Abstract
The cell viability and fermentation performance often deteriorate in fermentations of spent sulphite liquor (SSL). This investigation therefore addresses the question of how different cultivation conditions for yeast cells influence their ability to survive and boost the ethanol production capacity in an SSL-based fermentation process. The strains used as pitching agents were an industrially harvested Saccharomyces cerevisiae and commercial dry baker’s yeast. This study therefore suggests that exposure to SSL in combination with nutrients, prior to the fermentation step, is crucial for the performance of the yeast. Supplying 0.5 g/l fresh yeast cultivated under appropriate cultivation conditions may increase ethanol concentration more than 200%.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
At present, two methods are used industrially to separate cellulose from both lignin and hemicellulose in the forestry industry. The most common one is the sulphate process, which is used for almost all paper pulp production. The sulphate process, kraft pulping, is an alkali process in which a strong cellulose fiber is produced [22]. The black liquor derived from this process contains lignin, extractives, and organic acids as well as lignin and carbohydrate degradation products. The hemicellulose in the wood is consequently totally hydrolyzed and not fermentable [22]. The sulphite process, which includes acid treatment of the wood material, produces high-grade cellulose by different cooking steps of the wood. During the different process steps, hemicellulose and lignin are degraded, but not totally decomposed. This liquid, usually referred to as spent sulphite liquor (SSL), contains fermentable glucose and mannose in addition to degradation products and cooking chemicals [22].
Along with the monosaccharides, SSL contains 5-hydroxymetylfurfural and furfural, organic acids, wood extractives, dissolved solids, and residues from the cooking process such as sulphite, and is therefore a highly inhibitory fermentation medium [2, 6, 20]. In addition, the insufficient levels of available nitrogen, phosphor, and some vitamins may limit cell growth, and eventually results in energy deprivation and deteriorating fermentation capacity. While Saccharomyces cerevisiae (Baker’s yeast), the preferred industrial ethanol-producing organism, is known for its industrial robustness, it may be severely inhibited under these conditions, which will result in poor productivity and incomplete fermentation.
There are different methods to attack this problem, e.g., detoxification of the SSL medium [10–12, 18, 19, 25]. Other approaches may involve fermentation strategy, choice of yeast strain, manipulation of external conditions such as temperature, and increased nutrient concentration. It is also possible to boost the yeast concentration during ongoing fermentation [9, 19].
It is also important to keep in mind that industrial microbiological processing of monosaccharides is easily subjected to infections by undesired microbes. In the case of ethanol fermentation, these infections can be by bacteria such as lactic acid bacteria [23] and acetic acid bacteria [17], but also by wild yeast [4]. Infected fermentations result in decreased ethanol yield, but a boosting strategy can, to a large extent, reverse this effect.
Studies on spent sulphite liquor fermentations and yeast viability appear to be lacking, although it is known that yeast cells will respond positively to an adaptation or training period before being exposed to the challenges offered by, e.g., lignocellulosic media [1, 21]. This study therefore focuses on how the use of a pitching agent affects the fermentation of SSL. It also deals with the difference between commercial bakers yeast and yeast adapted to SSL when it comes to the fermentative capacity and tolerance to storage.
Materials and method
Organism
The fermenting organism was an industrial strain of the species Saccharomyces cerevisiae, obtained by isolation from Domsjö Fabriker industrial ethanol production plant located in Örnsköldsvik, Sweden, and it is deposited at the Culture Collection University Göteborg (CCUG53310). The start inoculum was a mixture of microorganisms harvested from the same site. This mixture (sludge) contained the complete microbiological community existing in an industrial ethanol fermentation plant; mainly yeast (S. cerevisiae), lactic acid bacteria [23], and acetic acid bacteria [17]. After harvest, the mixture was allowed to settle for approximately 1 h in order for the microorganisms to sediment. The supernatant was discarded and the microorganisms were concentrated by centrifugation at 2,910 g for 5 min in room temperature and weighed prior to inoculation.
A pure culture of the industrial strain of Saccharomyces cerevisiae was inoculated to each one of fermentations in order to boost the system with fresh yeast. Thus, a fixed amount of yeast is added to the fermentation in order to try to increase ethanol yield. Commercial dry bakers yeast (CBY) was used as reference.
Cultivation medium
Three different cultivation media were used for growth of fresh yeast. Medium one contained 20 g/l glucose, 3 g/l yeast extract and 3.42 g/l (NH4)2SO4 suspended in tap water (YD). Medium two consisted of spent sulphite liquor (SSL) from the Domsjö Fabriker plant supplemented with 3.6 g/l KH2PO4, 7.5 g/l (NH4)2SO4, 0.09 g/l ZnSO4•7 H2O, 0.97 g/l MgSO4•7 H2O, and 50 μg/l biotin. Medium three consisted of a 1/1 mixture of the two above described media. The synthetic medium was autoclaved in 120°C for 20 min and then cooled to 30°C before use. The SSL was used directly and was slowly heated to 30°C prior to inoculation.
Cultivation strategy
A preculture was started by inoculating yeast grown on an agar plate containing 20 g/l yeast extract agar (MERCK KGaA), 20 g/l glucose and 20 g/l peptone (YPD) to a liquid medium containing yeast extract, glucose and peptone with the same concentrations as the agar plate. The temperature was regulated to 30°C in a shake-bath. Subsequently, the preculture was further inoculated into an Infors HT Minifors fermentor with a working volume of 4 l, containing 500 ml of medium one. Cultivation of yeast was carried out by 24 h of aerobic batch cultivation followed by 34 h of feeding of one of the three media; SSL, YD or SSL/YD. The batch cultivation was performed with a temperature set to 30°C and pH continuously adjusted to 5.0 by 5 M NaOH and the air flow was set to 3.3 vvm. The feed rate was 85 ml/h. The temperature and the air flow were the same both during batch and fed-batch cultivation.
Fermentation medium and fermentation strategy
SSL supplemented with 10.2 ml/l 25% ammonium and 171 mg/l KH2PO4 was used as fermentation substrate. The pH was adjusted to 5.0 by 5 M NaOH prior to fermentation. The fermentations were performed in 300-ml Erlenmeyer flasks with a total fermentation volume of 150 ml. The fermentation time was 12 h, the temperature was 30°C, and the agitation (in an orbital shake) was 150 rpm. The pH was not regulated during fermentation. Starter culture (sludge) and pitching agents were inoculated together at time zero of all fermentations.
Sampling technique during cultivation and during fermentation
Samples were withdrawn from the cultivation of new yeast via a sterile vessel connected to the fermentor. Samples from the fermentations were withdrawn with a sterile syringe at the end of each fermentation. Glucose concentration was determined using a Boehringer Mannheim/R-BIOPHARM Glucose kit. The ethanol concentration was determined using a Boehringer Mannheim/R-BIOPHARM Ethanol kit.
Storage conditions
The pitching agent was stored for 86 h in the cultivation medium with no supply of air. Temperature was 30°C and the agitation was 150 rpm.
Cell viability
Measurements of the cell viability were performed using YPD-agar plates for yeast and MRS-agar supplemented with cycloheximide for lactic acid bacteria. The YPD-agar plate contained 20 g/l yeast extract agar (MERCK KGaA), 20 g/l glucose, and 20 g/l peptone. The MRS-agar plate contained MRS broth and 100 mg/l cycloheximide.
Reproducibility
The fermentations were performed with a base of industrial sludge harvested randomly during a period of 9 months. It is of utmost importance to harvest sludge at different times in order to validate the effect of the pitching agent in an industrial context where process variations in the rest of the factory can influence ethanol production and the composition of microbes. In order to determine the standard deviation of the ethanol concentration between the same sets of fermentations performed at different times, Student’s t test was used.
Results and discussion
The cell viability and fermentation performance is often deteriorating in SSL-based environments. Hence, in many cases it is necessary or at least beneficial to add fresh yeast to the fermentor. This investigation addresses the question of how different cultivation conditions for a yeast culture that subsequently will be used as pitching agent in SSL fermentations will influence its capacity to survive and improve the ethanol production capacity. Four different cultivation conditions were compared, commercial dry bakers yeast, and cells cultivated in YD, SSL or a 1/1 mixture of YD and SSL, respectively.
Influence of yeast additions in SSL-based fermentations
The ethanol concentration did increase with an addition of fresh-grown yeast (Fig. 1). Results obtained in this study indicate with 90% certainty (determined using Student's t test) that yeast grown on SSL produces a more than two-fold increase in ethanol concentration in subsequent fermentations with SSL as fermentation media compared to fermentations without any addition of fresh yeast. In order to ferment a harsh media like SSL within a reasonable time-span it therefore seems advantageous to cultivate the fermenting organism, S. cerevisiae, under conditions resembling the subsequent fermentation medium [1, 21].
Ethanol concentrations after 12 h of fermentation in SSL media using sludge from an industrial ethanol production plant with or without addition of fresh yeast. Bars 1 and 2 represent control experiments using only sludge with a concentration of 2.0 or 2.5 g/l (dw), respectively. Bars 3–6 show results when 2.0 g/l (dw) of sludge was pitched with 0.5 g/l (dw) of commercial dry baker’s yeast (CBY), or with cells cultivated in YD, SSL or a mixture of YD and SSL, respectively. Cultivation of yeast used for pitching was performed in fed-batch cultures and the cells were harvested immediately at the end of the feeding period. The error bars indicate the standard deviation of a minimum of four sets of experiments
An increased level of nutrients during growth did increase the ethanol concentration even more, although the difference between pure SSL and supplemented SSL is not statistically significant. After 12 h of fermentation, a more than two-fold increase in ethanol concentration was obtained with 95% certainty when yeast cultivated in a mixture of YD/SSL were used as pitching agents, compared to sludge as pitching agent (Fig. 1). If the fresh yeast is cultivated in a mix of rich nutrients (YD) and SSL it is suggested that the yeast will be able to produce a biomass with proper levels of e.g., nutrients, proteins, and energy to sustain a high performance and viability in a harsh, nutrient-poor environment offered by SSL. This may decrease the lag phase and result in a higher productivity as well as a longer life span, which would be of importance in e.g., continuous fermentations where the yeast is exposed to the challenging conditions during an extended time period. It has been shown that S. cerevisiae has the potential to adapt to harsh conditions and is therefore suitable for industrial fermentations [19, for review see 22]. Accordingly, a correlation between fermentation ability and stress tolerance has been shown in S. cerevisiae wine strains [8], and the results obtained in this investigation suggest that this may also be true for the industrially harvested S. cerevisiae used in this study. The addition of commercial bakers yeast results in a minimal increase in ethanol concentration (Fig. 1) and even though it is an attractive option due to its simplicity, it does not have any significant effect on the ethanol productivity under these conditions.
The effect of the amount of yeast added
An increased inoculum size affects ethanol productivity positively, independently of the growth media [9]. The increase in ethanol concentration is, however, not proportional to the increase in cell mass (Table 1). A fourfold increase in cell mass results in a two to three-fold increase in ethanol concentration. A difference is, however, noticed when comparing yeast grown with and without SSL present in the medium. Inoculation of 0.2 g/l fresh yeast grown in YD, to SSL fermentations does not affect ethanol concentration at all (Table 1). The same inoculation of yeast cultivated in the presence of SSL, on the other hand, affects ethanol concentration positively already at this limited cell concentration (Table 1). It has been proposed that an initial cell concentration of 107 CFU/ml is preferable [7], which roughly corresponds to an inoculation size of 0.5 g/l (dw). In fermentations inoculated with 0.2 g/l (dw) of fresh grown yeast, no difference in improved ethanol concentration can be seen between yeast grown in pure SSL and yeast grown in SSL supplemented with nutrients. However, at higher concentrations of yeast additions (0.5 and 0.8 g/l dw), a positive effect on ethanol productivity by addition of nutrients was observed. Hence, a cultivation medium based on SSL and supplemented with nutrients give rise to more active and resistant yeast cells.
How will the storage of yeast affect its capability as a pitching agent?
From a production process point of view, it would be most valuable if the good characteristics of yeast cultivated under proper conditions could prolong its life-span as a valuable pitching agent. However, when stored yeast cells were added to the SSL fermentation broth, there was no positive effect whatsoever in terms of ethanol production (Fig. 2). The results showed low fermentative capacity in subsequent SSL fermentations. Storage often includes exposure of the yeast to minimal concentrations of carbon and nutrients, which may lead to reduced energy content of the cell and in turn lower the fermentative capacity. This is further supported by Thomsson et al. [24], who suggests that carbon starvation prior to fermentation results in an almost complete loss of fermentative capacity of S. cerevisiae. Nilsson et al. [16] suggests that the physiological state from which the cells originate affects the fermentative capacity after storage. Storage conditions can be optimized [13, 14] but freshly grown yeast will still most likely be superior to stored yeast (Figs. 1, 2).
Ethanol concentrations after 12 h of fermentation in SSL media using sludge from an industrial ethanol production plant without or with addition of commercial dry baker’s yeast or yeast cells that were produced and stored for a period of 86 h. Bars 1 and 2 represent control experiments using only sludge with a concentration of 2.0 or 2.5 g/l (dw), respectively. Bar 3–6 shows results when 2.0 g/l (dw) of sludge was pitched with 0.5 g/l (dw) of commercial dry baker’s yeast (CBY) or with yeast cultivated in different media (YD, SSL YD/SSL) and stored for 86 h. The error bars indicate max/min values of two separate experiments
Comparison of increasing inherent yeast concentrations and addition of new yeast
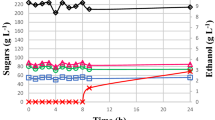
Figure 3 illustrates the effect of increasing the concentration of inherent cell mass. A fermentation with 6–8 g/l (dw) sludge results in the same ethanol concentration as fermentations inoculated with 2 g/l (dw) sludge and 0.5 g/l (dw) fresh yeast grown in SSL (Fig. 1). This suggests that almost a 2.5-fold increase in cell concentration is needed in order to increase ethanol concentration to the same level as when fresh grown yeast is added to the fermentation. This increases the need for very effective cell retention. The ethanol concentration will also most likely decrease with time due to a possible viability loss of the cells. Enhanced productivity may at the beginning be reached with increased concentration of inherent yeast, but in order to achieve continuous high-productivity pitching of fresh grown adapted yeast may be preferable.
Influence of yeast additions on the amount of bacteria
The amount of viable yeast cells does increase in the SSL fermentation when new yeast is added, the highest amount of viable cells being obtained by the addition of cells grown in YD/SSL (Table 2). Also, the addition of commercial dry baker's yeast results in an increase in the number of viable cells. Despite this, the ethanol concentration remains unaffected (Fig. 1). Hence, it seems as if the added commercial dry baker's yeast are surviving, at least for a limited amount of time, in the harsh environment but it is not very active in terms of ethanol production. Fermentations with a yeast inoculum grown in SSL/YD exhibited the highest viable yeast cell concentration and a tenfold decrease in bacterial cell concentration (Table 2). This also coincides with the highest ethanol concentration (Fig. 1). The addition of yeast cells cultivated under other conditions did not affect the number of viable bacterial cells (Table 2). Bacterial contaminants compete for the amount of fermentable sugars and micronutrients, as well as increase the amount of inhibitors, e.g., organic acids [15], which may influence ethanol production [5]. Even though the number of viable bacterial cells observed in our study is below the amount that is commonly believed to influence ethanol production [3, 15], it cannot be ruled out that the reduction of bacterial cells in our investigation is indeed important. Most probably, the substrate as well as the composition of the bacterial community will have an influence on the significance of the bacterial cell number. This study has focused on the concentration of lactic acid bacteria but we have indeed indications that Acetobacteria are especially troublesome in this respect and may lead to severe reductions in yeast-cell viability as well as ethanol production.
Conclusions
If cultivation of the yeast is performed with the same substrate as the subsequent fermentation, ethanol concentration will be increased. It is therefore suggested that, under such conditions, the yeast will be able to produce a biomass with proper levels of the specific enzymes and proteins needed under suboptimal fermentation conditions in order to sustain a high performance and viability in a harsh media like SSL. With a total hexose concentration of 36 g/l, an inoculation of 0.8 g/l (dw) of the pitching agent grown in SSL/YD produces an ethanol concentration of 78% of the theoretical ethanol concentration within 12 h of fermentation. A viable yeast culture may also indirectly suppress microbial infections, which may otherwise be a problem in continuous fermentations. This investigation also suggests that a relatively low concentration of bacterial cells can significantly decrease ethanol productivity by S. cerevisiae. In an ethanol production plant with a nutrient-poor and challenging substrate, it is of utmost interest to make a well-balanced calculation on how much and how well adapted yeast culture that is optimal for usage as pitching agents under these conditions.
References
Alkasrawi M, Rudolf A, Lidén G, Zacchi G (2006) Influence of strain and cultivation procedure on the performance of simultaneous saccharification and fermentation of steam pretreated spruce. Enzym Microbiol Technol 38:279–286
Almeida JRM, Modig T, Petersson A, Hahn-Hägerdal B, Lidén G, Gorwa-Grauslund MF (2007) Increased tolerance and conversion of inhibitors in lignocellulosic hydrolysates by Saccharomyces cerevisiae. J Chem Technol Biotechnol 82:340–349
Barbour EA, Priest FG (1988) Some effects of Lactobacillus contamination in scotch whiskey fermentations. J Inst Brew 94:89–92
Basilio ACM, de Araujo PRL, de Morais JOF, da Silva Filho EA, de Morais MA Jr, Simoes DA (2008) Detection and identification of wild yeast contaminants of the industrial fuel ethanol fermentation process. Curr Microbiol 56:322–326
Bischoff KM, Liu S, Leathers TD, Worthington RE, Rich JO (2009) Modeling bacterial contamination of fuel ethanol fermentation. Biotechnol Bioeng 103:117–122
Delgenes JP, Moletta R, Navarro JM (1996) Effects of lignocellulose degradation products on ethanol fermentations of glucose and xylose by Saccharomyces cerevisiae, Zymomonas mobilis, Pichia stipitis, and Candida shehatae. Enzym Microbiol Technol 19:220–225
Ingledew WM (2009) Yeasts: physiology, nutrition and ethanol production. In: Ingledew WM, Kelsall DR, Austin GD, Kluhspies C (eds) The alcohol textbook, 5th edn. Nottingham University Press, Nottingham, pp 101–113
Ivorra C, Pérez-Ortìn IE, del Olmo M (1999) An inverse correlation between stress resistance and stuck fermentations in wine yeasts. A molecular study. Biotechnol Bioeng 64:668–708
Laluce C, Tognolli JO, de Oliviera KF, Souza CS, Morais MR (2009) Optimization of temperature, sugar concentration, and inoculum size to maximize ethanol production without significant decrease in yeast cell viability. Appl Microbiol Biotechnol 83:627–637
Larsson S, Reimann A, Nilvebrant N-O, Jönsson LJ (1999) Comparison of different methods for the detoxification of lignocellulose hydrolyzates of spruce. Appl Biochem Biotechnol 77:91–103
Lopez MJ, Nichols NN, Dien BS, Moreno J, Bothast RJ (2004) Isolation of microorganisms for biological detoxification of lignocellulosic hydrolysates. Appl Microbiol Biotechnol 64:125–131
Martinez A, Rodriguez ME, York SW, Preston JF, Ingram LO (2000) Effects of Ca(OH)2 treatments (“overliming”) on the composition and toxicity of bagasse hemicellulose hydrolyzates. Biotechnol Bioeng 69(5):526–536
McCaig R, Bendiak DS, Dirk S (1985) Yeast handling studies. I. Agitation of stored pitching yeast. Techn Quart Master Brew Assoc Am 22:172–176
McCaig R, Bendiak DS (1985) Yeast handling studies. II. Temperature of storage of pitching yeast. Techn Quart Master Brew Assoc Am 22:177–180
Narendranath NV, Thomas KC, Ingledew WM (2001) Effects of acetic acid and lactic acid on the growth of Saccharomyces cerevisiae in minimal media. J Indust Microbiol Biotech 26:171–177
Nilsson A, Norbeck J, Oelz R, Blomberg A, Gustafsson L (2001) Fermentative capacity after cold storage of baker’s yeast is dependent on the initial physiological state but not correlated to the levels of glycolytic enzymes. Inter J Food Microbiol 71:111–124
Olsson L, Hahn-Hägerdal B (1993) Fermentative performance of bacteria and yeast in lignocellulose hydrolysates. Process Biochem 28:249–257
Palmqvist E, Hahn-Hägerdal B, Szengyel Z, Zacchi G, Rèczey K (1997) Simultaneous detoxification and enzyme production of hemicellulose hydrolyzates obtained after steam pretreatment. Enzyme Microbiol Technol 20:286–293
Parawira W, Tekere M (2010) Biotechnological strategies to overcome inhibitors in lignocellulose hydrolysates for ethanol production: review. Crit Rev Biotechnol. doi: 10.3109/07388551003757816
Schimz K-L (1980) The effect of sulfite on the yeast Saccharomyces cerevisiae. Arch Microbiol 125:89–95
Silva CJSM, Roberto IC (2001) Improvement of xylitol production by Candida guilliermondii FTI 20037 previously adapted to rice straw hemicellulosic hydrolysate. Lett Appl Microbiol 32:248–252
Sjöström E (1981) Wood chemistry—fundamentals and applications. Academic Press, New York
Skinner AK, Leathers TD (2004) Bacterial contaminants of fuel ethanol production. J Ind Microbiol Biotechnol 31:401–408
Thomsson E, Larsson C, Albers E, Nilsson A, Fransén CJ, Gustafsson L (2003) Carbon starvation can induce energy deprivation and loss of fermentative capacity in Saccharomyces cerevisiae. Appl Environ Microbiol 69:3251–3257
Zhu JJ, Yong Q, Xu Y, Yu S-Y (2009) Comparative detoxification of vacuum evaporation/steam stripping combined with overliming on corn stover prehydrolyzate. Energy Environ Technol ICEET `09. International conference. doi: 10.1109/ICEET.2009.523
Acknowledgments
Financial support by the Kempe Foundation and the county administrative board of Västernorrland is gratefully acknowledged. Special thanks are also given to Domsjö Fabriker for their cooperation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Johansson, E., Brandberg, T. & Larsson, C. Influence of cultivation procedure for Saccharomyces cerevisiae used as pitching agent in industrial spent sulphite liquor fermentations. J Ind Microbiol Biotechnol 38, 1787–1792 (2011). https://doi.org/10.1007/s10295-011-0965-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-011-0965-0