PPW by the Department of Biotechnology, Ministry of Science and Technology, Government of India. KPS was funded by a University Grants Commission fellowship, Government of India.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singh, K.P., Wangikar, P.P. & Jadhav, S. Correlation between pellet morphology and glycopeptide antibiotic balhimycin production by Amycolatopsis balhimycina DSM 5908. J Ind Microbiol Biotechnol 39, 27–35 (2012). https://doi.org/10.1007/s10295-011-0995-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-011-0995-7