Abstract
A total of 179 non-spore-forming bacteria aerobically growing on Nutrient Agar, Plate Count Agar or in specific enrichment conditions for salmonella, campylobacteria, listeria, yersinia or staphylococci, were isolated from 16 untreated paper mill pulps. After phenotypical screening the isolates were characterised by automated ribotyping and partial sequencing of the 16S rRNA gene. They could be divided into seven taxonomical classes representing 63 taxa (species): actinobacteria (11 species), bacilli (7), flavobacteria (3) alphaproteobacteria (10), betaproteobacteria (5), gammaproteobacteria (25) and sphingobacteria (2). Most of the gammaproteobacteria were enterobacteria, mainly species of the genera Enterobacter (7 species, 7 samples/3 mills) and Klebsiella (5 species, 6 samples/3 mills). Other commonly occurring bacteria were most closely related to Microbacterium barkeri (7 samples/3 mills), Cloacibacterium normanense (6 samples/2 mills), Pseudoxanthomonas taiwanensis (5 samples/2 mills) and Sphingobacterium composti (5 samples/1 mill). Sporadic isolates of Listeria innocua, L. monocytogenes, Enterococcus casseliflavus and Staphylococcus warneri were detected, from which only L. monocytogenes is considered to be a food pathogen. No isolates of the genera Campylobacter, Salmonella or Yersinia were detected. The detected bacteria may be harmful in process control, but the load of food pathogens with recycled fibres to paper machines is insignificant. Faecal contamination of the pulp samples was not indicated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
During the past 10 years hygiene in the paper and packaging industry has become important, because the use of recycled fibres in food packages has increased and the storage time of foodstuffs in packages has become significantly longer. In addition to food authorities, manufacturers of packaging materials and their end-users in food industry, who are responsible for the safety of their products, have been concerned about the health risks associated with recycled fibres. Most concern has been focused on chemical impurities, such as heavy metals, chlorinated organics, volatile compounds and mutagens [13, 23, 30]. From the risk assessment point of view, microbiological quality is also a crucial issue, because recycled fibres provide a good substrate for microbial growth during collection, storage and processing [3, 24]. Consumer safety is the principal concern when international regulations are prepared [2]. Hence, no harmful substances—including microbes, their spores and metabolites—should migrate from the packaging materials into foods even during long storage times in warehouses. The estimation of this risk requires relevant data on the occurrence of different microbes in recycled materials and their survival in papermaking processes. Data on the occurrence of non-spore-forming bacteria, especially hygiene indicators and food pathogens, has been insufficient.
Total numbers of aerobically grown microbes in recycled fibre pulps have been reported to be 108–1010 cfu g−1 d.w., the number of spore-forming bacilli being 103–106 cfu g−1 d.w. [26, 27]. Total numbers of anaerobically growing spore-forming bacteria, including strict (clostridia) and facultative anaerobes (bacilli) have been reported to be from 102 to 104 cfu g−1 d.w. [26]. Fungi [25, 26] and filamentous actinobacteria [26, 29] have been detected up to 102–106 and 102–105 cfu g−1 d.w., respectively. In addition to these bacterial groups, proteobacteria, including enterobacteria and pseudomonads, are also common in paper mills [9, 12, 16, 17, 19–21, 33], but their occurrence in recycled pulp samples has not hitherto been reported. New microbes enter the process via raw materials (e.g. recycled fibres) or papermaking chemicals (starch, sizing agents, kaolin, CaCO3). In addition, each mill has its own house-keeping microbiota, which occur as biofilms on surfaces and in recycling process water and are often difficult to remove. Paper mills control the quantity and quality of their microbes with different biocides, the use of which is not only an economic but also an environmental problem. From the process control point of view only those microbes are harmful which degrade raw materials and papermaking chemicals in order to generate nutrients for their growth (e.g. bacilli), induce corrosion (sulphate-reducing bacteria), produce odorous volatile fatty acids and/or explosive gases (clostridia) or produce extracellular slime (e.g. bacilli, proteobacteria) or filaments (fungi, actinobacteria), which may block filters and screens. The papermaking process drastically reduces the total counts of microbes. From the product safety point of view harmful microbes are those which form thermotolerant spores and may enter the end-products. Hence, microbiological risks are usually attributed to spore-forming bacteria, which may cause food spoilage and/or induce health problems in humans.
The phylum Proteobacteria, including enterobacteria with several hygiene indicators and also pseudomonads, constitutes the largest and phenotypically most diverse phylogenetic lineage [15]. Already in 2002 it included more than 460 genera (over 40% of all prokaryotic genera) and more than 1,600 species divided into the 5 classes alpha-, beta-, gamma-, delta- and epsilonproteobacteria. Together they comprise a major proportion of the Gram-negative bacteria and show extreme metabolic diversity. They include the majority of the known Gram-negative bacteria of medical, veterinary, industrial and agricultural interest.
Among the non-spore-forming bacteria there are also pathogenic food poisoning bacteria (food pathogens), which may cause food poisonings via an infection (Salmonella spp., Campylobacter spp., Listeria monocytogenes, Yersinia enterocolitica) or via producing bacterial toxins (Staphylococcus aureus).
The aim of this study was to detect and characterise by molecular methods viable aerobically growing non-spore-forming bacteria from paper mill pulps containing recycled fibres, with special reference to proteobacteria, including hygiene indicators and food pathogens.
Materials and methods
Sampling and isolation
A total of 16 untreated pulp samples were collected from four different mills located in Finland (coded A-C) and Spain (coded D; samples D2 and D3 were analysed only for food pathogens). Except for pulps A3 (95%) and D3 (85%), the pulps contained 100% recycled fibre materials, which originated from newspapers, magazines, milk and juice packages, corrugated cases, plastic-coated wrapping paper, craft paper and mill broke in various proportions. For the culture of bacteria, a 10 g sample was mixed with 90 ml of sterile Ringer’s solution and homogenised in a Stomacher (Stomacher 400, Seward Medical, UK) for 1 min. Appropriate dilutions of samples were cultivated on Nutrient Agar (Oxoid, Basingstoke, Hampshire, England) and Plate Count Agar (Difco, Becton Dickinson, Sparks, USA) including 100 mg l−1 cycloheximide (actidion) (Sigma, Steinheim, Germany). The incubation temperature was 30°C and growth was monitored from 3 to 14 days. From 10 to 15 colonies from different dilution plates of each sample were picked randomly. The number of species was based on the highest dilution factor of the plate from which the species was isolated (cfu g−1 of wet sample). The preliminary tests of isolates included colony (visual) and cell (microscope) morphology, Gram, oxidase and catalase reactions, after which the spore-forming bacilli were rejected. The isolates were stored in 5% glycerol at −70°C.
The proportion of samples to be used for detection of food pathogens was frozen after their arrival at the laboratory. The samples were mixed with equal volumes of 20% (v/v) glycerol and stored at −70°C. After thawing, samples of 25 g were suspended into relevant enrichment media. After specific enrichment procedures, the samples were cultivated on specific agars, from which all colonies were usually picked:
-
Detection of Salmonella spp. Two-phase enrichment [pre-enrichment in Buffered Peptone Water, 37°C, 20 h; enrichment in Selenite Broth (Difco), 37°C, 1–2 days and Tetrathionate Broth (Difco), 43°C, 1–2 days] followed by cultivation on two selective media: Brilliant Green Agar (Difco), 37°C, 1–2 days and Önöz Agar (Merck, Darmstadt, Germany), 37°C, 1–2 days.
-
Detection of Campylobacter spp. Enrichment in Preston Broth (Oxoid), 42°C, 24 h followed by cultivation on Campylobacter Selective Medium, improved blood free (LabM, Lancashire, UK), 42°C, 2 days.
-
Detection of Listeria spp. Enrichment in Listeria Enrichment Broth EB (LabM), 30°C, 2 days followed by cultivation on Listeria Isolation Medium Oxford (LabM), 37°C, 1–2 days, microaerophilic conditions.
-
Detection of Yersinia spp. Three-phase enrichment [Phosphate Buffered Saline (PBS) with 2% sorbitol and 0.15% bile salts, 22–25°C, 3 h; cold enrichment in PBS, 4°C, 8 days and Yersinia Enrichment Broth acc. to Wauters (Merck), 22–25°C, 4 days] followed by cultivation on CIN Agar (Oxoid), 30°C, 20 h.
-
Detection of coagulase-positive Staphylococcus spp. Cultivation on Baird Parker Agar (Difco) + Egg Yolk Tellurite Enrichment (Difco), 37°C, 2 days.
In addition to the preliminary tests above, these isolates were also identified by motility, physiological tests (API, bioMérieux, Marcy-l′Etoile, France) and FAME (fatty acid methyl ester profile, MIS Standard Libraries, MIDI Inc., Newark, DE, USA). Specific Ani™ agglutination tests (Ani Biotech Oy, Vantaa, Finland) were carried out for potential Salmonella and S. aureus isolates. The enrichment methods used gave only qualitative results, i.e. pathogens detected or not detected in a sample of 25 g.
Automated ribotyping of isolates
Characterisation of isolates was performed using the automated ribotyping device RiboPrinter® System (DuPont Qualicon, Wilmington, DE, USA) with PvuII (actinobacteria) or EcoRI (other bacteria) as a restriction enzyme [6]. A ribogroup is defined as a set of closely related patterns that are mathematically indistinguishable from one another by the system. A ribogroup may include one or more (composite) patterns generated from the same or different isolates (strains). At least one isolate from each ribogroup (ribotype) was subjected to partial sequencing of the 16S rRNA gene.
In order to compare the riboprint patterns of isolates and those of relevant type strains (chosen on the basis of the sequencing results), the patterns were transferred to the BioNumerics programme (Applied Maths, Sint-Martens-Latem, Belgium) and analysed by clustering methods using Pearson correlation and UPGMA. The relevant type strains were obtained from DSMZ (Deutsche Sammlung von Mikroorganismen und Zellkulturen, Braunschweig, Germany), LMG (BCCM™/LMG bacteria collection, Gent, Belgium), CIP (Pasteur Institut, bacteria collection, Paris, France) or ATCC (American Type Culture Collection, Manassas, VA, USA).
Sequencing of the 16S rRNA gene
Total genomic DNA was obtained by lysing the cells mechanically using a FastPrep™ FP120 cell homogeniser (Savant Instruments, Inc., Holbrook, NY, USA). PCR was performed on a crude cell lysate using primers BSF8/20 and BSR1541/20 (http://bioinformatics.psb.ugent.be/webtools/rRNA/primers/BS_lst.html) as described earlier [22]. Prior to sequencing, amplification products were purified using a QIAquick PCR purification kit (Qiagen GmbH, Hilden, Germany) according to the manufacturer’s instructions. Sequencing reactions of PCR products were performed with the ABI PRISM® BigDye® Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, Foster City, CA, USA) according to the manufacturer’s instructions using the forward primer BSF8/20. Sequences were analysed with an ABI PRISM 3100 Genetic Analyser (Applied Biosystems) and corrected manually (Chromas version 2.13, Technelysium, Australia). Similarity searching of sequences was performed using BLAST (NCBI) analysis (http://www.ncbi.nlm.nih.gov/blast/Blast.cgi). Most closely related type strains were also searched from RDPII database (http://rdp.cme.msu.edu/index.jsp). One sequence from each species was deposited in GeneBank with the accession numbers EU438937–EU438998.
Results
A total of 179 non-spore-forming isolates were phenotypically tested, 124 were ribotyped and 113 were partially sequenced by the 16S rRNA gene. A total of 16 Pvu and 59 Eco-ribogroups were generated representing 39 genera and 63 taxa or species (Table 1). The detected bacteria could be divided into seven taxonomical classes: actinobacteria (11 species), bacilli (7), flavobacteria (3), alphaproteobacteria (10), betaproteobacteria (5), gammaproteobacteria (25) and sphingobacteria (2) (Fig. 1).
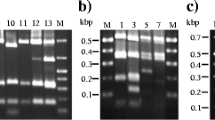
Most of the actinobacterial isolates (55%) belonged to the genus Microbacterium and the most frequently occurring bacterium was most closely related to M. barkeri (7 samples/3 mills) (Table 1). However, both the sequence and riboprint similarities to the type strain were low, indicating one or more new paper mill species close to M. barkeri. The obtained sequences were similar with each other, but ribotyping generated different ribotypes, which did not cluster with the type strain or with each other (Fig. 2). Digestion with PvuII was better than with EcoRI, but even with PvuII it was only satisfactory, generating only one or two clear fragments (at least 3–4 fragments should be generated). In addition, there was undigested DNA (e.g. the C. funkei isolate and type strain were not digested at all) and background, which reduced the similarity values (>0.85 required for identification). In addition to actinobacteria, the Brevundimonas isolates were digested with PvuII, because there was no digestion with EcoRI.
Among bacilli, sporadic isolates of Enterococcus casseliflavus, Listeria innocua, L. monocytogenes and Staphylococcus warneri were detected, of which only L. monocytogenes is a food pathogen. These isolates were reliably identified by conventional (data not shown) and by genetic methods (Fig. 3). From the enrichment conditions for Listeria and S. aureus, most isolates turned out to be Bacillus species, which were not considered here, because they are spore-formers.
The dominant flavobacterium was closely related to the recently described species Cloacibacterium normanense and was typical for mill B (Table 1). Here again, the obtained sequences were similar, but the riboprints were different (Fig. 3) and the similarities to the type strain were low. The patterns included only one or two fragments, indicating that the digestion with EcoRI (the most commonly used enzyme) was not good enough.
The largest class among proteobacteria (40 species) was gammaproteobacteria (25 species), which includes enterobacteria and most pseudomonads. Enterobacteria (Enterobacter, Escherichia, Klebsiella, Leclercia) were isolated especially from the enrichment conditions for salmonella, but also for campylobacteria and yersinia. Most enterobacteria were members of the genus Enterobacter (7 species) or Klebsiella (5 species) (Table 1). Among these bacteria the sequencing and ribotyping results agreed rather well in most cases (Fig. 4a). The partial 16S rRNA gene sequence of strain E-95644 was identical with those of different E. coli strains in GeneBank, but the closest type strain was Shigella flexneri. However, API (data not shown) and different riboprints identified it as E. coli (Fig. 4a). Hence, it is considered to be an atypical environmental strain of E. coli. Isolates of the genera Salmonella (gammaproteobacteria), Campylobacter (epsilonproteobacteria) or Yersinia (gammaproteobacteria) were not detected. Isolates of Ralstonia mannitolilytica and of Pseudoxanthomonas taiwanensis resulted in similar sequences and riboprints, respectively. Thus, their composite riboprint patterns are only represented in Fig. 4a. The clustering of generated riboprints of alpha- and betaproteobacteria are presented in Fig. 4b.
The dominant sphingobacteria were most closely related to the recently described species Sphingobacterium composti (Table 1, Fig. 3) and they were typical to mill A. On the basis of low similarities to the type strain, these isolates are also potential members of one or more new species.
Discussion
In this study, 16 untreated pulps containing recycled fibres were analysed for non-spore-forming aerobically grown bacteria, with special reference to proteobacteria including enterobacteria (hygiene indicators), pseudomonads and food pathogens. Many of the isolates were members of relatively new species validly described during the current decade, e.g. Pseudoxanthomonas taiwanensis [7], Cloacibacterium normanense [1] and Sphingobacterium composti [31]. Many isolates were atypical members of their closest species, or potential members of novel species or of species several times reclassified (such as the pseudomonads), which is confusing when comparing old and new literature. For example, Microbacterium barkeri was earlier known as Corynebacterium barkeri and Aureobacterium barkeri. In Genebank there are several sequences of proposed novel type strains, e.g. within the genera Chryseobacterium and Exiguobacterium, but when writing this article these species were not yet accepted to the list of valid names (http://www.dsmz.de/microorganisms/bacterial_nomenclature) and they were not considered in this study. However, this may explain why some sequencing and ribotyping results did not agree very well. The current closest described species are not actually the correct type strains for these isolates. Ribotyping is more discriminatory than partial sequencing of the 16S rRNA gene, because it analyses the whole operon of the ribosomal genes including the highly variable spacer regions lying between them [5] and it may characterise isolates below the species level. Hence, it is a good tool to support the sequencing results and to group the identical isolates.
Paper machine environments are favourable for microbial growth because of the suitable temperatures (30–50°C), pH values of process waters (4–10) and the presence of cellulose, starch, casein, resin sizers and other nutrients, which many microbes are able to degrade [9, 33]. The frequently occurring bacteria were most closely related to Microbacterium barkeri, Pseudoxanthomonas taiwanensis, Cloacibacterium normanense and Sphingobacterium composti. Members of the genus Microbacterium are widely distributed in various environments, such as dairy, soil, sewage, water [11] and paper mills [9, this study] and they can be associated with plants, insects and clinical specimens [11]. The type strain of thermophilic P. taiwanensis was isolated from a hot spring in Taiwan [7]. It exhibits an unusual denitrification reaction, reducing nitrate to N2O only. N2O is a gas with neuroleptic activity on humans, but it is unlikely to be produced in paper machines as a result of low or no nitrate or nitrite availability in the process water. These bacteria appear to be common in recycled fibre and other pulps [9, 28] this study. Isolates of C. normanense were isolated from untreated wastewater from a water treatment plant in Norman, OK, USA [1], but their presence in paper mills has not been reported earlier. It was postulated that the source of this bacterium may have been the human gastrointestinal tract, but this could not be confirmed. However, the organism was found in large numbers in untreated wastewater, where it may play a role in removal of phosphate. S. composti was recently isolated from a compost that was collected near Daejeon city in South Korea [31]. It can degrade DNA, but is negative for degradation of macromolecules such as casein, collagen, starch, chitin, cellulose and xylan. This bacterium was typical only for one mill and most probably represents a new species within the genus Sphingobacterium.
Family Enterobacteriaceae comprises a large but relatively homogeneous phylogenetic group of bacteria [15]. Several enterobacteria are opportunistic pathogens belonging to the risk (hazardous) group 2 (http://www.dsmz.de/microorganisms/bacterial_nomenclature). They may cause different inflammations, problematic hospital infections and also food poisoning, e.g. strains of genera Salmonella, Yersinia and Escherichia [18]. They are mainly present in the intestine and faeces (coliforms) of humans and animals, but also free-living in various environments, e.g. in waters and on plants. Most of our isolates were members of the complex Enterobacter cloacae or Klebsiella pneumoniae . In Genebank, the species Enterobacter hormaechei has two published novel subspecies “oharae” and “steigerwaltii” [14], which are not yet included in the list of valid names but were considered in this study. One isolate of E. sakazakii, an emergent food pathogen associated with infant milk formula and some severe infections (e.g. meningitis) with a high level of mortality (40–80%) [4, 10, 18], was detected. There was growth in enrichment conditions for Salmonella, Campylobacter, Listeria, Yersinia and Staphylococcus species, but almost all of the characterised isolates were enterobacteria or Bacillus species and only single isolates of L. innocua and L. monocytogenes were detected, which indicates that these pathogens are very rare or unlikely in recycled fibres.
Coliforms and faecal streptococci (enterococci) have conventionally been used as hygiene indicators, which indicate faecal contamination of samples [18]. Coliforms are usually detected on Violet Red Bile Agar at 37 and 44°C, and for detection of faecal streptococci mEnterococcus Agar (i.e. Slanetz Bartley Medium) has been applied. Coliform bacteria cover a range of genera belonging to the enterobacteria. E. coli has conventionally been the key faecal indicator, but other opportunistic pathogens are also found among other coliforms (non-faecal coliforms) belonging to genera Enterobacter, Klebsiella and Serratia, several isolates of which were also detected in this study. The numbers of these bacteria expressed as coliforms in some pulps have been reported earlier to be as high as 106 cfu g−1 d.w. [23], which is in good accordance with the results of this study, but only one atypical E. coli isolate (faecal coliform) was detected. The presence of faecal streptococci has also been reported in some pulps up to 104 cfu g−1 d.w. [23]. However, Enterococcus faecalis or E. faecium isolates, which indicate recent faecal contamination, were not detected in this study. Only the presence of E. casseliflavus was detected. The heat treatment (80–120°C) and drying at the end of the processes in the studied mills had eliminated all enterobacteria, which were no longer present in board samples made from these pulps [23]. The presence of these hygiene indicators (coliforms) has been reported to be common in Canadian paper mills and they grew very well on mill effluent [12]. However, E. coli isolates were recognised to be harmless non-toxigenic serotypes.
The traditional group of the pseudomonads has turned out to be phylogenetically extremely heterogeneous and the members have been scattered throughout the alpha-, beta- and gammaproteobacteria [15]. Pseudomonads include species of the genus Pseudomonas and genera reclassified from it (e.g. Brevundimonas, Burkholderia, Ralstonia/Cupriavidus, Sphingomonas/Novosphingobium, Stenotrophomonas). Several of them are opportunistic pathogens and members of risk group 2. For example Burkholderia cepacia occurs in environmental and clinical sources and may cause cystic fibrosis [32] as well as Cupriavidus (Ralstonia) respiraculi [8]. Pseudomonads may be detected using several universal media, but there are also selective agars for them. It is possible that the cultivation on universal agars in this study did not favour their detection and thus these results may underestimate their occurrence in recycled fibres. It has been reported that especially the presence of bacteria of the complex Burkholderia cepacia may be high in the wet end of paper mills [33]. The cell walls of Gram-negative bacteria contain toxic lipopolysaccharides (LPS) (http://www.cyberlipid.org/glycolip/glyl0005.htm), which if inhaled with aerosols may cause several symptoms, such as tiredness, fever, headache, diarrhoea and blood pressure decrease in sensitive individuals, and in some cases even serious syndromes, e.g. adult respiratory distress syndrome (ARDS) (http://www.msu.edu/~moulinfr/lps.html). In addition to enterobacteria, pseudomonads are efficient slime-formers in paper mills [9, 17, 20]. In this study, especially slimy were the Skermanella and Pedobacter isolates.
It can be concluded that the non-spore-forming microbiota of recycled fibres is diverse. Among these bacteria there are several microbes (e.g. enterobacteria and pseudomonads), which may be harmful from the process point of view, causing spoilage of papermaking chemicals and disturbing the runability of processes by slime formation. Furthermore, Gram-negative bacteria may cause symptoms among mill workers, if bacterial aerosols are inhaled. However, from the product safety point of view these bacteria are not harmful, because they are eliminated at the end of processes and do not enter the final products. On the basis of this study, the presence of non-spore-forming food pathogens in recycled fibres is sporadic or even unlikely. The occurrence of faecal contamination of these samples was not indicated.
References
Allen TD, Lawson PA, Collins MD, Falsen E, Tanner RS (2006) Cloacibacterium normanense gen. nov., sp. nov., a novel bacterium in the family Flavobacteriaceae isolated from municipal wastewater. Int J Syst Evol Microbiol 56:1311–1316. doi:10.1099/ijs.0.64218-0
Anonymous (2002) Paper and board. European list of grades of recovered paper and board. European standard EN 643, pp 1–12
Blanco MA, Negro C, Gaspar I, Tijero J (1996) Slime problems in paper and board industry. Appl Microbiol Biotechnol 46:203–208. doi:10.1007/s002530050806
Bowen AB, Braden CR (2006) Invasive Enterobacter sakazakii disease in infants. Emerg Infect Dis 12:1185–1189
Brosius J, Ullrich A, Raker MA, Gray A, Dull TJ, Gutell RR et al (1981) Construction and fine mapping of recombinant plasmids containing the rrnB ribosomal RNA operon of E. coli. Plasmid 6:112–118. doi:10.1016/0147-619X(81)90058-5
Bruce J (1996) Automated system rapidly identifies and characterizes micro-organisms in food. Food Technol 50:77–81
Chen MY, Tsay SS, Chen KY, Shi YC, Lin YT, Lin GH (2002) Pseudoxanthomonas taiwanensis sp. nov., a novel thermophilic, N2O-producing species isolated from hot spring. Int J Syst Evol Microbiol 52:2155–2161. doi:10.1099/ijs.0.02306-0
Coenye T, Vandamme P, LiPuma JJ (2003) Ralstonia respiraculi sp. nov., isolated from the respiratory tract of cystic fibrosis patients. Int J Syst Evol Microbiol 53:1339–1342. doi:10.1099/ijs.0.02440-0
Desjardins E, Beaulieu C (2003) Identification of bacteria contaminating pulp and a paper machine in a Canadian paper mill. J Ind Microbiol Biotechnol 30:141–145
Drudy D, Mullane NR, Quinn T, Wall PG, Fanning S (2006) Enterobacter sakazakii: an emerging pathogen in powdered infant formula. Food Saf 42:996–1002
Evtushenko LI, Takeuchi M (2006) The family Microbacteriaceae. The genus Microbacterium. Prokaryotes 3:1056–1066. doi:10.1007/0-357-30743-5_43. Springer, New York
Gauthier F, Archibald F (2001) The ecology of “fecal indicator” bacteria commonly found in pulp and paper mill water systems. Water Res 35:2207–2218. doi:10.1016/S0043-1354(00)00506-6
Hainje H (1990) Safety risks from paper and board. Papier Kunstoff-Verarbeiter Int 3:4–7
Hoffmann H, Stindl S, Ludwig W, Stumpf A, Mehlen A, Monget D et al (2005) Enterobacter hormaechei subsp. oharae subsp. nov., E. hormaechei subsp. hormaechei comb. nov., and E. hormaechei subsp. steigerwaltii subsp. nov., three new subspecies of clinical importance. J Clin Microbiol 43:3297–3303. doi:10.1128/JCM.43.7.3297-3303.2005
Kersters K, de Vos P, Gillis M, Swings J, Vandamme P, Stackerbrandt E (2006) Introduction to the proteobacteria. Prokaryotes 5:3–37. doi:10.1007/0-387-30745-1_1. Springer, New York
Lahtinen T, Kosonen M, Tiirola M, Vuento M, Oker-Blom C (2006) Diversity of bacteria contaminating paper machines. J Ind Microbiol Biotechnol 33:734–740. doi:10.1007/s10295-006-0105-4
Martin CH (1988) Identification and implication of troublesome slime-forming bacteria found in paper mill systems. TAPPI Proceedings, pp 91–95
Pandey A, Joshi VK, Nigam P, Soccol CR (2002) Enterobacteriaceae, coliforms and E. coli. In: Robinson RK, Batt CA, Patel PD (eds) Encyclopedia of food microbiology, vol 1. Academic Press, London, pp 604–610
Raaska L, Sillanpää J, Sjöberg AM, Suihko ML (2002) Potential microbiological hazards in the production of refined paper products for food applications. J Ind Microbiol Biotechnol 28:225–231. doi:10.1038/sj.jim.7000238
Rättö M, Suihko ML, Siika-aho M (2005) Polysaccharide-producing bacteria isolated from paper machine slime deposits. J Ind Microbiol Biotechnol 32:109–114. doi:10.1007/s10295-005-0210-9
Rättö M, Verhoef R, Suihko ML, Blanco A, Schols HA, Voragen AGJ et al (2006) Colanic acid is an exopolysaccharide common to many enterobacteria isolated from paper-machine slimes. J Ind Microbiol Biotechnol 33:359–367. doi:10.1007/s10295-005-0064-1
Saarela M, Alakomi HL, Suihko ML, Maunuksela L, Raaska L, Mattila-Sandholm T (2004) Heterotrophic microorganisms in air and biofilm samples from Roman catacombs with a special emphasis on actinobacteria and fungi. Int Biodet Biodeg 54:27–37. doi:10.1016/j.ibiod.2003.12.003
Sipiläinen-Malm T, Latva-Kala K, Tikkanen L, Suihko ML, Skyttä E (1997) Purity of recycled fibre-based materials. Food Addit Contam 14:695–703
Sorelle PH, Belgard WE (1991) The effect of recycled fiber use on paper machine biological control. TAPPI Proceedings, pp 569–575
Suihko ML, Hoekstra ES (1999) Fungi present in some recycled fibre pulps and paperboards. Nord Pulp Pap Res J 14:199–203. doi:10.3183/NPPRJ-1999-14-03-p199-203
Suihko ML, Skyttä E (1997) A study of the microflora of some recycled fibre pulps, boards and kitchen rolls. J Appl Microbiol 83:199–207. doi:10.1046/j.1365-2672.1997.00219.x
Suihko ML, Stackebrandt E (2003) Identification of aerobic mesophilic bacilli isolated from board and paper products containing recycled fibres. J Appl Microbiol 94:25–34. doi:10.1046/j.1365-2672.2003.01803.x
Suihko ML, Sinkko H, Partanen L, Mattila-Sandholm T, Salkinoja-Salonen M, Raaska L (2004) Description of heterotrophic bacteria occurring in paper mills and paper products. J Appl Microbiol 97:1228–1235. doi:10.1111/j.1365-2672.2004.02416.x
Suihko ML, Kroppenstedt RM, Stackebrandt E (2006) Occurrence and characterization of actinobacteria and thermoactinomycetes isolated from pulp and board samples containing recycled fibres. J Ind Microbiol Biotechnol 33:183–191. doi:10.1007/s10295-005-0055-2
Svensson K, Hallikainen A, Hammarling L, Hellström T, Lillemark L, Nielsen PA et al (1994) Paper and board based on recycled fibres in food contact. TemaNord 1994:649. Nordic Council of Ministers, Copenhagen
Ten LN, Liu QM, Im WT, Aslam Z, Lee ST (2006) Sphingobacterium composti sp. nov., a novel DNase-producing bacterium isolated from compost. J Microbiol Biotechnol 16:1728–1733
Vandamme P, Holmes B, Vancanneyt M, Coenye T, Hoste B, Coopman R et al (1997) Occurrence of multiple genomovars of Burkholderia cepacia in cystic fibrosis patients and proposal of Burkholderia multivorans sp. nov. Int J Syst Bacteriol 47:1188–1200
Väisänen OM, Weber A, Bennasar A, Rainey FA, Busse HJ, Salkinoja-Salonen MS (1998) Microbial communities of printing paper machines. J Appl Microbiol 84:1069–1084. doi:10.1046/j.1365-2672.1998.00447.x
Acknowledgments
The isolates studied were from the samples collected during the project Safety of Recycled Fibres, which was coordinated by the Association of Packaging Technology and Research and financed by the Technology Development Centre (TEKES) and the pulp and paper industry in Finland and Sweden. The ribotyping of these isolates was started in the TEKES project 40116. The technical assistance of Helena Hakuli and Tarja Vappula, Aila Tuomolin and Taina Holm is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Suihko, ML., Skyttä, E. Characterisation of aerobically grown non-spore-forming bacteria from paper mill pulps containing recycled fibres. J Ind Microbiol Biotechnol 36, 53–64 (2009). https://doi.org/10.1007/s10295-008-0472-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-008-0472-0