Abstract
By analyzing with X-ray diffraction and FT-IR spectroscopy, the main effective factors among common culture conditions on crystallinity index and I α fraction of cellulose produced by Gluconacetobacter xylinus were examined with Plackett–Burman design experiment. Varying pH value in the medium by adjusting the composition of citrate buffer or by adding HCl/NaOH solution indicates it is the content of citrate buffer rather than its function of pH buffering that gives the influence on crystallinity. Further experiment reveals that Na+ concentration of 0.174 mol/l in medium with citrate buffer added would decrease the crystallinity index significantly. Comparison of carbon sources shows that fructose leads to a higher crystallinity index than glucose, which suggests a relationship between crystallinity and production speed of bacterial cellulose affected by carbon sources. An interesting phenomenon was that a longer period of cultivation would decrease the crystallinity of bacterial cellulose. The reason is assumed to be the dense network of cellulose formed by bacterial cells that restrict the motion of themselves as the incubation period extends. Though the effect of inoculum age is still unclear, the influence on crystallinity of bacterial cellulose caused by variation of some ordinary culture conditions can be drawn out from data of this work.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bacterial cellulose (BC) is a natural pure cellulose aggregate produced by strains of the bacterium Gluconacetobacter xylinus (formerly Acetobacter xylinum), which has no impurities. It is composed of fibers of diameter ~3 nm grouped into bundles of diameter ~50 nm and forming a dense net. With a strong tensile strength, low toxicity, and high hydrophilicity, the BC fiber is ideally suited for use in different fields [9, 17], especially in medical device applications [2, 11], which are actively being pursued by numerous research groups worldwide.
There are several other microorganisms, such as strains of Pseudomonas, Achromobacter, Alcaligene, and Azotobacter, also found to produce cellulose, but A. xylinum is the only one that can form cellulose in sufficient abundance for industrial applications [20]. The main crystalline forms of native cellulose are Cellulose I and II [4]. Detailed structural characteristics demonstrated that Cellulose I is a composite of two different crystalline phases called cellulose I α (triclinic) and I β (monoclinic) unit cells [3]. Normally, A. xylinum produces I α-rich cellulose with highly crystalline characteristics [11].
Numerous papers published in the past showed that various water-soluble polymers or their fractions can interfere with the aggregation of microfibrils into a normal ribbon as they were added into the culture medium and thus affect the crystallinity degree and I α fraction of BC [21, 24]. Other physical cultivation conditions were also discussed as to whether they induce changes in crystal structure of BC [6, 22]. Some papers discussed the crystallinity of BC produced with various carbon sources, including molasses [8], processed rice bark [4], and saccharified food wastes [12]. However, few studies have focused on finding out which are the major effects among common culture conditions. Given this background, the present work screened the main factors that affect the crystallinity of BC by a Plackett–Burman design and analyzes their influence.
Materials and methods
Materials
The cellulose was produced by Gluconacetobacter xylinus (Acetobacter xylinum subsp. sucrofermentans BPR2001, ATCC #700178). Maple syrup, which is mainly composed of fructose, glucose, and sucrose [13], was purchased from Kirkland®. All other chemicals used were purchased from Sigma–Aldrich.
Sample preparation
The inocula was incubated at 27°C with a rotation speed of 160 rpm in a medium composed of fructose 20 g/l, (NH4)2SO4 3.3 g/l, yeast extract 20 g/l, KH2PO4 1 g/l, and MgSO4·7H2O 0.122 g/l. The bacterial cellulose was then produced in 500-ml Erlenmeyer flasks containing 250 ml of media. The product was purified by processing with 1% NaOH solution for 30 min in a boiling water bath to lyse the bacteria. After being washed to neutral, the BC was dried for XRD and FT-IR measurements. All reported quantitative data were the mean values and standard deviations corresponding to three triplicate samples.
Screening parameters by Plackett–Burman design
The Plackett–Burman design can identify the significant variables among a number of factors from the screening experiment by analysis of variance (ANOVA) [15]. This experimental design is based on a linear approach:
where Y is the response, M 0 is the model intercept, and M i the regression coefficients. This model assumes no interaction among factors, X i , in the range of variables under consideration. The main effect (the contrast coefficient) was calculated as the difference between the average of measurements made at the high level (+1), and the average of measurements made at the low level (−1) of each factor. The contrast coefficient provides the evaluation for the effect of each factor. A large contrast coefficient, positive or negative, indicates a factor of large impact, and a contrast coefficient close to zero implies a factor with little or no effect. The significance of each variable was determined by applying Student’s t test and a low p value indicates a significant effect. A 24-run Plackett–Burman design generated by the software Minitab 14 was used to screen 12 variables, which included nutrients, physical parameters and pH buffer at two levels (Tables 1, 2). The rows in Table 2 represent the 24 different trials and each column represents an independent or dependent variable. The signs 1 and −1 represent two different levels of the independent variables under investigation (Table 1). Except variables in the Plackett–Burman design, other culture conditions were same as described above in sample preparation. All experiments were carried out in triplicate and the averages of the crystallinity index and I α fraction were taken as the response.
Influence of citrate buffer and pH value
The experiment was designed with the pH buffer system varying in culture medium according to Fig. 1b. Other medium ingredients: fructose 30 g/l, yeast extract powder 20 g/l, (NH4)2SO4 3.3 g/l, KH2PO4 1.0 g/l, MgSO4·7H2O 0.8 g/l, ethanol 0.5% (v/v) and acetic acid 0.5 g/l. A 3-day inoculum was transferred in with an inoculum ratio of 6%. Then, with the same flasks and media volume as described above, the cultivation was conducted at 27°C with a rotation speed of 135 rpm for 10 days.
Effects of different carbon sources
The culture conditions were almost the same as above in pH value comparison except that the carbon source varied and pH buffer system was fixed to 1.60 g/l of citric acid and 2.40 g/l of trisodium citrate·2H2O. Each carbon source has an equivalent level of carbohydrate at 30 g/l. A mixture of sucrose, fructose, and glucose (SFG) to simulate the maple syrup composition was also tested with a proportion demonstrated in Fig. 2.
Crystallinity index and I α fraction of bacterial cellulose produced in media with different carbon sources (all have an equivalent level of carbohydrate at 30 g/l). MS maple syrup, Fru fructose, Glu glucose, Suc sucrose, SFG mixture of sucrose (89% of total sugar), fructose (5.5%) and glucose (5.5%); values are means ± SD; the connecting lines between symbols are just for distinguishing them conveniently
X-ray diffraction (XRD) analysis
Samples of BC were analyzed by a Rigaku MioniFlex X-ray diffractometer, using monochromated CuKα radiation (λ = 1.54059 Å) operated at 30 kV and 15 mA. The relative crystallinity index (CrI) was estimated by equation: CrI = 1−Iam/I002, in which I002 is the maximum intensity of the 002 lattice diffraction and Iam is the intensity at 2θ = 18° [12].
FT-IR measurements
FT-IR spectra were obtained using a Perkin-Elmer Spectrum 2000 FT-IR spectrometer. Yamamoto et al. [21] revealed a simple linear relationship exists between the CPMAS 13C NMR method (needs 100–150 mg of sample) and FT-IR spectroscopy (needs a few milligrams of sample) for determination of the mass fractions of celluloses I α and I β. By using this relationship, the I α fraction (f α) was derived from equations: f α = 2.55 × f IR − 0.32 and f IR = A 750/(A 750 + A 710), with A 750 and A 710 are band areas of the characteristic IR absorption bands at 750 cm−1 for I α and 710 cm−1 for I β that were deconvoluted by using a Gaussian-Lorentzian curve fitting analysis as presented according to Yamamoto et al. [21].
Results
Screen the main factors by Plackett–Burman design
Statistical methods for optimization of the culture condition have been proven to be a powerful and useful tool for biotechnology. There are many factors that may affect the crystal structure of BC, including medium composition as well as some physical factors. This experiment performed an attempt of screening out which one in culture conditions has more of an effect on BC crystallinity by applying the Plackett–Burman experimental design. Based on the factors that hold the most promise for affecting the crystal structure of BC, an experimental plan for a 24-run experiment was generated (Table 2) and then statistically analyzed (Table 3) with the software Minitab 14.
For the I α fraction of BC, none of the factors has shown a statistical significance at the significance level of 0.05. By decreasing the significance level to 0.10, parameter X10 and X4 exhibited relatively significant effects on I α fraction of BC (Table 3). From the positive values of the effects of these two factors, a longer inoculum age and higher content of citrate buffer would result in a higher I α fraction of BC. There were six parameters (X2, X5, X8, X10, X11, and X12) that affected the crystallinity index of BC at the significance level of 0.05 or 0.10 (Table 3), respectively. With the positive or negative values of effects, these parameters exhibited different influences on the crystallinity index. Namely, the lower content of agar, shorter age of inoculum, slower rotation speed and shorter incubation period would lead to a higher crystallinity index of BC while the ethanol content and culture temperature had a contrary trend. Among these parameters, the rotation speed and content of agar have an influence on BC crystallinity the same as in previous reports [3, 10, 22], while the cultivation period, citrate buffer, ethanol content, age of inoculum, and culture temperature need further study.
Role of citric acid radical and sodium ion
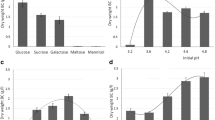
Figure 1 shows no significant difference in the I α fraction and crystallinity index of BC among groups Con, V, and VI, but significant differences (p < 0.05) between pH values before and after incubation were found among them. It showed that only the difference in concentration of H+ or OH− and their natural changes during the incubation would not affect the crystalline structure of BC. It’s obviously that in group III the crystallinity index and I α fraction of BC decreased with a higher Na+ concentration (calculated as 0.174 mol/l). Concentrations of Na+ were both 0.049 mol/l in groups II and IV, but there were increases in the crystallinity index and I α fraction of BC while citric acid radical raised from 0.033 to 0.062 mol/l. The different effect of concentration of Na+ and citric acid radical in the medium suggested that an appropriate content of citric acid radical would benefit the BC crystallinity but a higher Na+ content gave an adverse effect.
Influence of carbon source and cultivation period
As the period of incubation extended, trends of decline in I α fraction were plotted from the early to final stage except the line of glucose in Fig. 2. There was no statistical significance of I α fraction among carbon sources that contained fructose or sucrose, except glucose. For the crystallinity index, an obvious descent from about 0.50 at the initial stage to 0.44 at the late stage is shown in Fig. 2. In both stages, the crystallinity indexes of BC produced with fructose as the carbon source were higher than that merely with glucose.
Discussion
Czaja and Yan [3, 22] thought that a moderate agitation speed would increase production of BC as well as decrease the crystallinity index and the I α fraction of BC compared to static culture. The addition of agar in this study also induced a reduction in the crystallinity index of BC, for it interfered the piling up of sub-elementary fibrils of BC and further influenced the crystallinity of it [10]. Hirai et al. reported that [5] the strain Acetobacter xylinum (ATCC #23769) produced BC in a band shape composed of Cellulose II at 4°C, and ribbons of Cellulose I at 28°C. Here with the culture temperature of 25 and 30°C, the strain (ATCC #700178) yielded BC in Cellulose I and gave a higher crystallinity as the temperature rose.
Data of groups V and VI in Fig. 1 illustrate that the variation of the pH value would not influence the crystalline of BC significantly. In groups IV and V, with similar pH values, significant difference in BC crystallinity implies that the citrate buffer gives more effects than pH buffering. Compared to the control, a relatively lower content of citrate buffer (groups I and II) would not affect crystalline of BC much, while a higher content of citric acid radical and Na+ cation exhibit a contrary influence (groups III and IV). In experimental design, it was originally intended to adjust the pH value in group III to the same as that in group VI by adding additional trisodium citrate, but that failed for merely small increment in pH value as 17.06 g/l of trisodium citrate was added. Trisodium citrate was no more added for too much of Na+ would affect the growth of cells. However, the experiment result indicated that it is the change of citrate buffer composition in the culture medium but not the pH value that affects the crystallinity of BC.
In groups I to IV, with citrate buffer added, there was a remarkable drop in the crystallinity index and I α fraction of BC in group III with 0.174 mol/l of Na+ in medium, but no significant (p < 0.05) variation in the crystallinity index as Na+ content increased from 0.024 mol/l (group I) to 0.048 mol/l (groups II and IV). This indicated that a moderate rise of Na+ concentration would not influence the crystalline of BC greatly, but too much of that would make an obvious reduction. To understand the role of Na+ in the formation of cellulose crystal structure, one mechanism for mercerization of cellulose can be used for reference. The mechanism holds that many small voids are generated as the swelling agent, water or aqueous sodium hydroxide, separates elementary fibrils of the cellulose [1], and then the separation of the molecular chains destructs the cellulose I structure followed by a reforming of the crystal structure [16]. Though the concentration of Na+ in group III (0.174 mol/l) is much less than that in mercerization, which is often several mol per liter, it might play the same role in separating the elementary fibrils and thus cause a decrease in the crystallinity index of BC.
Whether media composition, especially carbon sources, had an influence on the crystallinity of BC was discussed by some papers but brought opposite opinions [4, 8]. Fructose is the optimal carbon source for the strain used here that was isolated from fruits originally [19]. The strain produced a much smaller amount of BC with only glucose in medium than those that contain fructose or sucrose (data not shown). Though ethanol content in Plackett–Burman experimental design (X5 in Table 1) had an influence on the crystallinity index of BC, it was also related to the carbon source for being considered to improve BC production as a substitute for glucose [23] or as an energy source for ATP generation [14]. By analyzing Fig. 2, it can be concluded that carbon sources that provide higher production of BC would result in a higher I α fraction and crystallinity index in BC.
Some papers chose the nascent BC at the same cultivation stage to conduct their experiments [6, 7]. However, few papers have discussed the crystallinity of BC at different stages of cultivation (Fig. 2). Shibazaki et al. [18] thought that the freedom of cell motion pushed by cellulose production could affect the piling up of cellulose microfibrils, which led to a change in the crystallite shape. It could be presumed from this hypothesis that in this study the bacterial cells moved freely at the earlier period of cultivation because the network formed by cellulose was not very dense, then the motion of cells got more and more restricted for the network of cellulose became intensive as the cultivation elongated. Thus, the change of accumulation of microfibrils caused by environmental variation induced an alteration on crystallinity of BC.
The age of inoculum (X10 in Table 1) was chosen to be a main factor in Plackett–Burman Design experiment, but the experiment data in this study is insufficient to clarify the reason. However, as far as the authors are aware, there are few reports examining the common conditions together that affect the crystallinity of BC. This finding will help to control that just by changing some common conditions in ordinary cultivation.
References
Crawshaw J, Bras W, Mant GR, Cameron RE (2002) Simultaneous SAXS and WAXS investigations of changes in native cellulose fiber microstructure on swelling in aqueous sodium hydroxide. J App Polym Sci 83(6):1209–1218
Czaja W, Krystynowicz A, Bielecki S, Brown RM (2006) Microbial cellulose: the natural power to heal wounds. Biomaterials 27(2):145–151
Czaja W, Romanovicz D, Brown RM (2004) Structural investigations of microbial cellulose produced in stationary and agitated culture. Cellulose 11(3–4):403–411
Goelzer FDE, Faria-Tischer PCS, Vitorino JC, Sierakowski MR, Tischer CA (2009) Production and characterization of nanospheres of bacterial cellulose from Acetobacter xylinum from processed rice bark. Mater Sci Eng C 29(2):546–551
Hirai A, Tsuji M, Horii F (1997) Culture conditions producing structure entities composed of cellulose I and II in bacterial cellulose. Cellulose 4(3):239–245
Hult E-L, Yamanaka S, Ishihara M, Sugiyama J (2003) Aggregation of ribbons in bacterial cellulose induced by high-pressure incubation. Carbohydr Polym 53(1):9–14
Kai A, Mondal IH (1997) Influence of substituent of direct dye having bisphenylenebis(azo) skeletal structure on structure of nascent cellulose produced by Acetobacter xylinum [I]: different influence of Direct Red 28, Blue 1 and 15 on nascent structure. Int J Biol Macromol 20(3):221–231
Keshk SMAS, Razek TMA, Sameshima K (2006) Bacterial cellulose production from beet Molasses. African J Biotechnol 5(17):1519–1523
Kim Y, Jung R, Kim H-S, Jin H-J (2009) Transparent nanocomposites prepared by incorporating microbial nanofibrils into poly(l-lactic acid). Curr App Phy 9:S69–S71
Klemm D, Schumann D, Kramer F, Hessler N, Hornung M, Schmauder HP, Marsch S (2006) Nanocelluloses as innovative polymers in research and application. Adv Polym Sci 205:49–96
Klemm D, Schumann D, Udhardt U, Marsch S (2001) Bacterial synthesized cellulose: artificial blood vessels for microsurgery. Prog Polym Sci 26(9):1561–1603
Moon SH, Park JM, Chun HY, Kim SJ (2006) Comparisons of physical properties of bacterial celluloses produced in different culture conditions using saccharified food wastes. Biotechnol Bioproc Eng 11(1):26–31
Morselli MF (1975) Nutritional value of maple syrup. Nat Maple Syrup Digest 14(2):12
Naritomi T, Kouda T, Yano H, Yoshinaga F (1998) Effect of ethanol on bacterial cellulose production from fructose in continuous culture. J Ferment Bioeng 85(6):598–603
Plackett RL, Burman JP (1946) The design of optimum multifactorial experiments. Biometrika 33(4):305–325
Revol JF, Dietrich A, Goring DA (1987) Effect of mercerization on the crystallite size and crystallinity index in cellulose from different sources. Can J Chem 65(8):1724–1725
Shah J, Brown R (2005) Towards electronic paper displays made from microbial cellulose. App Microbiol Biotechnol 66(4):352–355
Shibazaki H, Saito M, Kuga S, Okano T (1998) Native cellulose II production by Acetobacter xylinum under physical constrain. Cellulose 5(3):165–173
Toyosaki H, Naritomi T, Seto A, Matsuoka M, Tsuchida T, Yoshinaga F (1995) Screening of bacterial cellulose-producing Acetobacter strains suitable for agitated culture. Biosci Biotechnol Biochem 59(8):1498–1502
Wan WK, Hutter JL, Millon L, Guhados G (2006) Bacterial cellulose and its nanocomposites for biomedical applications. In: Oksman K, Sain M (eds) Cellulose nanocomposites: processing, characterization, and properties, ACS Symposium Series 938. American Chemical Society, Washington, DC, pp 221–241
Yamamoto H, Horii F, Hirai A (1996) In situ crystallization of bacterial cellulose II. Influences of different polymeric additives on the formation of celluloses Iα and Iβ at the early stage of incubation. Cellulose 3(1):229–242
Yan ZY, Chen SY, Wang HP, Wang BA, Jiang JM (2008) Biosynthesis of bacterial cellulose/multi-walled carbon nanotubes in agitated culture. Carbohydr Polym 74(3):659–665
Yunoki S, Osada Y, Kono H, Takai M (2004) Role of ethanol in improvement of bacterial cellulose production: analysis using C13-labeled carbon sources. Food Sci Technol Res 10(3):307–313
Zhou LL, Sun DP, Hu LY, Li YW, Yang JZ (2007) Effect of addition of sodium alginate on bacterial cellulose production by Acetobacter xylinum. J Ind Microbiol Biotechnol 34(7):483–489
Acknowledgments
Dr. Wankei Wan of the University of Western Ontario is gratefully acknowledged for his help in the experiments as well as his kind discussions about bacterial cellulose. The authors would like to thank the editor of this journal, Dr. Alan T. Bull, and anonymous reviewers for giving detailed comments and constructive suggestions on this paper. This research was supported by the National Natural Science Foundation of China (30900298), the Fundamental Research Funds for the Central Universities of China (3082013 in Wuhan University), and also by the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry of China.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zeng, X., Liu, J., Chen, J. et al. Screening of the common culture conditions affecting crystallinity of bacterial cellulose. J Ind Microbiol Biotechnol 38, 1993–1999 (2011). https://doi.org/10.1007/s10295-011-0989-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-011-0989-5