Abstract
In this study, we performed an analysis of the ability of four Saccharomyces cerevisiae and one S. bayanus var. uvarum strains, isolated from different industrial processes, to ferment increasing amounts of fructose (from 0 to 70%, w/v). Overall yeast growth was estimated by integration of the area under optical density vs. time curves. Subsequently, this parameter was modeled by means of a substrate inhibition model. All strains showed a similar behavior against fructose concentration in spite of their different origins, but with slight differences. The optimum fructose concentrations to stimulate yeast growth were obtained between 4.33 and 6.05%, while the maximum concentrations above which growth was completely inhibited were attained between 59.56 and 63.85%. Statistically, model parameters calculated for wine yeast strains were significantly different than those obtained for yeasts from Agave and table olive fermentations, except for the maximum inhibitory concentration. The methodology used in this work could be useful for the industry in general as a first procedure to select yeast strains with higher fructose preferences or tolerances, and especially for winemakers, where the risk of spoilage increases by the presence of a marked residual fructose concentration in the finished wine.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fructose is a simple reducing sugar found in many foods. This compound is an isomer of glucose with the same empirical formula (C6H12O6), but with a different structure, which considerably determines its physicochemical properties. It can be present in many foods either as a free monosaccharide or bound to glucose, forming the sucrose disaccharide [6]. In general, foods that contain free fructose have more or less the same amount of free glucose (grapes, peaches, bananas, etc.), but some of the fruits have larger proportions of fructose to glucose (for example, apples or pears) [28].
It is well known that both fructose and glucose can be fermented by yeasts, producing ethanol, water, carbon dioxide and other minor compounds. Glucose and fructose are transported into the cell by the same hexose transporters (Hxt) located in the plasma membrane [20]. These Hxt proteins are permeases that mediate facilitated diffusion of glucose and fructose through the membrane. Saccharomyces cerevisiae differs from other yeasts, which may exhibit, in addition to diffusion of glucose and fructose, a proton symport system for both sugars [21]. Specifically, fructose/H+ symport activities were found to coexist with a facilitated diffusion system for hexoses in diverse species of the Saccharomyces senso stricto complex [12, 23]. Once the sugars have been imported into the cell, they are phosphorylated by one of three sugar kinases, Hxk1, Hxk2 or Glk1 [8, 9], which also differ in their different glucose/fructose affinities. Finally, metabolism of hexoses (glucose, fructose and mannose) is general and is carried out largely by the glycolytic pathway.
Fleet [11] reported that yeasts had a slight preference for glucose uptake compared to fructose in wine fermentations, resulting in a difference between glucose and fructose consumption through the fermentative process. Metabolization of fructose by microorganisms in table olive fermentations is also slower than glucose [2]. For these reasons, a marked residual fructose concentration may be present in the last phase of the fermentative process, and sluggish or stuck fermentation can occur, which imparts important economic losses. One of the solutions to this serious problem is the reinoculation of the medium with a yeast starter culture that is both alcohol tolerant and a vigorous fructose fermenter [24]. Berthels et al. [7] showed that different wine yeast strains have strain-specific glucose/fructose consumption discrepancies. Differences in the glucose/fructose utilization by yeast strains could originate from the level of the transporter or phosphorylation into the cell, as mentioned above. Searching for yeast strains with higher fructose preference and tolerance is an important goal for the fermentation industry in general, and especially for winemakers. Belloch et al. [5] studied the fermentative stress adaptation of diverse yeasts within the Saccharomyces sensu stricto complex in laboratory medium. All yeasts tested by these authors were able to grow in 30% (w/v) of sugar. It is well known that “sensu stricto” species of the genus Saccharomyces are well adapted to grow on sugar concentrations around 20–30% [22].
A rapid method to detect candidate yeasts with different fructose utilization patterns is the study of their growth under a wide range of fructose concentrations. In this way, predictive microbiology can be a useful tool [19]. The effect of fructose concentration on yeast growth can be estimated in different ways, but the substrate inhibition model seems to be a good alternative. In most of the biotechnological processes, substrates can act as inhibitors at higher concentrations and behave as activators at lower levels. Several mathematical models have been developed for quantifying the inhibitory effect of substrate concentration on microbial growth. Generally, these models are adaptations of modified Monod equations for substrate inhibition of enzymatic reactions [16, 17, 25].
The goal of this work is to study the effect of a wide range of fructose concentrations on the overall growth of diverse S. cerevisiae strains isolated from different industrial fermentations. This paper introduces predictive microbiology techniques to objectively assess the yeast response, and it could be used easily by the industry to select, in a first stage, the best adapted strains to higher levels of fructose.
Materials and methods
Yeast strains
Five yeast strains isolated from different industrial fermentations and regions were used in this study. S. cerevisiae T73 and S. bayanus var. uvarum BM58 were isolated from wine fermentations in Alicante and Utiel–Requena wine regions (Spain), respectively. They are currently commercialized as active dry yeasts. S. cerevisiae IGAL01 was isolated from table olive fermentations in Málaga (Spain), while S. cerevisiae C6 and S. cerevisiae C9 were isolated from alcoholic fermentations of Agave duranguensis during the production of mezcal in Durango (México). These yeasts were deliberately chosen because they are well adapted to their respective fermentation conditions, which present different levels of fructose concentration (from 0.5 to 12.5% of fructose). BM58 was introduced in this work as an external species control for comparison with the response obtained for the diverse S. cerevisiae strains.
Inoculum preparation
Single colonies from pure cultures of each strain were inoculated separately into 5 ml of Yeast Nitrogen Base (YNB) medium (Difco™, Becton and Dickinson Co., Sparks, MD) supplemented with 1% of fructose as the only carbon source, and then incubated at 25°C for 48 h. After this period, the tubes were centrifuged at 9,000 g for 10 min; the pellets were washed with sterile saline solution (0.9% NaCl), centrifuged and re-suspended again in sterile saline solution to obtain a concentration of about 8 log10 CFU/ml.
Media and growth conditions
Experiments for determining the effects of fructose concentration were carried out under semi-anaerobic conditions in 10 ml of YNB medium supplemented with 12 different fructose concentrations (0.00, 0.55, 1.37, 2.75, 5.50, 11.01, 16.52, 27.54, 38.56, 55.09, 62.65 and 69.57%). Media were sterilized by filtration (0.2 μm) to avoid fructose caramelization, inoculated independently with the diverse yeasts suspension reaching an initial concentration of 5.74 ± 0.10 log10 CFU/ml, and subsequently incubated without shaking at 25°C. All experiments were carried out in duplicate and monitored for 7 days until growth curves reached the stationary phase. Therefore, a total of 120 growth curves (12 fructose concentrations × 5 strains × 2 replicates) were obtained and analyzed. When no growth was recorded, samples were spread on yeast-malt-peptone-glucose (YM, Difco) agar plates to compare the current yeast population to the initial inoculum level and to confirm, in this way, a possible inhibitory effect of high fructose concentrations.
Growth measurement
Yeast growth was determined by optical density (OD) at 600 nm in an Eppendorf BioPhotometer (Hamburg, Germany), using a non-inoculated YNB medium as blank. A previous calibration curve was developed to obtain correlations between OD and plate counts for the five yeasts, showing a linear correlation between 0.1 and 1.7 OD corresponding to 5.6 and 7.1 log10 CFU/ml, respectively. For this reason, when OD was higher than a value of 1.5, dilutions were obtained in the same blank medium. At least 11 measurements were taken for each experiment to build the OD vs. time plots. Subsequently, the overall yeast growth was estimated as the area under the OD vs. time curve. This parameter was calculated by integration using the OriginPro 7.5 software (OriginLab Corp., Northampton, MA).
Substrate inhibition model
A non-linear mathematical model, used previously by Luong [17] and more recently by Lin et al. [16], was also applied in this work to estimate the effect of fructose concentration on yeast growth. The model considers that substrates will act as inhibitors at higher concentrations and behave as activators at lower levels, and it is derived from a generalization of a Monod type model, with the following expression:
where A (dependent variable) is the area under the OD vs. time curve, S (independent variable) is the substrate concentration, expressed as percentage (%) equivalent to grams per 100 ml, U is the maximum area value calculated by the model, K s is the monod or substrate saturation constant (%), and S max is the maximum substrate concentration above which growth is completely inhibited (%). These parameters were obtained by a non-linear regression procedure, minimizing the sum of squares of the difference between the experimental data and the fitted model, i.e., loss function (observed–predicted)2. This task was accomplished using the non-linear module of the Statistica 7.0 software package (StatSoft, Tulsa, OK) and its Quasi-Newton option. Fit adequacy was checked by the proportion of variance explained by the model (R 2) with respect to experimental data.
The model has the capability to predict the value of substrate concentration where maximum growth is obtained, denoted in this work as S opt. It can be obtained from the following equation according to Luong [17]:
Statistical analysis
The one-way ANOVA module of the Statistica 7.0 software package was used to assess if the parameters obtained for yeast growth were statistically different from each other. This task was carried out by means of a post hoc comparison with the Scheffé test, which is considered to be one of the most conservative post hoc tests [29]. An alternative advantage of the Scheffé test is that it can also be used with unequal sample sizes.
Results and discussion
Estimating the overall yeast growth by means of the area under the OD/time curves
Optical density measurement is a common procedure usually used in food and predictive microbiology to follow microbial growth [19]. It is easily applicable, rapid and inexpensive with respect to the plate count methods. For these reasons, several authors have used the area under the OD/time curves as a valuable procedure to estimate the inhibitory effects of diverse substances (essential oils, chloride salts, weak acids) on microbial growth [3, 4, 14, 27]. As the amount of inhibitor increases, the effect on the growth of the organism also increases, and this effect on the growth is reflected by a reduction in the area under the OD/time curve. However, if the substance has a stimulating effect, an increase in the area under the OD/time curve is observed [4]. Comparing area values between different strains and conditions, it is necessary always to use the same time period (in this work 7 days) because this parameter is strongly influenced by the duration of the experiments.
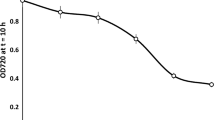
As a graphic example, Fig. 1 shows the plot of OD vs. time for yeast T73 growing under different fructose concentrations. Similar results were also obtained for the other yeasts (data not shown). As can be seen, the area under the growth curve decreased as the inhibitor effect of fructose concentration increased, obtaining the minimum area at 62.65% of fructose. Their values, estimated directly by integration of the curves with an appropriate software (see "Materials and methods"), were 684.69, 440.07, 284.14, 60.89 and 11.89 for 5.50, 27.54, 38.56, 55.09 and 62.65% of fructose, respectively. In Table 1, the area under the OD/time curve is shown to be inversely related to the lag phase and linearly related to both the maximum exponential growth rate and maximum OD reached. In this way, the maximum area value was obtained when the slope of the growth curve and the upper OD asymptote were higher, but the lag phase was shorter. Therefore, in this work the area under the OD/time curve was considered as an appropriate indicator of the overall yeast growth because this value showed a clear proportionality (direct or inverse) with the kinetic growth parameters. All area values were obtained for each initial fructose concentration and yeast. Table 2 summarizes the means of these values for the strains T73, IGAL01, C6, C9 and BM58, showing also their respective standard deviations calculated from duplicated experiments. In general, area values were higher for yeast T73 than for the other strains, regardless the concentrations assayed.
Fit of experimental data to the substrate inhibition model
Diverse substrates can act as activators at low levels and as inhibitors at higher concentrations, showing a non-linear dose response. Luong [17] reported the inhibitory effect of butanol on Candida utilis growth. A butanol level around 0.1% produced a maximum on the maximum specific growth rate of C. utilis, but higher levels of this compound produced a marked decrease of the growth, resulting in a total inhibition at 0.9%. Bautista-Gallego et al. [4] also mentioned a similar behavior of yeast S. cerevisiae as a function of increasing levels of potassium chloride, showing an activation shoulder of around 2% and a total inhibition at 22%. According to Fig. 2, which represents the areas under the growth curve vs. fructose concentrations between 0 and 70%, a similar trend was also found for yeasts T73, IGAL01, C6, C9 and BM58. At low substrate concentrations, the areas increased with increasing substrate concentration, but after the maximum value was reached, the areas decreased linearly as substrate concentration was further increased. Graphically, maximum area values were attained around 5% of fructose concentration, while growth was completely inhibited above 60%.
Area under growth curves vs. fructose concentration for yeasts S. cerevisiae T73, S. bayanus var. uvarum BM58, S. cerevisiae IGAL01, S. cerevisiae C6 and S. cerevisiae C9 fitted by means of a substrate inhibition model [17]
Experimental data obtained in Table 2 were fitted with the Luong model [17] by means of a non-linear regression procedure (Fig. 2). The fit was very good, with a proportion of variance accounted (R 2) of 0.996, 0.995, 0.990, 0.996 and 0.978 for yeasts T73, IGAL01, C6, C9 and BM58, respectively. The probability for the F obtained in the ANOVA regression was always below 0.000001. Therefore, the model used accurately represents the experimental data, and the obtained parameters (U, S opt, S max and K s; Table 3) can be used for a quantitative comparison of the yeast response against fructose concentration variation.
The optimal fructose concentrations for growth (S opt) were obtained at 6.05, 5.43, 4.65, 4.54 and 4.33% for yeasts BM58, T73, C6, IGAL01 and C9, respectively. The effects of glucose and sucrose concentration on microbial growth had been previously studied and fitted with a substrate inhibition model by Sivakumar et al. [25]. These authors showed that a glucose concentration around 2% produced a maximum in Klebsiella oxytoca growth, but over this value the growth decreased linearly. A similar result was also obtained for glucose and Actinobacillus succinogenes in a semi-defined medium by Lin et al. [16]. The optimum sucrose concentration for growth of a mixed culture of microorganism was attained at 0.2% [25]. In this work, optimum fructose concentrations for S. cerevisiae and S. bayanus strains were always above these values.
The maximum fructose concentrations above which growth was completely inhibited (S max) were attained at 63.85, 61.90, 61.57, 61.20 and 59.56% for yeasts C9, IGAL01, T73, C6 and BM58, respectively. Yeast population decreased after 7 days at these fructose levels approximately in 3.7 log10 CFU/ml with respect to the initial inoculum (5.74 log10 CFU/ml), which confirmed the inhibitory effect of this sugar at high levels. S. cerevisiae and S. bayanus are not normally isolated from high-sugar foods (>50%) [22]. According to Tilbury [26], yeasts with the capacity to grow up to 60% of sugar can be considered as osmotolerant microorganisms, while osmophilic species are those able to growth above 60% of sugar [22]. Resistance to high sugar is largely based on the accumulation of high concentrations of compatible solutes in the cytoplasm, such as glycerol, trehalose or arabitol [22]. Although most yeasts can happily grow in 20% of sugar, only diverse Zygosaccharomyces and Candida yeast strains can tolerate glucose concentrations between 72 and 90% [18], values higher than those obtained in this work and those reported by Lin et al. [16] for Actinobacillus succinogenes in a semi-defined medium (15%). Unfortunately, we have not found numerical data in the literature about the effects of high levels of glucose on S. cerevisiae growth in order to compare them with the results obtained in this work for fructose. However, glucose and fructose have the same molecular weight (180.16), and we can presume that a given quantity of glucose in solution should result in approximately the same osmotic pressure as a fructose solution at the same concentration, producing similar effects. Finally, the maximum area values estimated by the model (U) were 804.04, 745.16, 641.22, 595.43 and 559.74 for yeasts T73, BM58, C9, IGAL01 and C6, while K s (the Monod or substrate saturation constant) was higher for BM58 and T73 than for the other yeasts (see Table 3). K s represents the sugar concentration for which half of the maximum area value is reached.
All yeasts studied in this work showed very similar responses although they were isolated from diverse continents and fermentations with different fructose proportions. The normal levels of fructose in wines, the environment for the yeasts T73 and BM58, are around 12.5%. IGAL01 was isolated from table olive fermentation with levels of fructose around 0.5%, while the yeasts C6 and C9 were obtained from Agave fermentation, where fructose is the main sugar and reaches concentrations of around 9%. The five yeasts were able to grow in fructose concentrations up to 60%, a limit far above the normal fructose concentrations found in their respective fermentative environments. However, significant differences were found among strains for the model parameters, and consequently in the yeast response, according to ANOVA-Scheffé tests (Table 3). In this way, the yeasts IGAL01, C6 and C9 did not show differences among each other for the parameters U, K s and S opt, but they were significantly different with respect to T73 and BM58. Wine yeasts, adapted to fermentative environments with higher fructose levels, also showed higher S opt. Moreover, T73 and BM58 were the yeasts with higher areas for all fructose concentrations assayed. The S. bayanus strain (BM58), used in this work as an external species control, was statistically different from S. cerevisiae strains for parameters K s and S opt. Finally, no statistical differences among yeasts were found for the maximum substrate inhibitory concentration (S max).
Fay and Benavides [10] found evidence of genetic diversity between S. cerevisiae wine yeasts and other Saccharomyces strains isolated from sake fermentations, analyzing the sequences at five loci in 49 strains. Similar results were also found by Legras et al. [15], who analyzed 651 strains from different foods by microsatellite analysis, and by Arias [1], who showed a genetic differentiation between Saccharomyces wine yeasts and other Saccharomyces strains isolated from diverse fermentations such as olives, beer, Agave and sake by using sequence analysis of four nuclear genes and one mitochondria from more than 230 strains. However, no studies were carried out to determine if different physiological properties were associated with these genetic divergences. In this work, we have found slight but significant differences for some biological parameters related to fructose response in S. cerevisiae strains isolated from wine, table olive and Agave fermentations. These differences could be related to genetic divergences, either to the level of sugar transport across the plasma membrane [13] or in the hexose phosphorylation inside the cell [8].
Conclusions
The model used in this work turned out to be useful to fit, on a small scale, the effect of fructose concentration on the growth of four S. cerevisiae and one S. bayanus strains, allowing at the same time a quantitative comparison among them. Levels of fructose between 4.33 and 6.05% stimulated yeast growth, but values above 59.56 and 63.85% were inhibitory, with a reduction on yeast population with respect to the initial inoculum of ≈3.7 log10 CFU/ml. Apparently, parameters obtained by means of the substrate inhibition model for wine yeast strains were significantly different than those obtained for the other yeasts, except for the maximum fructose concentration, where growth was inhibited. The different physiological sugar responses between S. cerevisiae wine yeast and other S. cerevisiae fermentative strains could have a genetic origin, although further studies are necessary to confirm these hypotheses. The methodology used in this work could be useful for the industry to select, in a first stage, yeast strains with higher fructose preferences or tolerances. However, these yeasts must be subsequently studied under real fermentation conditions to validate the preliminary results, paying special attention to their fermentative properties. Further studies are also necessary to check if this procedure result is appropriate to estimate the effect of fructose for the rest of the Saccharomyces species.
References
Arias JA (2008) Diversidad genética en las especies del complejo Saccharomyces sensu stricto de fermentaciones tradicionales. Doctoral Thesis. Facultat de Ciències Biològiques. Universitat de València
Arroyo-López FN, Durán-Quintana MC, Romero C, Rodríguez-Gómez F, Garrido-Fernández A (2007) Effect of storage process on the sugars, polyphenols, color and microbiological changes in cracked Manzanilla-Aloreña table olives. J Agric Food Chem 55:7434–7444. doi:10.1021/jf070571b
Arroyo-López FN, Bautista-Gallego J, Durán-Quintana MC, Garrido-Fernández A (2008) Modelling the inhibition of sorbic and benzoic acids on a native yeast cocktail from table olives. Food Microbiol 25:566–574. doi:10.1016/j.fm.2008.02.007
Bautista-Gallego J, Arroyo-López FN, Durán-Quintana MC, Garrido-Fernández A (2008) Individual effects of sodium, potassium, calcium and magnesium chloride salts on Lactobacillus pentosus and Saccharomyces cerevisiae growth. J Food Prot 71:1412–1421
Belloch C, Orlic S, Barrio E, Querol A (2008) Fermentative stress adaptation of hybrids within the Saccharomyces sensu stricto complex. Int J Food Microbiol 122:188–195. doi:10.1016/j.ijfoodmicro.2007.11.083
BeMiller JN (2007) Carbohydrate chemistry for food scientists, Second Edition edn. American Association of Cereal Chemists (AACC), St Paul
Berthels NJ, Cordero Otero RR, Bauer FF, Thevelein JM, Pretorius IS (2004) Discrepancy in glucose and fructose utilisation during fermentation by Saccharomyces cerevisiae wine yeast strains. FEMS Yeast Res 4:683–689. doi:10.1016/j.femsyr.2004.02.005
Berthels NJ, Cordero Otero RR, Bauer FF, Pretorius IS, Thevelein JM (2008) Correlation between glucose/fructose discrepancy and hexokinase kinetic properties in different Saccharomyces cerevisiae wine yeast strains. Appl Microbiol Biotechnol 77:1083–1091. doi:10.1007/s00253-007-1231-2
Entian KD, Barnett JA (1992) Regulation of sugar utilization by Saccharomyces cerevisiae. Trends Biochem Sci 17:506–510. doi:10.1016/0968-0004(92)90341-6
Fay JC, Benavides JA (2005) Evidence for domesticated and wild populations of Saccharomyces cerevisiae. PLoS Genet 1:1–6. doi:10.1371/journal.pgen.0010005
Fleet GH (1998) The microbiology of alcoholic beverages. In: Wood BJB (ed) Microbiology of fermented foods. Blackie, Glasgow, pp 217–262
Gonçalves P, Rodrigues de Sousa H, Spencer-Martins I (2000) FSY1, a novel gene encoding a specific fructose/H+ symporter in the type strain of Saccharomyces carlsbergensis. J Bacteriol 182:5628–5630. doi:10.1128/JB.182.19.5628-5630.2000
Guillaume C, Delobel P, Sablayrolles JM, Blondin B (2007) Molecular basis of fructose utilization by the wine yeast Saccharomyces cerevisiae: a mutated HXT3 allele enhances fructose fermentation. Appl Environ Microbiol 73:2432–2439. doi:10.1128/AEM.02269-06
Lambert RJW, Pearson J (2000) Susceptibility testing: accurate and reproducible minimum inhibitory concentration (MIC) and non-inhibitory concentration (NIC) values. J Appl Microbiol 88:784–790. doi:10.1046/j.1365-2672.2000.01017.x
Legras YL, Merdinoglu D, Cornuet YM, Karst F (2007) Bread, beer and wine: Saccharomyces cerevisiae diversity reflects human history. Mol Ecol 16:2091–2192. doi:10.1111/j.1365-294X.2007.03266.x
Lin SKC, Du C, Koutinas A, Wang R, Webb C (2008) Substrate and product inhibition kinetics in succinic acid production by Actinobacillus succioneges. Biochem Eng J 41:128–135. doi:10.1016/j.bej.2008.03.013
Luong JHT (1987) Generalization of monod kinetics for analysis of growth data with substrate inhibition. Biotechnol Bioeng 29:242–248. doi:10.1002/bit.260290215
Martorell P, Stratford M, Steels H, Fernández-Espinar MT, Querol A (2007) Physiological characterization of spoilage strains of Zygosaccharomyces bailii and Z. rouxii isolated from high sugar environments. Int J Food Microbiol 114:234–242. doi:10.1016/j.ijfoodmicro.2006.09.014
McMeekin TA, Olley JN, Ross T, Ratkosky DA (1993) Predictive Microbiology: theory and application. Wiley, New York
Özcan S, Johnston M (1999) Function and regulation of yeast hexose transporters. Microbiol Mol Biol Rev 63:554–569
Postma E, van den Broek PJ (1990) Continuos-culture study of the regulation of glucose and fructose transport in Kluyveromyces marxianus CBS 6556. J Bacteriol 172:2871–2876
Querol A, Fleet G (2006) Yeasts in food and beverages. Springer, Berlin
Rodrigues de Sousa H, Madeira-Lopes A, Spencer-Martins I (1995) The significance of active fructose transport and maximum temperature for growth in the taxonomy of Saccharomyces sensu stricto. Syst Appl Microbiol 18:44–51
Santos J, Sousa MJ, Cardoso H, Inácio J, Silva S, Spencer-Martins I, Leão C (2008) Ethanol tolerance of sugar transport, and the rectification of stuck wine fermentations. Microbiol 154:422–430. doi:10.1099/mic.0.2007/011445-0
Sivakumar A, Srinivasaraghavan T, Swaminathan T, Baradarajan A (1994) Extended monod kinetics for substrate inhibited systems. Bioprocess Eng 11:185–188. doi:10.1007/BF00369628
Tilbury RH (1980) Xerotolerant yeasts at high sugar concentrations. In: Gould GW, Corry JEL (eds) Microbial growth and survival in extremes of environment, Society for Applied bacteriology technical series, vol 15. Academic, London, pp 103–128
Tiina M, Sandholm M (1989) Antibacterial effect of the glucose oxidase–glucose system on food poisoning organisms. Int J Food Microbiol 8:165–174. doi:10.1016/0168-1605(89)90071-8
USDA (United States Department of Agriculture) (2008) National Nutrient Database for Standard reference. Available on-line at: http://www.nal.usda.gov/fnic/foodcomp/search. Last visit 30 September 2008
Winer BJ (1962) Statistical principles in experimental design. McGraw-Hill, New York
Acknowledgments
This work was supported by Generalitat Valenciana (project GV2008-037) and the Spanish Government (projects AGL2006-12703-CO2-01 and 02/ALI). The authors wish to thank to Jesús Páez Lerma from Instituto Tecnológico de Durango (México) for kindly supplying S. cerevisiae yeasts from Agave fermentation and Dr Antonio Garrido Fernández for his appreciated comments during the preparation of this manuscript. F.N. Arroyo-López also thanks the Ministry of Education and Science of Spain (MEC) for his “Juan de la Cierva” program postdoctoral research contracts.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Arroyo-López, F.N., Querol, A. & Barrio, E. Application of a substrate inhibition model to estimate the effect of fructose concentration on the growth of diverse Saccharomyces cerevisiae strains. J Ind Microbiol Biotechnol 36, 663–669 (2009). https://doi.org/10.1007/s10295-009-0535-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-009-0535-x