Abstract
Phenotypic mutants of Sporosarcina pasteurii (previously known as Bacillus pasteurii) (MTCC 1761) were developed by UV irradiation to test their ability to enhance urease activity and calcite production. Among the mutants, Bp M-3 was found to be more efficient compared to other mutants and wild-type strain. It produced the highest urease activity and calcite production compared to other isolates. The production of extracellular polymeric substances and biofilm was also higher in this mutant than other isolates. Microbial sand plugging results showed the highest calcite precipitation by Bp M-3 mutant. Scanning electron micrography, energy-dispersive X-ray and X-ray diffraction analyses evidenced the direct involvement of bacteria in CaCO3 precipitation. This study suggests that calcite production by the mutant through biomineralization processes is highly effective and may provide a useful strategy as a sealing agent for filling the gaps or cracks and fissures in any construction structures.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Microorganisms and microbially mediated mineralization processes are active in almost every environment on earth [20, 32] and possibly in extraterrestrial systems as well [21]. In natural environments, chemical CaCO3 precipitation (Ca2+ + CO3 2− → CaCO3↓) is accompanied by biological processes, both of which often occur simultaneously or sequentially. Microbes from soils and aqueous media have been frequently reported to induce the precipitation of calcium carbonate mineral phases in both natural and laboratory settings [19]. Because of this, microbial activity is regarded as an important player in the formation of carbonate sediments and soil carbonate deposits [30]. A number of studies have investigated carbonate mineralization induced by microbes (see [39] and references therein), including that by soil bacteria [9, 29, 34]. An endospore-forming soil bacterium, S. pasteurii, participates in calcite precipitation in the environment by producing the urease enzyme. Urease (urea amidohydrolase; E.C. 3.5.1.5) catalyzes urea to produce CO2 and ammonia, resulting in an increase of pH in the surroundings where mineral ions (Ca2+ and CO3 2−) precipitate as CaCO3.
A novel technique for the remediation of damaged structural formations has been developed by employing a selective microbial plugging process in which microbial metabolic activities promote precipitation of CaCO3 in the form of calcite [17, 34]. Microbial calcite has potential for technical and industrial applications. Microbial mineral precipitation (biodeposition) technologies have already been used for consolidation of sand columns [27, 34], for repair of limestone monuments [12, 31, 37], and for remediation of cracks in concrete [4, 6, 28].
Cracking of concrete is a common phenomenon. Without immediate and proper treatments, cracks in concrete structures tend to expand further and eventually require costly repair. Use of bacteria in concrete remediation is an unorthodox concept in current concrete research. It is, however, a new approach to an old idea that a microbial mineral deposit constantly occurs in natural environments. Specifically, microbiologically induced calcite is environmentally innocuous, compared to synthetic polymers currently used for concrete repairs. The high alkaline pH of concrete is a major hindering factor to the growth of a moderate alkaliphile, Bacillus pasteurii, the commonly used soil bacterium. It grows well at an optimum pH of 9.0 and also has the ability to produce the endospore, a dormant form of the cell, to endure extreme environments. However, due to the high pH of concrete, the growth and urease activity may not be optimal to produce more calcite. Moreover, microbial CaCO3 precipitation is a complex phenomenon, and calcite precipitation is a function of the cell concentration, ionic strength, and pH of the medium [28]. In our previous study, lactose mother liquor was used as an alternative nutrient source for urease and calcite production [1]. In this study, the phenotypic mutants of S. pasteurii were developed to increase the efficiency of this bacterium to grow at high pH and to produce more urease for maximum calcite formation. The results obtained from mutants were compared with the wild type.
Materials and methods
Microorganisms, growth conditions and culture media
Sporosarcina pasteurii MTCC 1761 (Bp W) was obtained from the Institute of Microbial Technology, Chandigarh, India, and maintained in nutrient medium (pH 8.0) at 37°C. To carry out urease production and calcite precipitation experiments, filter-sterilized CaCl2 (25.2 mM) and urea solution (2%, w/v) were added to 8 g nutrient broth (HiMedia, India).
Mutagenesis
The phenotypic mutants of S. pasteurii were developed by UV irradiation. S. pasteurii was grown overnight in nutrient broth containing 2% urea solution at 37°C under shaking condition. The cells were washed twice with 0.5 M phosphate buffer (pH 8.0) and resuspended in 1 ml of the same buffer. The cells were diluted in phosphate buffer to obtain ≈4 × 10 7 cfu/ml and exposed to UV using a Philips 20-W germicidal lamp for 20 min, where a less than 10% survival rate was observed. The colonies were randomly selected and transferred onto urea agar base medium (HiMedia, India) to check the production of urease based on the intensity of pink color. Four colonies, Bp M-1, Bp M-2, Bp M-3, and Bp M-4, were selected for further studies based on their ability to produce intense pink color. The mutants were cultured at least five times to assure incapability of reverse mutation and compared with wild-type strain (Bp W) for quantitative urease production, calcite precipitation, and other parameters. The effect of different pH (in buffered media) on the growth of these mutants was also studied.
Urease assay
The urease activity was determined for all the phenotypic mutants of S. pasteurii, including the wild type, by measuring the amount of ammonia released from urea according to the phenol-hypochlorite assay method [26] at different time intervals as described by Achal et al. [1].
Protease activity
Alkaline protease activity was determined according to the method of Fukumoto et al. [16]. One milliliter of the culture was added to 5 ml of 0.6% casein and incubated at 37°C for 10 min. The reaction was stopped by adding 5 ml of a TCA mixture (36 ml of 50% (w/v) TCA, 220 ml of 1 M sodium acetate, and 330 ml of 1 M acetic acid in a total volume of 1,000 ml). The unreacted casein was precipitated, and the resulting precipitate was filtered and the optical density of the filtrate was measured at 275 nm against a blank. A standard graph was generated using 5–50 μg/ml of tyrosine. One unit of protease activity was defined as the amount of enzyme that liberated 1 μg tyrosine per min at 37°C.
Extracellular polymeric substances production
Extracellular polymeric substances (EPS) production was tested according to the procedure described by Friedman et al. [15]. Briefly, the dye Congo red (CR) was mixed in a bacterial culture (approximately 108 cfu/ml) at a concentration of 3.5 mg/l and was incubated for 30 min. The colored culture was centrifuged for 5 min at 8,000 rpm to harvest the dyed cells. The cells were washed with 1 ml milliQ water to release adsorbed CR into the water. To remove the cells, the dyed culture was centrifuged for 5 min at 8,000 rpm. Optical density was measured at 430 nm. The increase in absorbance is due to the uptake of CR by the EPS producing bacteria. The quantity (nmol) of EPS production was calculated by CR bound/108 cfu/ml.
Biofilm production
Biofilm production from all the isolates was established aseptically in nutrient broth containing calcium chloride and urea on glass plates (25 × 75 mm) using crystal violet (CV) staining method as described by Morikawa et al. [24] with minor modification. Briefly, an overnight culture of all the isolates was diluted to an OD600 of 0.5, and 1 μl was added to 99 μl nutrient medium on the glass plate and incubated at 37°C for 24 h. Then, media and loosely bound cells were removed from the glass plate by gently rinsing with sterile milliQ water, and the remaining cells and matrices were stained with 150 μl of 1% CV solution for 25 min at room temperature. After washing twice with distilled water, the CV attached to the biofilm was solubilized in 150 μl DMSO, quantified by measuring its absorbance at 570 nm and expressed in cfu/mm2.
Sand consolidation and microbial cementation
Microbiological sand plugging by all isolates was performed to study the calcite precipitation as described by Achal et al. [1]. The sand columns were divided into three layers (upper, middle, and lower layer), and each layer was individually ground and sieved through a 45-μm-diameter mesh prior to calcite estimation. Precipitated calcite from each layer was measured by the EDTA titration method [2].
SEM-EDX and XRD analysis
The morphology and chemical constituents of the bacteria and sand consolidated column was analyzed with SEM-EDX and XRD. Samples were completely dried at room temperature, then examined at accelerating voltages ranging from 30 to 35 kV by a SEM (Zeiss EVO50), which was equipped with an energy-dispersive X-ray analyzer (Bruker-AXS, QuanTax 200) for elemental analysis. Samples were gold coated with a sputter coating Emitech K575 prior to examination. Consolidated sand cores of the microbial sand column were cut open prior to examination.
XRD spectra were obtained using an X’Pert PRO diffractometer with a Cu anode (40 kV and 30 mA) and scanning from 3° to 60° 2θ. Each sand-consolidated sample was crushed and ground using a motor pestle before mounting onto a glass fiber filter using a tubular aerosol suspension chamber (TASC). The components of the sample were identified by comparing them with standards established by the International Center for Diffraction Data.
All experiments were performed in triplicate. Data were analyzed by analysis of variance (ANOVA), and the means were compared by Tukey’s test using GraphPad Prism software (version 4.0).
Results and discussions
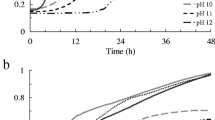
The phenotypic mutants of S. pasteurii were developed by UV irradiation and compared with the wild-type strain for their ability to grow at high pH and produce urease activity and calcite precipitation. The growth profile studied up to 168 h showed that all the mutants increased their growth till 72 h and remained stationary up to 168 h. The maximum growth was observed with mutant Bp M-3 (Fig. 1a). The pH of the medium was significantly increased with the increase in growth of these isolates. The maximum pH increase of 11.94 was recorded in the case of the Bp M-3 mutant after 168 h (Fig. 1b). To test the efficacy of these mutants to grow at different pHs, these isolates were grown in nutrient media amended with different buffers. The results showed that the wild-type strain (Bp W) and the mutant Bp M-2 were able to grow at pH ranging from 6 to 10, two mutants Bp M-1 and Bp M-4 at pH from 6.5 to 10, whereas the mutant Bp M-3 was able to grow at pH from 5 to 11. The ability to grow at high pH by Bp M-3 suggests that it can be used in building materials such as cement to enhance the calcite precipitation where the pH of the proximal environment is highly alkaline (pH 11–12).
Urease and protease activity
The mutant Bp M-3 showed the maximum urease activity (550 U/ml) compared to the other isolates, including the wild type (Fig. 2a). After 120 h, urease activity was decreased in all the isolates. The alkaline protease activity increased slowly with an increase in time till 120 h and significantly increased thereafter. The maximum protease activity was observed with the mutant Bp M-3 (Fig. 2b).
Bacteria are known to hydrolyze urea by urease for the purposes of: (1) increasing the ambient pH [8], (2) utilizing it as a nitrogen source [7], and (3) using it as a source of energy [23]. B. pasteurii is known to produce a large amount of urease in soil environments [10]. The solubility of calcite is a function of pH and affected by ionic strength in the aqueous medium [36]. We added urea and calcium chloride in the media that supports microbial growth. The bacterial cell surface with a variety of ions could nonspecifically induce mineral deposition by providing nucleation sites [13, 14]. Especially Ca2+ is not likely utilized by microbial metabolic processes, but rather accumulates outside the cell [33]. In medium it is possible that individual microorganisms produce ammonia as a result of enzymatic urea hydrolysis to create an alkaline micro-environment around the cell. The high pH of these localized areas, without a significant increase in pH in the entire medium at the beginning, apparently commences the growth of calcite crystals around the cell [5]. In this study, due to hydrolysis of urea, the pH of the medium was significantly increased, and the isolates were able to survive in this environment. Aono et al. [3] suggested that certain structural components of the cell wall of some alkalophiles, such as teichuronopeptide, may contribute to pH homeostasis at alkaline pH and aid bacteria to survive in alkaline environments. The urease activity was significantly decreased and the protease activity increased after 120 h in this study. Protease might have an adverse effect on urease activity as its accumulation in the culture media might have affected the urease and ultimately lowered the urease production.
Extracellular polymeric substances and biofilm production
A significant difference between UV-induced bacterial isolates and wild type was found in terms of EPS and biofilm production. The mutant Bp M-3 was able to produce greater amounts of EPS (37 nmol/ml) compared to other isolates (Fig. 3). The mutant Bp M-3 also showed a higher monoxenic biofilm (352 cfu/mm2) compared to other mutants, including the wild type (Fig. 3). Kawaguchi and Decho [18] showed the influence of extracellular polymeric substance (EPS) secretions on calcium carbonate precipitation. EPS seems to play an important role in the coverage of the surface by biofilms, cell adhesion [38], and possibly the capturing of the produced calcium carbonate, which might result in a homogeneous layer of calcium carbonate. Merz-Preiss and Riding [22] showed that a biofilm is to colonize the surface of the stones and react as nucleation site for extracellular calcium carbonate precipitation. Dick et al. [12] showed that the production of EPS and biofilm was not significant among the bacterial isolates used to remediate the degraded limestones. The structure of the biofilm is clearly influenced by a number of biological factors, such as twitching motility, growth rate, cell signaling, and EPS production [35]. The biofilm structure appears to be largely determined by the production of a slime-like matrix of EPS, which provides the structural support for the biofilm. In our study, however, we found significant differences in the ability of the UV-induced bacterial isolates compared to the wild type to produce EPS and form biofilms. This result suggests that EPS and biofilm produced by these isolates could serve as potential factors for cementing the sand.
SEM-EDX and XRD analysis of microbial sand columns
The results of sand plugging showed that all columns were found to be tightly packed except the control sand column. The sand column consolidated with Bp M-3 was found to be the most compact and the tightest one, as it required more physical pressure to break. The maximum calcite content was recorded in the upper layer of the sand column with all isolates, and the highest accumulation was observed with Bp M-3 (Fig. 4). There were 40, 24, and 13% calcite depositions in the upper, middle, and lower layer of the sand column, respectively, with Bp M-3 compared to the wild type where it was 34, 20, and 10%. The influence of microbial cementation on granular behavior is dependent on the ability of microbes to move freely throughout the pore space and on sufficient particle-particle contacts per unit volume at which cementation will occur [11]. Calcite precipitation occurred predominantly in the areas close to the surface of the sand column. It is mainly due to the fact that facultatively anaerobic S. pasteurii grows at a higher rate in the presence of oxygen and consequently induces active precipitation of CaCO3 around the surface area. To determine the presence of microbial calcite precipitation, the sand consolidated samples were examined under SEM. Based on the physiological characterization of the wild-type strain and all four UV-induced mutants, Bp M-3 was found to produce the maximum urease activity; hence, this strain was chosen for further analysis by SEM-EDX and XRD. This strain was deposited with the Institute of Microbial Technology Chandigarh, India, and was assigned an accession number MTCC 5428 under the International Budapest Treaty. A patent was filed [25]. SEM analysis of microbial involvement in sand consolidation is depicted in Fig. 5, where distinct calcite crystals embedded with bacteria can be found between and on the surfaces of sand grains. The presence of crystalline calcite associated with bacteria indicates that bacteria served as nucleation sites during the mineralization process [34]. Mineral constituents of the microbial sand columns were further characterized by EDX analysis. Presence of high amounts of calcium in all the bacterial-treated sand columns confirmed that calcite was present in the form of calcium carbonate. The maximum amount of calcium was found to be in the sand column consolidated with Bp M-3, which was clearly shown by EDX spectra (Fig. 6). The majority of the carbonate deposits were present as calcite crystals, as was confirmed by XRD analysis, although the major component was quartz. The maximum number of calcite peaks was observed in Bp M-3 compared to the wild type (Fig. 7). From these results, it can be concluded that the UV-induced mutant of S. pasteurii (Bp M-3) is more efficient than its wild-type strain (Bp W) with respect to calcite precipitation.
Improvement of microbial strains for the overproduction of enzymes has been the hallmark of all commercial processes. Such improved strains can reduce the cost of the process with increased productivity and may also possess some specialized desirable characteristics. The effectiveness of physical mutagenesis by UV radiation in strain improvement for enhanced urease productivity and calcite precipitation was demonstrated in this investigation. A significant increase in the urease activity, calcite amount, and also survival at higher pH was observed in case of UV-induced mutants of S. pasteurii compared with the wild type. These results suggest that the mutant strain of S. pasteurii can be exploited commercially for remediation of cracks in structures and fissures and also for surface treatment. Future work is required based on the use of these mutants for the remediation of cracks in building materials and to check the compressive strength in cement mortars.
Conclusion
In this study, we successfully improved the strain of S. pasteurii by mutagenesis. The mutant (Bp M-3) showed increased urease activity, calcite precipitation, and survival at higher pH, which could be used in the remediation of cracks in building materials.
References
Achal V, Mukherjee A, Basu PC, Reddy MS (2009) Lactose mother liquor as an alternative nutrient source for microbial concrete production by Sporosarcina pasteurii. J Ind Microbiol Biotechnol 36:433–438. doi:10.1007/s10295-008-0514-7
American Public Health Association (APHA) (1989) Standard methods for the examination of water and wastewater, 17th edn. American Public Health Association, Washington
Aono R, Ito M, Machida T (1999) Contribution of cell wall component teichuronopeptide to pH homeostasis and alkaliphily in the alkaliphile Bacillus lentus C-125. J Bacteriol 181:6600–6606
Bachmeier KL, Williams AE, Warmington JR, Bang SS (2002) Urease activity in microbiologically-induced calcite precipitation. J Biotechnol 93:171–181. doi:10.1016/S0168-1656(01)00393-5
Bang SS, Ramakrishnan V (2001) Microbiologically-enhanced crack remediation (MECR). In: Proceedings of the international symposium on industrial application of microbial genomes. Daegu, Korea, pp 3–13
Bang SS, Galinat JK, Ramakrishnan V (2001) Calcite precipitation induced by polyurethane-immobilized Bacillus pasteurii. Enzyme Microb Technol 28:404–409. doi:10.1016/S0141-0229(00)00348-3
Burne RA, Chen RE (2001) Bacterial ureases in infectious diseases. Microbes Infect 2:533–542. doi:10.1016/S1286-4579(00)00312-9
Burne RA, Marquis RE (2000) Alkali production by oral bacteria and protection against dental caries. FEMS Microbiol Lett 193:1–6. doi:10.1111/j.1574-6968.2000.tb09393.x
Cacchio P, Ercole C, Cappuccio G, Lepidi A (2003) Calcium carbonate precipitation by bacterial strains isolated from a limestone cave and from a loamy soil. Geomicrobiol J 20:85–99. doi:10.1080/01490450303883
Ciurli S, Marzadori C, Benini S, Deiana S, Gessa C (1996) Urease from the soil bacterium Bacillus pasteurii: immobilization on Ca-polygalacturonate. Soil Biol Biochem 28:811–817. doi:10.1016/0038-0717(96)00020-X
De Jong JT, Fritzges MB, Nein K (2006) Microbial induced cementation to control sand response to undrained shear. J Geotech Geoenviron Eng 132(11):1381–1392. doi:10.1061/(ASCE)1090-0241(2006)132:11(1381)
Dick J, De Windt W, De Graef B, Saveyn H, Van der Meeren P, De Belie N, Verstraete W (2006) Bio-deposition of a calcium carbonate layer on degraded limestone by Bacillus species. Biodegradation 17:357–367. doi:10.1007/s10532-005-9006-x
Ferris FG, Beveridge TJ, Fyfe WS (1986) Iron-silica crystallite nucleation by bacteria in a geothermal sediment. Nature 320:609–611. doi:10.1038/320609a0
Ferris FG, Fyfe WS, Beveridge TJ (1987) Bacteria as nucleation sites for authigenic minerals in a metal-contaminated lake sediment. Chem Geol 63:225–232. doi:10.1016/0009-2541(87)90165-3
Friedman LE, De Passerini Rossi BN, Messina MT, Franco MA (2001) Phenotype evaluation of Bordetella bronchiseptica cultures by urease activity and congo red affinity. Lett Appl Microbiol 33:285–290. doi:10.1046/j.1472-765X.2001.00997.x
Fukumoto J, Yamamoto T, Tsuru D (1971) Process for producing detergent resisting alkaline protease. Can Pat 910:214
Gollapudi UK, Knutson CL, Bang SS, Islam MR (1995) A new method for controlling leaching through permeable channels. Chemosphere 30(4):695–705. doi:10.1016/0045-6535(94)00435-W
Kawaguchi T, Decho AW (2002) A laboratory investigation of cyanobacterial extracellular polymeric secretions (EPS) in influencing CaCO3 polymorphism. J Cryst Growth 240:230–235. doi:10.1016/S0022-0248(02)00918-1
Lian B, Hu Q, Chen J, Ji J, Teng HH (2006) Carbonate biomineralization induced by soil bacterium Bacillus megaterium. Geochim Cosmochim Acta 70:5522–5535. doi:10.1016/j.gca.2006.08.044
Lopez-Garcia P, Kazmierczak J, Benzerara K, Kempe S, Guyot F, Moreira D (2005) Bacterial diversity and carbonate precipitation in the giant microbialites from the highly alkaline Lake Van, Turkey. Extremophiles 9:263–274. doi:10.1007/s00792-005-0457-0
McKay DS, Gibson EK Jr, Thomas-Keprta KL, Vali H, Romanek CS, Clemett SJ, Chillier XDF, Maechling CR, Zare RN (1996) Search for past life on Mars: possible relic biogenic activity in martian meteorite ALH84001. Science 273:924–930. doi:10.1126/science.273.5277.924
Merz-Preiss M, Riding R (1999) Cyanobacterial tufa calcification in two freshwater streams: ambient environment, chemical thresholds and biological processes. Sediment Geol 126(1–4):103–124. doi:10.1016/S0037-0738(99)00035-4
Mobley HLT, Hausinger RP (1989) Microbial ureases: significance, regulation and molecular characterisation. Microbiol Rev 53:85–108
Morikawa M, Kagihiro S, Haruki M, Takano K, Branda S, Kolter R, Kanaya S (2006) Biofilm formation by a Bacillus subtilis strain that produces γ-polyglutamate. Microbiology 152:2801–2807. doi:10.1099/mic.0.29060-0
Mukherjee A, Reddy MS, Achal V (2008) A novel additive for building materials and methods of preparation & application there of. Indian Patent Application No. 2191/DEL/2008
Natarajan KR (1995) Kinetic study of the enzyme urease from Dolichos biflorus. J Chem Educ 72:556–557
Nemati M, Voordouw G (2003) Modification of porous media permeability, using calcium carbonate produced enzymatically in situ. Enz Microb Tech 33:635–642. doi:10.1016/S0141-0229(03)00191-1
Ramachandran SK, Ramakrishnan V, Bang SS (2001) Remediation of concrete using microorganisms. Am Concr Inst Mater J 98:3–9
Rivadeneyra MA, Delgado G, Ramos-Cormenzana A, Delgado R (1997) Precipitation of carbonates by Deleya halophila in liquid media: pedological implications in saline soils. Arid Soil Res Rehabil 11:35–49
Rivadeneyra MA, Delgado G, Ramos-Cormenzana A, Delgado R (1998) Biomineralisation of carbonates by Halomonas eurihalina in solid and liquid media with different salinities: crystal formation sequence. Res Microbiol 149:277–287. doi:10.1016/S0923-2508(98)80303-3
Rodriguez-Navarro C, Rodriguez-Gallego M, Ben Chekroun K, Gonzalez-Munoz MT (2003) Conservation of ornamental stone by Myxococcus xanthus—induced carbonate biomineralization. Appl Environ Microbiol 69(4):2182–2193. doi:10.1128/AEM.69.4.2182-2193.2003
Shen Y, Buick R, Canfield DE (2001) Isotopic evidence for microbial sulphate reduction in the early Archaean era. Nature 410:77–81. doi:10.1038/35065071
Silver S, Toth K, Scribner H (1975) Facilitated transport of calcium by cells and subcellular membranes of Bacillus subtilis and Escherichia coli. J Bacteriol 12:880–885
Stocks-Fischer S, Galinat JK, Bang SS (1999) Microbiological precipitation of CaCO3. Soil Biol Biochem 31(11):1563–1571. doi:10.1016/S0038-0717(99)00082-6
Stoodley P, Sauer K, Davies DG, Costerton JW (2002) Biofilms as complex differentiated communities. Annu Rev Microbiol 56:187–209. doi:10.1146/annurev.micro.56.012302.160705
Stumm W, Morgan JJ (1981) Aquatic chemistry. Wiley, NY
Tiano P, Biagiotti L, Mastromei G (1999) Bacterial bio-mediated calcite precipitation for monumental stones conservation: methods of evaluation. J Microbiol Methods 36:138–145. doi:10.1016/S0167-7012(99)00019-6
Tsuneda S, Jung J, Hayashi H, Aikawa H, Hirata A, Sasaki H (2003) Influence of extracellular polymers on electrokinetic properties of heterotrophic bacterial cells examined by soft particle electrophoresis theory. Colloid Surf B 29:181–188. doi:10.1016/S0927-7765(02)00188-1
Wright DT, Oren A (2005) Non-photosynthetic bacteria and the formation of carbonates and evaporates through time. Geomicrobiol J 22:27–53. doi:10.1080/01490450590922532
Acknowledgements
Financial assistance for this study received from the Atomic Energy Regulatory Board, Department of Atomic Energy and Department of Science & Technology, India, is gratefully acknowledged. The authors thank TIFAC-CORE for the experimental facilities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Achal, V., Mukherjee, A., Basu, P.C. et al. Strain improvement of Sporosarcina pasteurii for enhanced urease and calcite production. J Ind Microbiol Biotechnol 36, 981–988 (2009). https://doi.org/10.1007/s10295-009-0578-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-009-0578-z