Abstract
In the region of Murcia (southeast Spain), sweet pepper has been grown as a monoculture in greenhouses for many years. Until 2005, when it was banned, soils were disinfested with methyl bromide (MB) to control pathogens and to prevent soil fatigue effects. The genus Fusarium plays an important role in the microbiological component associated with yield decline in pepper monocultures. In the present study, soils were treated with manure amendments, alone (biofumigation, B) or in combination with solarization (biosolarization, BS), with or without the addition of pepper plant residues. The B and BS treatments were compared with a treatment using MB. The extent of disinfestation was measured from the density of Fusarium spp. isolated from the soil before and after the respective treatments. Three different species were systematically isolated: Fusarium oxysporum, Fusarium solani and Fusarium equiseti. The repeated use of manure amendments with pepper crop residues, without solarization, was unable to decrease the Fusarium spp. density (which increased from 2,047.17 CFU g−1 to 3,157.24 CFU g−1 before and after soil disinfestation, respectively), unlike MB-treated soil (in which the fungi decreased from 481.39 CFU g−1 to 23.98 CFU g−1). However, the effectiveness of the repeated application of BS in diminishing doses (with or without adding plant residues) on Fusarium populations (reductions greater than 72%) was similar to or even greater than the effect of MB.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sweet pepper (Capsicum annuum L.) has been grown as a monoculture in greenhouses in Murcia (southeast Spain) for many years [29]. The crop-growing season runs from November/December to August/September [30].
To control the main pathogens of the crop (Phytophthora spp. and Meloidogyne incognita Chitwood) [48] and to avoid crop decline effects [56] associated with repeated monoculture, soils used to be disinfested every year with methyl bromide (MB) until it was banned in 2005 [49]. Losses in yield of 20% have been attributed to the lack of disinfestations after two consecutive sweet pepper crops in soils without soil-borne pathogens [18]. Bouhot [6] reported this effect in horticultural crops and referred to “soil fatigue” (soil sickness according to Katan and Vanachter [25]) as a reduction in plant development and a loss of productive capacity. Hartemink [22] described losses of yield in many soils associated with the intensification or crop repetition, which can be recovered with soil disinfestation or the addition of organic matter. The mitigation of soil fatigue with soil disinfestation underlines the importance of the microbiological component of soils [6], which is, perhaps, even more important than any change produced in their chemical and physical properties [41]. This effect, which is specific to sweet pepper [20] and with a very strong microbiological character [31, 32], may be associated with a build-up of the density of non-pathogenic fungi during the growing season [33], Fusarium spp. being of particular relevance in this respect, as supported by previous observations [54]. In other crops, such as sugar cane, this phenomenon is called yield decline [7, 8, 16] or soil fertility decline in the soils of Sub-Saharan Africa [22]. In sweet pepper, Fusarium species seem to have no parasitic implications per se [2]. According to Bonanomi et al. [5], non-pathogenic Fusaria are common components of soil microbial communities and are strongly antagonistic to pathogenic Fusaria. In crops such as ginseng, Fusarium solani (Mart.), Fusarium oxysporum (Schlechtend.) and Fusarium equiseti (Corda) are reported to cause seed decay and the damping-off of seedlings [43].
Since the prohibition of MB, finding alternatives has been a great challenge [24]. One such alternative, the application of amendments of different origin (biofumigation, B) alone [8] or in combination with soil solarization (biosolarization, BS), has been broadly studied in many crops as a method of soil disinfestation [3, 15, 45] and its effectiveness has been demonstrated.
Biofumigation (B) is a term that refers to the suppression of soil-borne pathogens and pests by decomposing organic material, including agricultural by-products or manure [14, 27, 35]. The volatile chemicals released during the process have the capacity to reduce fungal, bacterial and nematode pathogens [39] and weeds [34]. The response of pathogen populations to organic manure amendments may be a reliable indicator only for some organic matter types, e.g. crop residues and wastes with a low C/N ratio [5]. Soil solarization (S) is a hydrothermal method based on trapping the radiant energy of soil by placing plastic sheets on moistened soil during periods of high ambient temperature [4, 11], and, as such, it is indicated for areas characterized by a warm climate [52]. Heating the soil surface over a period of several weeks helps to control pathogenic fungi and phytoparasitic nematodes, but may also have several indirect effects on soil biota, increasing competition or stimulating the density of thermal-tolerant organisms [10]. The combination of these two treatments, B and S, is called biosolarization (BS) and is considered to be a suitable soil disinfestation method for sweet pepper greenhouses [18, 21, 42, 50]. The effectiveness of soil disinfestation has been shown to be stable with time [19, 20]. Other beneficial aspects of BS are that the chemical and physical properties of soil are enhanced [13, 50] and that good levels of pathogen control are achieved, accompanied by an acceptable marketable yield, the overall effects being similar to those of MB [50].
The effect of soil disinfestation on fungal populations in general [33] and on Fusarium populations in particular has been assessed. In this work, the effect on the genus Fusarium is only part of the overall effect of fumigation measured on total non-pathogenic fungi in greenhouse-grown sweet pepper. The main objective of this research was to evaluate the effect of organic amendments, with or without S and the repeated application, on the diversity and density of soil populations of Fusarium spp. in sweet pepper crops and to compare the results with those obtained using MB.
Materials and methods
Treatments and experimental design
The experiments were performed over 3 years in greenhouse A and over 1 year in greenhouse B. Both greenhouses are located at the same experimental station, in the middle of a pepper-growing area at Campo de Cartagena, Murcia (southeast Spain), with a mean annual temperature of 20°C and an average rainfall of 300 mm. The soil is clay loam, with the following characteristics: pH 7.5 and 2.15% organic matter in greenhouse A and pH 7.8 and 1.83% organic matter in greenhouse B. When soil sampling began in 2001, a monoculture of pepper had been grown for 2 years in greenhouse B and for 4 years in greenhouse A. The soil of both greenhouses was infested by M. incognita. The effect of the application of a variety of organic amendments, alone or in combination with S, has previously been evaluated for the main pathogens of the crop [29, 57]. All treatments based on the addition of manure amendments, alone (B) or in combination with S (BS), were compared with the use of MB (Table 1). In two treatments in greenhouse B, the use of plant residues, in combination with manure amendments (B + pepper plant residues) or with biosolarization (BS + pepper plant residues), was evaluated. A randomised complete-block design with three replicates was used in each greenhouse. In greenhouse B, four treatments were evaluated in 2003. While the same number of treatments were also evaluated in greenhouse A in 2001 and 2002, five treatments were assessed in greenhouse A in 2003. In the case of BS, in greenhouse A, a repetition of the process was evaluated in the same plots every year (BS 2nd, 3rd, 4th, 5th and 6th reiteration). The BS plots that, in a particular year, had been subjected to “x” reiterations were noted to have been subjected to “x + 1” reiterations of BS the following year, although at the same time, the rate of amendment was reduced each year, while the C/N ratio was maintained. The organic amendment reduction programme was: 7.5 kg m−2 for plots previously subjected to two applications of BS, 6 kg m−2 for plots subjected to three applications of BS, 4.5 kg m−2 for plots subjected to four applications of BS and 2.5 kg m−2 for plots subjected to five or six applications of BS. The surface of each plot was 54 m2 in greenhouse A and 60 m2 in greenhouse B.
The soil was prepared with deep ploughing, the same as when chemical fumigation is to be performed. Soils fumigated with MB (30 g m−2 year−1) during the preceding three growing seasons received 2.5 kg m−2 of manure (2 kg m−2 of fresh sheep manure, FSM, + 0.5 kg m−2 of chicken manure, CM) to maintain the organic matter level. The applied manure had the following characteristics every year: pH 8.24 and EC 5.06 dS m−1 for sheep manure and pH 7.44 and EC 5.50 dS m−1 for chicken manure, organic C content of 403 g kg−1 for sheep manure and 247 g kg−1 for chicken manure, total N 25.2 g kg−1 and 27.0 g kg−1, total P 5.8 g kg−1 and 34.2 g kg−1, and total K 26.4 g kg−1 and 18.4 g kg−1, respectively. The mixed manure was added to the soil to a depth of 20 cm using a rotary tiller and, just after the incorporation of the organic amendments, a drip-irrigation system was installed to moisten the soil (using 3 L h−1 emitters spaced 0.40 m apart in the same row, with 0.50 m between drip rows). Subsequently, a plastic cover was applied (for BS treatments) and the soil was irrigated for 3 h on two consecutive days. The soil remained covered by plastic sheets for about 10 weeks (Table 2). Therefore, the only chemical product used (MB) was applied in November, when the soil was covered for about 7 days, just before the beginning of a new growing season. MB (Metabrom, 98% a.i., Dead Sea Bromide, Israel) at 30 g m−2 was applied as a cold fumigation. In BS plots, the soil remained covered by a transparent, low-density polyethylene film (PE, 50 μm) and the MB-treated soils were covered by a co-extruded, three-layer, virtually-impermeable film (VIF, composed of two polyethylene layers with one polyamide film, 50-μm thick, in the middle).
In Table 2, the dates of collecting soil samples before and after the fumigation treatments are indicated for both greenhouses and for the different years of the study (in the case of greenhouse A).
Soil sampling
Populations of Fusarium spp. were estimated in each plot before and after treatment as colony-forming units per gram of soil (CFU g−1).
Five points were selected randomly from the upper 15 cm depth in each plot. A soil sub-sample (approximately 250 g) was taken from each point and the five sub-samples were mixed together. Samples (1,250 g) were air-dried in the laboratory for 1 week, crushed and sieved (0.2-mm mesh size).
Isolation, quantification and identification of Fusarium spp.
Fusarium population densities in soil samples were assessed by dilution plating, using Komada medium [28]. Although this medium was designed to be selective for Fusarium oxysporum, it is also suitable for the recovery of several other species of Fusarium from soils [3, 43, 47, 54, 56]. Each soil sample was held in a plastic bag that was shaken just before carrying out the next step. For each sample to be analysed, four plastic flasks were prepared with 10 g of soil each. Each flask was weighed before and after analysis [46, 51]. The difference determined the weight of the analysed soil in each flask. Ten millilitres of medium cooled at 40°C were poured into each Petri dish and then a small amount of soil (20 mg per plate) was transferred to it. The plate was rotated by hand in a broad and slow, swirling motion to disperse the soil suspension [12, 47]. Four Petri dishes were prepared for each plastic flask: 16 dishes per soil sample or repetition (one repetition corresponds to one block in the greenhouse; 48 dishes per fumigation treatment). The plates were incubated under continuous fluorescent light at 25–28°C for 5–7 days. Then, the number of Fusarium colonies was counted and classified as F. oxysporum, F. solani and F. equiseti. The Fusarium population density was expressed as the number of colony-forming units (CFUs) per gram of dried soil (mean of 16 replicates). Fusarium species were identified according to Gerlach and Nirenberg [17], Nelson et al. [38] and Nelson [37], using a microscope at 100–400× magnification. With respect to the identification of the three species, F. oxysporum on Komada medium shows rapid growth and the white aerial mycelium becomes tinged with purple. On the same medium, F. solani growth is rapid, with abundant aerial mycelium. The surface is soon covered with confluent sporodochia that give the surface a cream, blue–green or blue colour. The growth of F. equiseti is also rapid, with dense aerial mycelium that is white at first and becomes tan to brown as the culture ages. The description is similar using potato dextrose agar (PDA) medium [38].
Statistical analysis
The statistical treatment of data was carried out using the STATGRAPHICS Plus 5.1 statistical package software (StatPoint, Inc., 2325 Dulles Corner Boulevard, Suite 500 Herndon, VA 20171, USA). The data obtained were subjected to statistical analysis using analysis of variance (ANOVA), comparing the means of the treatments with the least significant difference (LSD) test (P < 0.05). The √(x + 0.5) transformation was used to normalise the data for all of the analyses.
Results and discussion
Three species of Fusarium were isolated systematically from the soil sample in Komada medium in the 3 years of the study: F. oxysporum, F. solani and F. equiseti. Generally, the most common species isolated was F. solani, for all treatments, before and after the disinfestation treatments. F. moniliforme was sporadically isolated in only one analysis (2.78 CFU g−1; in greenhouse A before disinfestation treatment with MB in the first year of study, Table 4), and, for this reason, was not expressed in the analysis.
The columns labelled “Before” in Tables 3–6 represent the density of soil Fusarium spp. isolated just before the fumigation treatments. These data are related to the accumulative density after one sweet pepper crop. The columns labelled “After” in Tables 3–6 refer to the density of Fusarium spp. isolated after the fumigation treatments and it is related to the density before the plantation of a new sweet pepper crop.
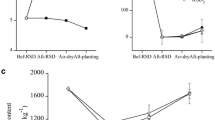
For greenhouse B (Table 3), when the applications of manure amendments (with or without a plastic cover and with or without the addition of pepper plant residues of the previous crop) were compared, the results showed that only the second year of BS with plant residues was able to reduce the Fusarium spp. inoculum to approximately the same extent as MB, although the percentage of inoculum reduction was inferior (72% reduction compared with MB 95.02% reduction). In addition, it is important to indicate that only BS was able to reduce F. solani populations, presumably the species that was mainly responsible for the crop decline effects attributed to the Fusarium genus. However, Fusarium showed an increased density in plots repeatedly biofumigated with manure and pepper plant residues of the previous crop. Particularly, in the latter case, the initial Fusarium spp. density before carrying out soil fumigation was higher (2,047.17 CFU g−1) with respect to the other treatments (481.39 CFU g−1 for MB, 52.10 CFU g−1 for BS 2nd and 61.89 CFU g−1 for BS 2nd + plants). In most cases, F. solani was the most abundant species isolated both before and after disinfestation treatments, although F. oxysporum provided two exceptions to this (Table 3).
In greenhouse A, all BS treatments and their reiterations had the same effect as MB in 2001 and 2002 (Tables 4 and 5), with even better percentages of reduction (>96%) of the Fusarium spp. inoculum. In 2001 in particular, all treatments involving repeated application of BS led to significantly decreased (P < 0.05) Fusarium spp. densities (with starting amounts of above 6,000 CFU g−1 soil), again with better effectiveness than MB (with starting amounts of above 5,000 CFU g−1 soil, Table 4). The effect on BS plots in the second year of application (BS 2nd reiteration) must be emphasised, since no Fusarium colony was isolated on Komada medium for any plot after this treatment. In the following year, 2002, all treatments reduced the Fusarium spp. density, the plots subjected to 4 years of repeated biosolarization (BS 4th reiteration) being particularly effective. The analyses made for plots that had been biosolarized five times (BS 5th reiteration) pointed to the absence of Fusarium spp. colonies either before or after soil disinfestation.
In 2003, MB did not produce any significant differences (P > 0.05) between the pre- and post-fumigation populations (Table 6). However, all treatments involving repeated application of BS significantly decreased (P < 0.05) Fusarium populations, with reductions exceeding 90%, although the average Fusarium spp. density before fumigation treatments was below 1,000 CFU g−1 in all cases. In repeatedly treated BS plots, Fusarium populations were significantly reduced (P < 0.05) and the effect was the same every year, underlining the stability of the effect.
Selective analysis of Fusarium spp. was performed to better differentiate the species and to provide a quantitative comparison between treatments. Fusarium species are not all equally adapted to survival in soil. Burgess [9] separated the genus into three groups according to the mode of survival: air Fusaria, soil Fusaria and Fusaria of subterranean habitats. Soil Fusaria show some characteristics that make them suitable to life in this substrate. Both F. solani and F. oxysporum are included in this group. Fusarium equiseti is a cosmopolitan, soil-inhabiting fungus that has frequently been reported in arid and semi-arid regions [9, 47].
The addition of soil to a medium in sub-fusion state has been used to study the dynamics of the most common species of Fusarium. However, the dilution plate method [58] has some weaknesses, due to its selectiveness for some Fusarium species and some propagules in the soil [46]. McMullen and Stack [36] reported that the recovery of Fusarium was consistently influenced by the isolation technique and not by variable site factors (grazed/ungrazed or cultivated field) or sampling year. On the other hand, some authors, such as Parkinson et al. [40], suggest that it is impossible to devise a single isolation technique by which all fungal forms present in the soil can be isolated, since any isolation technique is selective to a certain extent. Despite these limitations, the technique has been used in qualitative studies of soil fungi from cultivated soils: tomato crops [54], asparagus [44, 57], soybean [26], maize [53], ginseng [43] or carnation [54, 59, 60], and from uncultivated soils: prairies [36], Pinus [46] and beach sands [55]. The main advantage is its great simplicity and speed. As Santos et al. [51] mentioned, the medium used has high selectivity for Fusarium species and it is easy to differentiate some of them based on pigmentation, aspect and colony growth rate. This method has also been used by Blok et al. [3] and is particularly suited to the analysis of soils from southeast Spain.
Soils in southeast Spain tend to be alkaline and the annual average temperature is relatively high, aspects which are very similar to places such as Egypt [1] and quite favourable for Fusarium spp., which may account for its high frequency. According to Steinkellner and Langer [53], environmental conditions such as climate, soil moisture, soil type and fertility are known to affect microbial soil populations and especially the range of Fusarium isolated from soil [9]. The genus Fusarium survives better in soil in dry than in wet conditions and may prefer a combination of such conditions with high temperatures.
Comparison of mean values of the isolated colonies for the different microenvironments reveals that there was no difference in species composition of the Fusarium spp. between chemical (MB) and non-chemical treatments (B and BS). Fusarium oxysporum, F. solani and F. equiseti were the only species isolated systematically, with F. solani being the most abundant and frequent Fusarium species isolated from soil repeatedly cultivated with sweet pepper. In this sense, the results obtained contrast with those of other works, where F. equiseti [43, 53] or F. oxysporum [23, 26, 47], respectively, were the common species isolated. In soils cultivated with tomato (another horticultural crop which is intensively cultivated in the region of Murcia), F. oxysporum was isolated abundantly and frequently, while F. oxysporum and F. roseum were the prevalent species in areas near cultivated soils [40]. All of these aspects seem to implicate F. solani as playing an essential role in the decline effects specific to sweet pepper crops.
The results indicate the following: BS has the same or an even better effect than MB for reducing populations of Fusarium spp., particularly when a moistened manure amendment is covered with a plastic film. In this sense, the results are similar to those of Blok et al. [3], who observed significant reductions in the CFU g−1 of F. oxysporum in plots cultivated with asparagus [3] and where organic amendment was incorporated and a plastic cover applied. With regards to the repetition of BS, the stability of the process with time was evident (Tables 4–6), and the Fusarium density was consistently reduced every year during which the method was repeated in the same plots, with the same effectiveness as (Tables 5 and 6) or even better than MB (96.34% reduction, Table 4). A representative example is the 2-year application of BS (BS 2nd year), the effect of which was studied three times (Tables 3, 4 and 6). The only time the Fusarium density increased was in greenhouse B (Table 3), where, it must be emphasised, the inoculum levels before the fumigation treatment were much lower (52.10 CFU g−1) than in other cases, such as greenhouse A in 2001 (6,093.81 CFU g−1; Table 4) and 2003 (705.59 CFU g−1; Table 6).
The results point to high Fusarium densities at the end of a growing season, which could be related with “soil fatigue” (Table 4) before soil disinfestation. Moreover, it might be an indicator of a cumulative effect that is produced in the absence of fumigation. In this sense, preliminary works have shown that, when some isolates of F. solani are used to inoculate young plants, symptoms such as yellowing and a reduction in plant growth have reoccurred [26].
With only one exception in plots subjected to 2 years of BS (Table 3), the effect of BS on Fusarium populations was the same, regardless of the amount of amendment added. A comparison of the same treatment with (BS 2nd + plants) or without (B 2nd + plants) a plastic cover highlights the action of the plastic: without it, the Fusarium density significantly (P < 0.05) increased (64.84% higher). Results with B alone are similar to those obtained by Scopa et al. [52] for S, indicating that this practice is beneficial to soil microflora, although other authors maintain that soil temperatures of around 50°C are detrimental to microbial biomass. In conclusion, the results contribute to our understanding of the effect of this non-chemical, simple, safe and effective method of soil disinfestations, which can be considered as suitable for organic agriculture, reducing or even eliminating Fusarium populations in soil to an extent that is similar and comparable to that obtained with MB. However, more studies are needed to understand the functionality of Fusaria and their repercussion on the loss of diversity with BS treatments and its continued reiteration.
References
Abdul Wahid OA, Moustafa AF, Ibrahim ME (1997) Soil mycoflora in tomato fields. Mycoscience 38:237–241
Alfaro A, Vegh I (1971) La “tristeza” o “seca” del pimiento producida por Phytophthora capsici Leonian. Ann INIA Ser Prot Veg 1:9–42
Blok WJ, Lamers JG, Termorshuizen AJ, Bollen GJ (2000) Control of soilborne plant pathogens by incorporating fresh organic amendments followed by tarping. Phytopathology 90:253–259. doi:10.1094/phyto.2000.90.3.253
Bonanomi G, Antignani V, Pane C, Scala F (2007) Suppression of soilborne fungal diseases with organic amendments. J Plant Pathol 89(3):311–324
Bonanomi G, Antignani V, Capodilupo M, Scala F (2010) Identifying the characteristics of organic soil amendments that suppress soilborne plant diseases. Soil Biol Biochem 42:136–144. doi:10.1016/j.soilbio.2009.10.012
Bouhot D (1982) La fatigue des sols. Position du problème et principe du diagnostic. Colloques de lNRA, INRA, París 17:9–12
Bramley RGV, Ellis N, Nable RO, Garside AL (1996) Changes in soil chemical properties under long-term sugar cane monoculture and their possible role in sugar yield decline. Aust J Soil Res 34(6):967–984
Bulluck LR III, Brosius M, Evanylo GK, Ristanio JB (2002) Organic and synthetic fertility amendments influence soil microbial, physical and chemical properties on organic and conventional farms. Appl Soil Ecol 19:147–160. doi:10.1016/S0929-1393(01)00187-1
Burgess LW (1981) General ecology of the Fusaria. In: Nelson PE, Toussoun TA, Cook RJ (eds) Fusarium: diseases, biology, and taxonomy. Pennsylvania State University Press, University Park, PA, pp 225–235
Culman SW, Duxbury JM, Lauren JG, Thies JE (2006) Microbial community response to soil solarization in Nepal’s rice–wheat cropping system. Soil Biol Biochem 38:3359–3371. doi:10.1016/j.soilbio.2006.04.053
DeVay JE, Katan J (1991) Soil solarization: historical perspectives, principles and uses. In: DeVay JE, Katan J (eds) Soil solarization. CRC Press, Boca Raton, FL, USA, pp 23–37, 267
El-Amin AN, Saadabi AMA (2007) Contribution to the knowledge of soil fungi in Sudan rhizosphere mycoflora of sugarcane at Kenana Sugar Estate. Int J Botany 3(1):97–102. doi:10.3923/ijb.2007.97.102
Fernández P, Guerrero MM, Ros C, Bello A, García A, Lacasa A (2004) Efecto de la biofumigación con solarización sobre la características físicas y químicas de los suelos de pimiento del sureste español. Actas de Horticultura 42:6–12
Flores P, Lacasa A, Fernández P, Hellín P, Fenoll J (2008) Impact of biofumigation with solarization on degradation of pesticides and heavy metal accumulation. J Environ Sci Health B 43:513–518. doi:10.1080/03601230802174698
Gamliel A, Stapleton JJ (1993) Effect of chicken compost or ammonium phosphate and solarization on pathogen control, rhizosphere microorganisms, and lettuce growth. Plant Dis 77:886–891. doi:10.1094/PD-77-00886
Garside AL (1996) Soil components of yield decline in sugarcane. In: MacEwan RJ, Carter MR (eds) Proceedings of the international symposium on advances in soil quality for land management: science, practice, and policy, University of Ballarat, Ballarat, Victoria, Australia, 17–19 April 1996, pp 93–96
Gerlach W, Nirenberg H (1982) The genus Fusarium. A pictorial atlas. Mitt Biol Bundesanst Land-Forstw 209:1–406
Guerrero MM, Ros C, Martínez MA, Martínez MC, Lacasa A (2005) Effects of biofumigation plus solarization on crop production. In: Proceedings of the annual meeting of the association for the advancement of industrial crops: international conference on industrial crops and rural development, Murcia, Spain, 17–21 September 2005, pp 225–228
Guerrero MM, Ros C, Martínez MA, Martínez MC, Barceló N, Lacasa A (2005) Biofumigación con solarización. Un método estable de desinfección de suelos de invernadero. Actas Portuguesas de Horticultura. V Congreso Ibérico de Ciencias Hortícolas. IV Congreso Iberoamericano de Ciencias Hortícolas. Oporto, Portugal. Resúmenes 7(3):111–115
Guerrero MM, Martínez MA, Martínez MC, Barceló N, Lacasa A, Ros C, Guirao P, Bello A, López JA (2005) Biofumigation plus solarisation efficacy for soil disinfestation in sweet pepper greenhouses in the Southeast of Spain. In: Vanachter A (ed) Proceedings of the VI International Symposium on Chemical and Non-Chemical Soil and Substrate Fumigation. Acta Hortic 698:293–298
Guerrero MM, Martínez MA, Ros C, Martínez MC, Bello A, Lacasa A (2006) Biofumigation vs. biofumigation plus solarization to control Meloidogyne incognita in sweet pepper. In: Proceedings of the meeting of the IOBC/WPRS working group integrated control in protected crops, Mediterranean climate, Murcia, Spain, 14–18 May 2006, pp 313–318
Hartemink AE (2006) Soil fertility decline: definitions and assessment. In: Lal R (ed) Encyclopedia of soil science, vol 2, 2nd edn. CRC Press, Boca Raton, FL, USA, p 1923. doi:10.1081/E-ESS-120041235
James RL (2000) Effects of a 2-year fallow period on soil populations of Fusarium, Trichoderma and Pythium species alter incorporating corn plant-residues. USDA Forest Service, Coeur D′Alene Nursery, Idaho, report 00-17, p 11
Katan J (2005) Soil disinfestation: one minute before methyl bromide phase out. In: Vanachter A (ed) Acta Hortic 698:19–26
Katan J, Vanachter A (2010) Crop and soil health following soil disinfestation. In: Vanachter A (ed) Acta Hortic (in press)
Killebrew JF, Roy KW, Abney TS (1993) Fusaria and other fungi on soybean seedlings and roots of older plants and interrelationships among fungi, symptoms and soil characteristics. Can J Plant Pathol 15:139–146
Kirkegaard JA, Matthiessen JN (2004) Developing and refining the biofumigation concept. Agroindustria 3:233–239
Komada H (1975) Development of a selective medium for quantitative isolation of Fusarium oxysporum from natural soil. Rev Plant Protect Res 8:114–125
Lacasa A, Guerrero MM, Guirao P, Ros C (2002) Alternatives to methyl bromide in sweet pepper crops in Spain. In: Proceedings of the 3rd international conference on alternatives to methyl bromide, Sevilla, Spain, 5–8 March 2002
López A, Guirao P (1998) El bromuro de metilo y el cultivo del pimiento en el Campo de Cartagena. FECOAM Informa 101:26–31
Martínez MA, Guerrero MM, Martínez MC, Barceló N, Guirao P, Ros C, Lacasa A, Tello J (2003) La fatiga del suelo en cultivos convencionales y ecológicos de pimiento en invernadero. Actas de Horticultura 39:36–37
Martínez MA, Lacasa A, Guerrero MM, Ros C, Martínez MC, Bielza P, Tello J (2006) Effects of soil disinfestation on fungi in greenhouses planted with sweet peppers. In: Castañé C, Sánchez JA (eds) Integrated control in protected crops, mediterranean climate. IOBC OILB Bull 29(4):301–306
Martínez MA, Lacasa A, Tello JC (2010) Ecología de la microbiota fúngica de los suelos de los invernaderos de pimiento y su interés agronómico. Publicaciones del Ministerio de Medio Ambiente y Medio Rural y Marino, Ambienta, p 374
Mattner SW, Porter IJ, Gounder RK, Shanks AL, Wren DJ, Allen D (2008) Factors that impact on the ability of biofumigants to suppress fungal pathogens and weeds of strawberry. Crop Prot 27:1165–1173. doi:10.1016/j.cropro.2008.02.002
Matthiessen JN, Kirkegaard JA (2006) Biofumigation and enhanced biodegradation: opportunity and challenge in soilborne pest and disease management. Crit Rev Plant Sci 25:235–265. doi:10.1080/07352680600611543
McMullen MP, Stack RW (1983) Effects of isolation techniques and media on the differential isolation of Fusarium species. Phytopathol 73(3):458–462
Nelson PE (1992) Taxonomy and biology of Fusarium moniliforme. Mycopathology 117:29–36. doi:10.1007/BF00497276
Nelson PE, Touson TA, Marasas WFO (1983) Fusarium species. An illustrated manual for identification. The Pennsylvania University Press, University Park, PA, USA and London, UK, 193 pp
Oka Y (2010) Mechanisms of nematode suppression by organic soil amendments—a review. Appl Soil Ecol 44:101–115. doi:10.1016/j.apsoil.2009.11.003
Parkinson D, Gray TRG, Williams ST (1971) Methods for studying the ecology of soil-microorganisms. International Biological Programme (IBP) handbook 19. Blackwell Scientific Publications, Oxford, 116 pp
Pérez-Piqueres A, Edel-Hermann V, Alabouvette C, Steinberg C (2006) Response of soil microbial communities to compost amendments. Soil Biol Biochem 38(3):460–470
Piedra Buena A, García-Álvarez A, Díez-Rojo MA, Ros C, Fernández P, Lacasa A, Bello A (2007) Use of pepper crop residues for the control of root-knot nematodes. Bioresour Technol 98:2846–2851. doi:10.1016/j.biortech.2006.09.042
Punja ZK, Wan A, Rahman M, Goswami RS, Barasubiye T, Seifert KA, Lévesque CA (2008) Growth, population dynamics, and diversity of Fusarium equiseti in ginseng fields. Eur J Plant Pathol 121:173–184. doi:10.1007/s10658-007-9261-2
Quilambaqui MA (2005) Aislamiento e identificación de especies de Fusarium spp. asociadas al declinamiento del espárrago (Asparagus officinalis L.) en cinco municipios de Guanajuato, México). Revista Tecnológica ESPOL 18:135–140
Ramírez-Villapudua J, Munnecke DE (1988) Effect of solar heating and soil amendments of cruciferous residues on Fusarium oxysporum f. sp. conglutinans and other organisms. Phytopathology 78:289–295
Rodríguez-Molina MC, Torres-Vila LM, Tello Marquina JC, Blanco Santos A, Palo Núñez EJ (2001) Caracterización de las poblaciones de Fusarium Link de suelos de dehesas de Badajoz. Bol San Veg Plagas 27:433–437
Rodríguez-Molina MC, Tello-Marquina J, Torres-Vila LM, Bielza-Lino P (2000) Micro-scale systematic sampling of soil: heterogeneity in populations of Fusarium oxysporum, F. solani, F. roseum and F. moniliforme. J Phytopathol 148:609–614. doi:10.1111/j.1439-0434.2000.00575.x
Ros C, Guerrero MM, Martínez MA, Barceló N, Martínez MC, Rodríguez I, Lacasa A, Guirao P, Bello A (2005) Resistant sweet pepper rootstocks integrated into the management of soilborne pathogens in greenhouse. In: Vanachter A (ed) Proceedings of the VI International Symposium on Chemical and Non-Chemical Soil and Substrate Fumigation. Acta Hortic 698:305–310
Ros M, Hernández MT, García C, Bernal A, Pascual JA (2005) Biopesticide effect of green compost against Fusarium wilt on melon plants. J Appl Microbiol 98:845–854
Ros M, García C, Hernández MT, Lacasa A, Fernández P, Pascual JA (2008) Effects of biosolarization as methyl bromide alternative for Meloidogyne incognita control on quality of soil under pepper. Biol Fertil Soils 45:37–44. doi:10.1007/s00374-008-0307-1
Santos M, Diánez F, de Cara M, Tello JC (2008) Possibilities of the use of vinasses in the control of fungi phytopathogens. Bioresour Technol 99:9040–9043. doi:10.1016/j.biortech.2008.04.032
Scopa A, Candido V, Dumontet S, Miccolis V (2008) Greenhouse solarization: effects on soil microbiological parameters and agronomic aspects. Sci Hortic 116:98–103. doi:10.1016/j.scienta.2007.11.008
Steinkellner S, Langer I (2004) Impact of tillage on the incidence of Fusarium spp. in soil. Plant Soil 267:13–22
Tello JC, Lacasa A (1990) Fusarium oxysporum en los cultivos intensivos del litoral mediterráneo de España. Bol San Veg Plagas Fuera de Serie 19:190
Tello JC, Rodríguez MC, Lacasa A (1992) Importancia de Fusarium en las arenas de playas de España. ITEA 88:77–92
Tello JC, Lacasa A (2004) Las enfermedades de origen edáfico y su control en los pimentonales del Campo de Cartagena: una interpretación retrospectiva del sexenio 1979–1985. Phytoma España 157:17–27
Vujanovic V, Hamel C, Yergeau E, St-Arnaud M (2006) Biodiversity and biogeography of Fusarium species from northeastern North American asparagus fields based on microbiological and molecular approaches. Microbial Ecol 51(2):242–255. doi:10.1007/s00248-005-0046-x
Warcup JH (1957) Studies on the occurrence and activity of fungi in a wheat-field soil. Trans Brit Mycol Soc 40:237–262
Wolcan SM, Lori GA, Monaco CI (1999) Fusarium moniliforme, nuevo patógeno de los cultivares asiáticos de Lilium. Investigación Agraria Producción Vegetal 14:117–130
Wolcan SM, Lori GA, Ronco BL, Perelló AE, Alippi HE (2000) Density of Fusarium spp. in soils from commercial carnation crops in Argentina. Fitopatologia Brasileira 25:161–167
Acknowledgments
M.A. Martínez acknowledges a Ph.D. grant and financial support from the Instituto Nacional de Investigación y Tecnología Agraria y Alimentaria (INIA), Ministerio de Ciencia y Tecnología, research project 0T03-006-C05-03, and the Consejería de Agricultura, Agua y Medio Ambiente de la Región de Murcia, project PR00-003. The authors wish to thank Dr. David Walker and Phillip Thomas for the manuscript revision.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the BioMicroWorld 2009 Special Issue.
Rights and permissions
About this article
Cite this article
Martínez, M.A., Martínez, M.C., Bielza, P. et al. Effect of biofumigation with manure amendments and repeated biosolarization on Fusarium densities in pepper crops. J Ind Microbiol Biotechnol 38, 3–11 (2011). https://doi.org/10.1007/s10295-010-0826-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-010-0826-2