Abstract
Streptomyces clavuligerus produces a large array of natural compounds with antibiotic, antitumor, β-lactamase inhibition or inmunomodulating activities. The production of cephamycin C, clavulanic acid and other compounds with a clavam structure has been studied for many years. A network of regulatory mechanisms is present in S. clavuligerus to control the formation of different compounds by pathway-specific regulators or pleiotropic regulators. The possible existence of a γ-butyrolactone signaling system in this streptomycete is emerging. In addition, S. clavuligerus possesses a stringent control mechanism somehow different from those previously reported in other Streptomyces species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
S. clavuligerus produces cephamycin C, a β-lactam antibiotic, the β-lactamase inhibitor clavulanic acid, and several compounds with a clavam structure and (3S, 5S) stereochemistry. All these compounds have the typical four-membered β-lactam ring. The 5S-clavam compounds, such as clavam-2-carboxylate, 2-formyloxymethylclavam, 2-hydroxymethylclavam, hydroxyethylclavam and alanylclavam, lack β-lactamase-inhibitory activity, which is apparently linked to the (3R, 5R) stereochemistry of clavulanic acid, but possess antifungal or antibacterial activities (for reviews see [4, 41]). In addition, S. clavuligerus produces the antibiotic holomycin with a pyrrothine structure [31], which has been described to have antitumor activity (Fig. 1). The formation of these compounds appears to be interrelated and controlled by a network of regulatory molecules. In this article, we consider some of the aspects that are known at present to be involved in these regulatory mechanisms. An antibiotic compound related to tunicamycin [31] and tacrolimus, an inmunomodulator [34], has also been reported to be produced by S. clavuligerus, but no studies have been carried out on these compounds and they will be not considered in this study.
Structure of some compounds with biological activity produced by S. clavuligerus. The structure of additional compounds with clavam structure and (3S, 5S) stereochemistry is shown at the bottom of Fig. 2
Antibiotic biosynthesis and genetics in S. clavuligerus
Cephamycin C and clavulanic acid biosynthesis
Production of cephamycin C in S. clavuligerus occurs in parallel with that of clavulanic acid. Biosynthetic genes for both compounds are adjacent in the genome forming a supercluster of about 60 kb. The cephamycin C gene cluster (Fig. 2a) contains biosynthetic enzyme-encoding genes such as pcbAB, pcbC, cefD, cefE, cefF, cmcI, cmcJ, cmcH [36, 40], genes for the biosynthesis of the α-aminoadipic precursor such as lat and pcd, genes for β-lactam resistance such as bla, pcbR and pbp74, the cmcT gene encoding a putative cephamycin transport protein, and the regulatory gene ccaR, encoding a SARP regulatory protein (Streptomyces-activator regulatory protein, see below; rewieved by [40]). The clavulanic acid cluster (Fig. 2b-I) carries biosynthetic enzyme-encoding genes ceaS2, bls2, cas2, pah2, gcaS and car [3, 4] (Figs. 2, 3), genes pbpA and pbp2 encoding penicillin-binding proteins [25], genes of unknown function such as cyp, oppA1 and oppA2, which are required for clavulanic acid biosynthesis, and gene claR, which encodes a LysR-type regulatory protein (see [41]).
Gene clusters encoding enzymes for cephamycin C (a), clavulanic acid (b-I) and clavams (b-II). A gene cluster containing genes paralogous to those of the clavulanic acid cluster is shown in (b-III). Notice, by the position of the pcbR gene, that the cephamycin C gene cluster is adjacent to the clavulanic acid gene cluster
Clavam biosynthesis
Three clusters of genes for clavulanic acid–clavam biosynthesis have been isolated in S. clavuligerus. The clavulanic acid cluster (already mentioned) was initially isolated by hybridization with the cas2 gene, encoding clavaminate synthase. A second cluster, named the clavam gene cluster, was isolated by using the cas1 gene, a duplicate gene encoding a clavaminate synthase isoenzyme, as probe [46]. This gene cluster contains genes cvm1 to cvm13, cvmG and cvmP (Fig. 2b-II). Their involvement in clavam biosynthesis was revealed by the disruption of certain genes (cvm1, cvm2 and cvm5) that resulted in mutants impaired in the production of some of the 5S clavams but still able to synthesize clavulanic acid. Disruption of the cvm2 gene resulted in a strain with low production of 2-hydroxymethylclavam and alanylclavam and completely unable to produce clavam-2-carboxylate, while disruption of cvm5 showed that synthesis of both hydroxymethylclavam and clavam-2-carboxylate was impaired (Fig. 3). Mutants disrupted in cvm1 produce undetectable levels of clavam-2-carboxylate, clavaminic acid or 2-hydroxymethylclavam [46]. The function of Cvm1, Cvm2 or Cvm5 in clavam biosynthesis is still unclear. No clear involvement of other genes of the clavam cluster in clavam or clavulanic acid formation has been found.
Hybridization of S. clavuligerus DNA with the pah2 gene of the clavulanic acid gene cluster, encoding proclavaminic acid hydrolase, allowed the identification of a second copy of the pah gene (later named pah1). A third cluster of genes for clavulanic acid biosynthesis, named the paralogous gene cluster, has been located surrounding pah1 [26, 27]. This cluster (Fig. 2b-III) contains genes duplicated from those of the first clavulanic acid cluster that were named ceaS1, bls1, pah1 and oat1 [61, 62]. Two additional genes named cvm6P and cvm7P have been located in this cluster. These last two genes are paralogous genes of cvm6 and cvm7 (located in the clavam gene cluster). Cvm7P is a regulatory protein of the LAL family and functions as a specific regulator for formation of 5S clavam structures. In spite of their similarity to cvm7, only the disruption of cvm7P (but not that of cvm7) results in complete loss of all the clavam structures without affecting clavulanic acid production [63].
Holomycin biosynthesis
The structure of holomycin is completely unrelated to that of the β-lactam compounds produced by S. clavuligerus. Holomycin is a yellow-colored, organic solvent-extractable compound that contains a disulfur bond and shows antibiotic activity against Gram-positive and Gram-negative bacteria. The holomycin producing cultures also contain small amounts of holothin, the deacylated precursor of holomycin. The wild-type strain, S. clavuligerus ATCC 27064, produces only traces of both compounds, close to their detection limits. However, mutants blocked in the regulatory gene ccaR or claR, and especially mutants blocked in the late steps of the clavulanic acid biosynthesis pathway (car, cyp, orf12, oppA2), produce levels of holomycin that range between 5 and 1,200-fold higher than the wild-type strain. All these mutants are clavulanic acid non-producers; however, surprisingly, clavulanic acid non-producer mutants blocked in early steps of the pathway (ceaS2, bls2) did not overproduce holomycin [16]. The enzymatic activity holomycin synthase, forming holomycin from holothin, increases proportionally with the levels of holomycin produced by the different mutants. Addition of arginine stimulates the production of holomycin. It is possible that a late intermediate of the clavulanic acid pathway (e.g., clavaminic acid) might trigger the formation of holomycin. This intermediate will be accumulated by the cells blocked in the late steps of the pathway, and the intracellular levels will increase in the presence of arginine, a precursor of clavulanic acid, which also stimulates the formation of holomycin. The genes for holomycin production have not yet been localized in S. clavuligerus genome.
Biosynthesis pathway for clavulanic acid and clavams. The steps between N-glycylclavaminic acid and clavaldehyde, or between clavaminic acid and 2-carboxymethylodenemclavam, are still unknown. The sequential formation of the different clavams has been deduced from the products formed by different mutants, but there is no knowledge of the enzymes involved
Regulation of antibiotic production in S. clavuligerus
The knowledge about the regulation of antibiotic production in S. clavuligerus is far from complete, but some insight has been obtained, both at the global regulatory level and at pathway-specific levels. These mechanisms are analyzed in the following sections (Fig. 4).
Pleiotropic regulatory factors
The activation of antibiotic biosynthesis is genetically controlled at several levels. The most fundamental level involves genes encoding pleiotropic regulators, which control both secondary metabolism and morphological differentiation. Many bld genes are known to control both sporulation and antibiotic production in S. coelicolor, but only bldA, bldH, bldN and bldG have been studied in other Streptomyces spp. The bldA gene encodes the tRNALeu for translation of the rare UUA codon [38] and its absence in the bldA-null mutant render this strain unable to produce actinorhodin and undecylprodigiosin and yields a bald phenotype [12, 69]. Expression of almost 150 genes, involved in different aspects of Streptomyces biology, appears to be affected by bldA in S. coelicolor, of which only two contain a TTA codon, indicating an upper level of regulation by TTA-containing transcriptional factors on polycistronic operons [19]. Although CcaR, the pathway-specific activator for cephamycin C-clavulanic acid production in S. clavuligerus, also contains a TTA codon, the S. clavuligerus bldA mutant has its aerial mycelium formation blocked, but there is no effect on translation of the ccaR gene or on antibiotic production levels [67]. The TTA codon context appears to have an important role on efficient mistranslation (i.e., in the context of a TTAC or TTAT sequence; [7]). Therefore, the CcaR bldA-independent translation might be determined by an in-frame mistranslation or frame-shift due to the G 3′ after the ccaR TTA [67].
The first reported bld gene affecting cephamycin C and clavulanic acid production in S. clavuligerus was bldG [9]. These authors demonstrated that expression of ccaR is bldG-dependent, thus placing bldG above ccaR and claR in the regulatory cascade that controls antibiotic production in S. clavuligerus. The bldG gene encodes an anti-anti-sigma factor [8] and, although the function of these factors remains unclear, they normally act close to an anti-sigma factor regulating the activity of the target sigma factor [14]. A key aspect of this sigma factor regulation system is the activation by phosphorylation at a conserved serine residue, since only the un-phosphorylated form is able to bind to the anti-sigma factor [8, 13].
One of the main targets through which bldA influences morphological differentiation is bldH; this gene has been renamed adpAc (in S. coelicolor). Both adpAc and adpAg (from S. griseus) contain a TTA codon [32, 64]. However, the regulation of both genes is different: while the S. griseus adpAg gene is directly regulated by ArpA, the receptor protein for the γ-butyrolactone signaling molecule A-factor [48, 49], adpAc transcription is independent of the γ-butyrolactone signaling system [64]. Once genes homologous to those involved in the γ-butyrolactone signal transduction are detected in the S. clavuligerus genome (M. T. López-García and N. Nardiz, personal communication), additional studies will allow the elucidation of the complex antibiotic regulatory cascade in this microorganism.
Pathway-specific regulatory factors
The ccaR gene identified within the cephamycin C biosynthetic gene cluster [52] encodes the positive regulatory factor for both cephamycin C and clavulanic acid production in S. clavuligerus. Disruption of ccaR gene results in an inability to produce cephamycin C and clavulanic acid that is restored by trans-complementation. CcaR, as other known regulatory factors included in the SARP family of proteins [70] such as RedD, ActII-ORF4, AfsR and DnrI [see in 23], does not contain helix-turn-helix DNA binding motifs but does possess an N-terminal DNA binding domain similar to that in the C-terminal end of the OmpR-family of proteins. However, unlike other reported cross-complementation events [60], ccaR does not complement an actII-ORF4 mutant of S. coelicolor [52].
Kyung et al. [37] identified the specific temporal-spatial expression profile of CcaR. Following the typical role of pathway-specific activators, CcaR controls antibiotic biosynthesis in S. clavuligerus by regulating the transcription of biosynthetic genes.
Several cephamycin C biosynthetic enzymes, such as lysine aminotransferase (LAT), isopenicillin-N synthase (IPNS), isopenicillin-N epimerase (IPNE) and desacetoxycephalosporin-C synthase (DAOCS), are clearly CcaR-dependent [1]. DNA-binding studies have demonstrated that CcaR interacts with the bidirectional cefD-cmcI promoter region of the cephamycin gene cluster [56]. Bearing in mind that the cefD promoter expresses a large transcript carrying transcriptionally coupled cefDE-pcd [53], CcaR could therefore simultaneously control early, middle and late genes of the pathway. Since it has been proposed that factors from SARP family recognize specific heptameric sequences sometimes overlapping with the -35 regions of structural genes [70], SARP boxes similar to those reported for genes controlled by ActII-ORF4 [2] and by DnrI [66] have been identified in the bidirectional region between the ATG start codon of cefD and cmcI. In contrast, CcaR does not show binding ability to other cephamycin C cluster promoter regions, such as those for blp and lat [56].
Binding sequences for CcaR within the clavulanic acid gene cluster remain to be identified; CcaR may act indirectly on the pathway-specific regulatory gene of this cluster, claR. This gene, located within the clavulanic acid gene cluster, encodes a protein similar to members of the LysR family of transcriptional activators and is specifically required for clavulanic acid production [54, 50]. Direct binding of CcaR to the claR promoter region has not been demonstrated and hypothetical targets, such as heptameric sequences SARP-like, are not evident.
Indeed, the ClaR transcriptional regulator is essential only for expression of the late genes of clavulanic acid biosynthesis, since a claR-null mutant accumulates the last intermediate of the pathway, clavaminic acid [50]. These authors identified the claR-dependent transcription of genes located immediately upstream and downstream of claR that have been later characterized as essential for clavulanic acid production. They encode an oligopeptide permease (oppA1) [42], the clavulanic-9-aldehyde reductase (car) [54] and a cytochrome P450 (cyp) [39, 43]. Expression of claR in ccaR-disrupted mutants is null as measured by Northern hybridization or S1-analysis. However PCR studies show that a claR-disrupted mutant expresses all the genes for clavulanic acid biosynthesis, although perhaps at lower levels (López-García, M.T., unpublished results).
It has been shown that CcaR controls expression from the ceaS2 promoter, since transcription of ceaS2, encoding the precursor-forming carboxyethyl-arginine-synthase, is almost eliminated in the ccaR mutant [61]. The ceaS2 promoter leads to a polycistronic transcript encompassing the genes ceaS2-bls2-pah2-cas2 encoding early enzymatic activities for clavulanic acid biosynthesis and is not controlled by ClaR [50, 51].
Therefore, while CcaR directly controls the production of cephamycin C by binding to promoter regions within the cephamycin C gene cluster, clavulanic acid regulation may be exerted in at least two ways: an indirect one mediated by a specific role of ClaR, and a second way that may involve CcaR directly regulating ceaS2 promoter activity.
The γ-butyrolactone signaling system
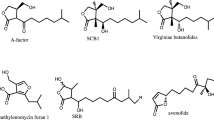
The existence of γ-butyrolactone type autoregulators acting as “microbial hormones” for the regulation of the onset of antibiotic production appears to be widespread in actinomycetes [15, 24]. Most of these autoregulators share a characteristic 2,3-disubstituted-γ-butyrolactone skeleton and are chemically classified into three types according to differences in the C2 side chain: (1) VB-type, which contains a 6-α-hydroxy group [35]; (2) the IM-2 type that contains a 6-β-hydroxy group [59, 65] and (3) the A-factor type, which contains a 6-keto group [45]. Additional autoinducer molecules different from the classical butyrolactone type have also been identified [55].
A gene encoding a butyrolactone receptor has been cloned from S. clavuligerus. Kim et al. [33] named this gene scaR and described studies supporting a dimeric form of the receptor as well as major binding affinity to IM-2 type molecules. Almost simultaneously, Santamarta et al. [57] characterized the function of the same orf, named brp, on clavulanic acid and cephamycin C production through gene disruption and DNA-protein mobility shift assays. Brp of S. clavuligerus has a repressor role, shown by a clear overproduction of both cephamycin C and clavulanic acid in the brp null mutant S. clavuligerus Δbrp. This is in agreement with the negative model of butyrolactone receptor proteins proposed in other Streptomyces species; in fact, the brp gene is similar to scbR from S. coelicolor [65], although the genomic environment in both genes is very different.
Specific sequences for binding of butyrolactone receptor proteins, named ARE boxes and consisting of palindromic inverted repeats [22, 24], have been described in several Streptomyces species, although the binding of receptors to those sequences has been demonstrated in only a few cases. Two different ARE sequences as targets for Brp have been identified in S. clavuligerus [57]. Brp directly binds an ARE target located in its own promoter region suggesting autoregulation as reported in ScbR in S. coelicolor, BarA in S. virginiae, and FarA in S. lavendulae [65]. The second ARE target sequence for Brp is located 815 nt upstream of the ccaR transcription start point, which is 75 nt upstream of the ccaR ATG codon [68]. In spite of being a great distance, clear additional open reading frames have not been identified between cmcH and ccaR; thus Brp might exert its repressor role through ccaR expression levels.
Very recently, the first example of multiple proteins binding to a γ-butyrolactone-receptor ARE target sequence was reported and characterized [58]. The AreB protein was isolated from S. clavuligerus through DNA-affinity chromatography, at the ARE sequence located upstream of the ccaR gene; it was identified as a member of the IclR family of regulatory proteins [44]. The areB gene is expressed from a divergent promoter region in an opposite orientation to the leuCD cluster for leucine biosynthesis. As reported in several back-to-back promoters [6], AreB plays a regulatory role in its own gene expression and also in the transcriptional regulation of the leuCD gene cluster. However, unlike the repressor role of the orthologous LtbR regulator in Corynebacterium glutamicum [10], AreB in S. clavuligerus is required for effective leucine assimilation and biosynthesis; in addition, AreB is required for fatty acid utilization as carbon source.
Cephamycin C and clavulanic acid levels are slightly increased in the absence of areB, as shown by antibiotic production analysis of the mutant strain S. clavuligerus ΔareB. Only a very small increase in ccaR transcription level, in contrast to a clear downregulation of brp expression, has been shown in the ΔareB mutant, and an indirect modulation role of AreB through brp has been proposed. Also, the characterization of the ΔareB mutant strain indicates a role for AreB in connecting primary and secondary metabolism. Underexpression of leucine biosynthetic genes in the ΔareB mutant leads to increased pools of valine precursor available for cephamycin C biosynthesis.
The most interesting finding about the role of this AreB additional protein in secondary metabolism regulation is the specific requirement of a small molecule for the binding of AreB to the ARE sequence, but not for the interaction with the areB-leuCD intergenic region. These findings suggest a differential effect of AreB on modulating antibiotic production, once the critical level of the small molecule is reached. Thus, AreB is identified as a member of a novel IclR-like subfamily specialized in connecting primary and secondary metabolism in S. clavuligerus.
Stringent response in S. clavuligerus
An important system in prokaryotes for sensing nutritional starvation and adapting to new environmental conditions is the “stringent response”. Under amino acid starvation conditions, the presence of uncharged tRNAs in the A-site of the ribosome activates the activity of the ribosome-associated protein RelA, which produces a burst in the level of the polyphosphorylated guanine nucleotide ppGpp with a concomitant decrease in the intracellular level of GTP. In E. coli, ppGpp binds the beta-subunit of RNA polymerase and provokes a global reduction of transcription, while genes for amino acid biosynthesis and response to stress are induced [11].
In Streptomyces, the decrease in intracellular GTP level is required for the onset of morphological differentiation, while the burst of ppGpp has been linked to the elicitation of secondary metabolism [47]. The first evidence of the link between ppGpp and secondary metabolism arose from the study of mutants lacking the stringent response in different Streptomyces species. These “relaxed” mutants are defective either in the RelA protein (i.e., relA mutants lacking ppGpp synthetase activity) or in the L11 ribosomal protein (mutants named either rplK or relC). Relaxed mutants are typically impaired in both antibiotic production and sporulation. Many of these mutants were initially obtained as thiopeptine-(a thiostrepton analogue) resistant clones [30]. In S. coelicolor, ppGpp synthesis switches the transcription from rapid-growth genes to stationary phase associated genes [20], including the induction of the actinorhodin pathway specific activator, but not that of undecylprodigiosin biosynthesis [21].
The stringent response in S. clavuligerus was initially studied by Bascarán et al. [5]. Fermentation studies did not establish a clear correlation between the onset of antibiotic production (either cephamycin C or clavulanic acid), and ppGpp intracellular levels [5, 29]. This lack of correlation between bursts of ppGpp and onset of antibiotic formation was later confirmed using the expression of structural genes (ceaS2, cefD) or regulatory genes (ccaR, claR) [17, 18]. It was shown that in typical batch fermentation in SA medium, S. clavuligerus wild-type strain produced antibiotic during the rapid growth phase, when the ppGpp level was still low, and stopped producing antibiotic after entering the stationary phase, concomitant with the highest level of ppGpp and a reduction in the mRNA abundance of antibiotic biosynthesis genes.
The rplK gene of S. clavuligerus encodes a 15.2 kDa protein with 93.7% identity to the homologous protein of S. coelicolor. Using this gene, a well-characterized relC mutant was obtained by deletion of the sequence encoding amino acids 29PALG32 in the RplK (L11) protein [17]. The mutant, S. clavuligerus rplK 29PALG32, shows a growth pattern only slightly delayed in relation to the wild-type strain and is more resistant to thiostrepton. As is usual in relC mutants, this strain produces very low levels of ppGpp in relation to the wild-type strain, and is unable to sporulate but does produce aerial mycelium. Cephamycin C and clavulanic acid production is impaired in S. clavuligerus rplK 29PALG32, which correlates well with the poor expression of the ccaR and claR regulatory genes, ceaS2 (for clavulanic acid biosynthesis) and cefD (for cephamycin biosynthesis). The impaired antibiotic production and sporulation of the relC mutant can be restored by complementation with the wild-type gene. These results agree with those found for other relC relaxed mutants of different antibiotic-producing Streptomyces [30, 47].
The relA gene of S. clavuligerus, that encodes an 843 amino acid protein, has been cloned and deleted or disrupted by two independent research groups. Jin et al. [28] disrupted relA by insertion of the hygromycin resistance gene to produce S. clavuligerus relA::hyg. Gomez-Escribano et al. [18] disrupted and completely deleted relA. Every relA-null mutant, S. clavuligerus relA::hyg [28], S. clavuligerus relA::neo or S. clavuligerus ΔrelA [18] is completely impaired in the formation of ppGpp, unable to sporulate and severely reduced in aerial mycelium formation. These phenotypes are reversed by reconstruction of the genetic organization of the relA region [28], or by complementation with the wild-type relA gene, or with a 804 bp DNA fragment encoding amino acids 228–495 of the RelA protein, which are expected to have ribosome-independent ppGpp-synthetase activity [18]. Surprisingly, although the S. clavuligerus relA::hyg mutant was unable to produce either cephamycin C or clavulanic acid in defined production medium (DP medium) containing glycerol as carbon source and arginine as nitrogen source, strains S. clavuligerus relA::neo and S. clavuligerus ΔrelA overproduce cephamycin C (sixfold) and clavulanic acid (fourfold) in defined SA medium, containing starch and asparagine as carbon and nitrogen sources. Similar results were also found in TSB complex medium. The data on higher levels of cephamycin C and clavulanic acid production by S. clavuligerus ΔrelA are supported by high resolution S1 nuclease protection analysis. Transcripts of cefD, and especially of ceaS2, are at much higher level in the relA-deleted mutant than in the wild type (Fig. 5). This was the first report of a relA-null mutant that overproduces antibiotics, but S. clavuligerus relaxed mutants, impaired in ppGpp synthesis, with higher cephamycin C production than the wild type had been previously found among spontaneous thiostrepton-resistant clones [5].
Comparison of S. clavuligerus ATCC 27064 and S. clavuligerus ΔrelA cultures grown in defined SA medium. a Growth (black circles), clavulanic acid production (black triangles), cephamycin C production (white triangles), intracellular ppGpp (white squares), intracellular GTP (black squares). b S1 nuclease protection analysis of the expression of ccaR (from tsp1), claR (from tsp2), cefD and ceaS2 in the wild type (left panel) and the ΔrelA::neo mutant (right panel) at 24, 36, 48 and 60 h of culture in SA medium. Notice the stronger expression and longer time of expression of the genes in the ΔrelA::neo mutant, concomitant with the higher antibiotic production shown in panel (a) for this strain
The different behavior of the relA-null mutants obtained by genetic engineering from the same parental strain, S. clavuligerus ATCC 27064, is surprising. However, sequencing of the intergenic region upstream of relA (147nt) shows marked differences between the parental strains used by both research groups. The nucleotide sequence of the intergenic region in S. clavuligerus ATCC 27064 used by Gomez-Escribano et al. [18] is identical to that of S. clavuligerus ATCC 27064 obtained independently in DSM (The Netherlands), while the sequence upstream of relA published for S. clavuligerus ATCC 27064 by Jin et al. [28] has an apparent deletion that affects the promoter region [18]. However, more differences between the strains, due to subculturing and differences in conservation, cannot be ruled out.
It is clear that the stringent response in S. clavuligerus plays a negative role in the control of antibiotic production. It is generally accepted that ppGpp is necessary for secondary metabolite production, which usually occurs during stationary growth phase. However, antibiotic production in S. clavuligerus takes place during rapid growth phase. Thus, the lack of correlation between ppGpp levels and antibiotic production is not surprising. More intriguing is the antibiotic overproduction by relA-null mutants, and it reveals the possibility of directed strain improvement by manipulating ppGpp metabolism in S. clavuligerus.
In summary, antibiotic production is subject to a complex network of interactions between small molecules, regulatory proteins and promoters of key genes. These molecules transmit the physiological and nutritional state of the cell to the antibiotic biosynthesis genes. At present, we know only little about these complex networks, which might be general and/or specific for every antibiotic-producing strain.
References
Alexander DC, Jensen SE (1998) Investigation of the Streptomyces clavuligerus cephamycin C gene cluster and its regulation by the CcaR protein. J Bacteriol 180:4068–4079
Arias P, Fernández-Moreno MA, Malpartida F (1999) Characterization of the pathway-specific positive transcriptional regulator for actinorhodin biosynthesis in Streptomyces coelicolor A3(2) as a DNA-binding protein. J Bacteriol 181:6958–6968
Arulanantham H, Kershaw NJ, Hewitson KS, Hughes CE, Thirkettle JE, Schofield CJ (2006) ORF17 from the clavulanic acid biosynthesis gene cluster catalyzes the ATP-dependent formation of N-glycyl-clavaminic acid. J Biol Chem 6:279–287
Baggaley KH, Brown AG, Schofield CJ (1997) Chemistry and biosynthesis of clavulanic acid and other clavams. Natl Prod Rep 140:309–333
Bascarán V, Sánchez L, Hardisson C, Braña AF (1991) Stringent response and initiation of secondary metabolism in Streptomyces clavuligerus. J Gen Microbiol 137:1625–1634
Beck CF, Warren RA (1988) Divergent promoters, a common form of gene organization. Microbiol Rev 52:318–326
Belcourt MF, Farabaugh PJ (1990) Ribosomal frameshifting in the yeast retrotransposon Ty: tRNAs induce slippage on a 7 nucleotide minimal site. Cell 62:339–352
Bignell DR, Warawa JL, Strap JL, Chater KF, Leskiw BK (2000) Study of the bldG locus suggests that an anti-anti-sigma factor and an anti-sigma factor may be involved in Streptomyces coelicolor antibiotic production and sporulation. Microbiology 146:2161–2173
Bignell DRD, Tahlan K, Colvin KR, Jensen SE, Leskiw BK (2005) Expression of ccaR, encoding the positive activator of cephamycin C and clavulanic acid production in Streptomyces clavuligerus, is dependent on bldG. Antimicrob Agents Chemother 49:1529–1541
Brune I, Jochmann N, Brinkrolf K, Hüser AT, Gerstmeir R, Eikmanns BJ, Kalinowski J, Pühler A, Tauch A (2007) The IclR-type transcriptional repressor LtbR regulates the expression of leucine and tryptophan biosynthesis genes in the amino acid producer Corynebacterium glutamicum. J Bacteriol 189:2720–2733
Cashel M, Gentry DR, Hernandez VJ, Vinella D (1996) The Stringent Response. In: Neidhardt FC, Curtiss III R, Ingraham JL, Lin ECC, Low KB, Magasanik B, Reznikoff WS, Riley M, Schaechter M, Umbarger HE (eds) Escherichia coli and Salmonella typhimurium: cellular and molecular biology. American Society for Microbiology, Washington DC, pp 1458–1496
Champness WC (1988) New loci required for Streptomyces coelicolor morphological and physiological differentiation. J Bacteriol 170:1168–1174
Diederich B, Wilkinson JF, Magnin T, Najafi M, Erringston J, Yudkin MD (1994) Role of interactions between SpoIIAA and SpoIIAB in regulating cell-specific transcription factor sigma F of Bacillus subtilis. Genes Dev 8:2653–2663
Duncan L, Losick R (1993) SpoIIAB is an anti-sigma factor that binds to and inhibits transcription by regulatory protein sigma F from Bacillus subtilis. Proc Natl Acad Sci 90:2325–2329
Folcher M, Gaillard H, Nguyen LT, Nguyen KT, Lacroix P, Bamas-Jacques N, Rinkel M, Thompson CJ (2001) Pleiotropic functions of a Streptomyces pristinaespiralis autoregulator receptor in development, antibiotic biosynthesis, and expression of a superoxide dismutase. J Biol Chem 276:44297–44306
Fuente A de la, Lorenzana LM, Martín JF, Liras P (2002) Mutants of Streptomyces clavuligerus with disruptions in different genes for clavulanic acid biosynthesis produce large amounts of holomycin: possible cross-regulation of two unrelated secondary metabolic pathways. J Bacteriol 184:6559–6565
Gomez-Escribano JP, Liras P, Pisabarro A, Martín JF (2006) An rplKΔ29−PALG−32 mutation leads to reduced expression of the regulatory genes ccaR and claR and very low transcription of the ceaS2 gene for clavulanic acid biosynthesis in Streptomyces clavuligerus. Mol Microbiol 61:758–770
Gomez-Escribano JP, Martin JF, Hesketh A, Bibb MJ, Liras P (2008) Streptomyces clavuligerus relA-null mutants overproduce clavulanic acid and cephamycin C: negative regulation of secondary metabolism by (p)ppGpp. Microbiology 154:744–755
Hesketh A, Bucca G, Laing E, Flett F, Hotchkiss G, Smith CP, Chater KF (2007) New pleiotropic effects of eliminating a rare tRNA from Streptomyces coelicolor, revealed by combined proteomic and transcriptomic analysis of liquid cultures. BMC Genomics 8:261
Hesketh A, Chen WJ, Ryding J, Chang S, Bibb M (2007) The global role of ppGpp synthesis in morphological differentiation and antibiotic production in Streptomyces coelicolor A3(2). Genome Biol 8:R161
Hesketh A, Sun J, Bibb M (2001) Induction of ppGpp synthesis in Streptomyces coelicolor A3(2) grown under conditions of nutritional sufficiency elicits actII-ORF4 transcription and actinorhodin biosynthesis. Mol Microbiol 39:136–144
Horinouchi S (2002) A microbial hormone, A-factor, as a master switch for morphological differentiation and secondary metabolism in Streptomyces griseus. Front Biosci 7:2045–2057
Horinouchi S (2003) AfsR as an integrator of signals that are sensed by multiple serine/threonine kinases in Streptomyces coelicolor A3(2). J Ind Microbiol Biotechnol 30:462–467
Horinouchi S (2007) Mining and polishing of the treasure trove in the bacterial genus Streptomyces. Biosci Biotechnol Biochem 71:283–299
Ishida K, Hung TV, Liou K, Lee HC, Shin CH, Sohng JK (2006) Characterization of pbpA and pbp2 encoding penicillin-binding proteins located on the downstream of clavulanic acid gene cluster in Streptomyces clavuligerus. Biotechnol Lett 28:409–417
Jensen SE, Elder KJ, Aidoo KA, Paradkar AS (2000) Enzymes catalyzing the early steps of clavulanic acid biosynthesis are encoded by two sets of paralogous genes in Streptomyces clavuligerus. Antimicrob Agents Chemother 44:720–726
Jensen SE, Paradkar AS, Mosher RH, Anders C, Beatty PH, Brumlik MJ, Griffin A, Barton B (2004) Five additional genes are involved in clavulanic acid biosynthesis in Streptomyces clavuligerus. Antimicrob Agents Chemother 48:192–202
Jin W, Ryu YG, Kang SG, Kim SK, Saito N, Ochi K, Lee SH, Lee KJ (2004) Two relA/spoT homologous genes are involved in the morphological and physiological differentiation of Streptomyces clavuligerus. Microbiology 150:1485–1493
Jones D, Thompson A, England R (1996) Guanosine 5′-diphosphate 3′-diphosphate (ppGpp), guanosine 5′diphosphate 3′monophosphate (ppGp) and antibiotic production in Streptomyces clavuligerus. Microbiology 142:1789–1795
Kawamoto S, Zhang D, Ochi K (1998) Sequence analysis of the ribosomal L11 protein gene (rplK = relC) in Streptomyces lavendulae using a deletion allele. J Antibiot 51:954–957
Kenig M, Reading C (1979) Holomycin and an antibiotic (MM 19290) related to tunicamycin, metabolites of Streptomyces clavuligerus. J Antibiot 32:549–554
Kim D-W, Chater K, Lee K-J, Hesketh A (2005) Changes in the extracellular proteome caused by the absence of the bldA gene product, a developmentally significant tRNA, reveal a new target for the pleiotropic regulator AdpA in Streptomyces coelicolor. J Bacteriol 187:2957–2966
Kim HS, Lee YJ, Lee CK, Choi SU, Yeo S, Hwang YI, Yu TS, Kinoshita H, Nihira T (2004) Cloning and characterization of a gene encoding the gamma-butyrolactone autoregulator receptor from Streptomyces clavuligerus. Arch Microbiol 182:44–50
Kim HS, Park YI (2007) Lipase activity and tacrolimus production in Streptomyces clavuligerus CKD1119 mutant strains. J Microbiol Biotechnol 17:1638–1644
Kondo K, Higuchi Y, Sakuda S, Nihira T, Yamada Y (1989) New virginiae butanolides from Streptomyces virginiae. J Antibiot 42:1873–1876
Kovacevic S, Tobin MB, Miller JR (1990) The beta-lactam biosynthesis genes for isopenicillin N epimerase and deacetoxycephalosporin C synthetase are expressed from a single transcript in Streptomyces clavuligerus. J Bacteriol 172:3952–3958
Kyung YS, Hu WS, Sherman DH (2001) Analysis of temporal and spatial expression of the CcaR regulatory element in the cephamycin C biosynthetic pathway using green fluorescent protein. Mol Microbiol 40:530–541
Lawlor E J, Baylis HA, Chater KF (1987) Pleiotropic morphological and antibiotic deficiencies result from mutations in a gene encoding a tRNA-like product in Streptomyces coelicolor A3(2). Genes Dev 1:1305–1310
Li R, Khaleeli N, Townsend CA (2000) Expansion of the clavulanic acid gene cluster: identification and in vivo functional analysis of three new genes required for biosynthesis of clavulanic acid by Streptomyces clavuligerus. J Bacteriol 182:4087–4095
Liras P (1999) Biosynthesis and molecular genetics of cephamycins. Cephamycins produced by actinomycetes. Antonie Van Leeuwenhoek 75:109–124
Liras P, Rodríguez-García A (2000) Clavulanic acid, a beta-lactamase inhibitor: biosynthesis and molecular genetics. Appl Microbiol Biotechnol 54:467–475
Lorenzana LM, Pérez-Redondo R, Santamarta I, Martín JF, Liras P (2004) Two oligopeptide-permease-encoding genes in the clavulanic acid cluster of Streptomyces clavuligerus are essential for production of the beta-lactamase inhibitor. J Bacteriol 186:3431–3438
Mellado E, Lorenzana LM, Rodríguez-Sáiz M, Díez B, Liras P, Barredo JL (2002) The clavulanic acid biosynthetic cluster of Streptomyces clavuligerus: genetic organization of the region upstream of the car gene. Microbiology 148:1427–1438
Molina-Henares AJ, Krell T, Guazzaroni ME, Segura A, Ramos JL (2006) Members of the IclR family of bacterial transcriptional regulators function as activators and/or repressors. FEMS Microbiol Rev 30:157–186
Mori K (1983) Revision of the absolute configuration of A-factor, the inducer of streptomycin biosynthesis, basing on the reconfirmed (R)-configuration of (+)-paraconic acid. Tetrahedron 39:3107–3109
Mosher RH, Paradkar AS, Anders C, Barton B, Jensen SE (1999) Genes specific for the biosynthesis of clavam metabolites antipodal to clavulanic acid are clustered with the gene for clavaminate synthase 1 in Streptomyces clavuligerus. Antimicrob Agents Chemother 43:1215–1224
Ochi K (1986) Occurrence of the stringent response in Streptomyces sp. and its significance for the initiation of morphological and physiological differentiation. J Gen Microbiol 132:2621–2631
Ohnishi Y, Kameyama S, Onaka H, Horinouchi S (1999) The A-factor regulatory cascade leading to streptomycin biosynthesis in Streptomyces griseus: identification of a target gene of the A-factor receptor. Mol Microbiol 34:102–111
Ohnishi Y, Yamazaki H, Kato J, Tomono A, Horinouchi S (2005) AdpA, a central transcriptional regulator in the A-factor regulatory cascade that leads to morphological development and secondary metabolism in Streptomyces griseus. Biosci Biotechnol Biochem 69:431–439
Paradkar AS, Aidoo KA Jensen SE (1998) A pathway-specific transcriptional activator regulates late steps of clavulanic acid biosynthesis in Streptomyces clavuligerus. Mol Microbiol 27:831–843
Paradkar AS, Jensen SE (1995) Functional analysis of the gene encoding the clavaminate synthase 2 isoenzyme involved in clavulanic acid biosynthesis in Streptomyces clavuligerus. J Bacteriol 177:1307–1314
Pérez-Llarena FJ, Liras P, Rodríguez-García A, Martín JF (1997) A regulatory gene (ccaR) required for cephamycin and clavulanic acid production in Streptomyces clavuligerus: amplification results in overproduction of both beta-lactam compounds. J Bacteriol 179:2053–2059
Pérez-Llarena FJ, Rodríguez-García A, Enguita FJ, Martín JF, Liras P (1998) The pcd gene encoding piperideine-6-carboxylate dehydrogenase involved in biosynthesis of alpha-aminoadipic acid is located in the cephamycin cluster of Streptomyces clavuligerus. J Bacteriol 180:4753–4756
Pérez-Redondo R, Rodríguez-García A, Martín JF, Liras P (1998) The claR gene of Streptomyces clavuligerus, encoding a LysR-type regulatory protein controlling clavulanic acid biosynthesis, is linked to the clavulanate-9-aldehyde reductase (car) gene. Gene 211:311–321
Recio E, Colinas A, Rumbero A, Aparicio JF, Martín JF (2004) PI factor, a novel type quorum-sensing inducer elicits pimaricin production in Streptomyces natalensis. J Biol Chem 279:41586–41593
Santamarta I, Rodríguez-García A, Pérez-Redondo R, Martín JF, Liras P (2002) CcaR is an autoregulatory protein that binds to the ccaR and cefD-cmcI promoters of the cephamycin C-clavulanic acid cluster in Streptomyces clavuligerus. J Bacteriol 184:3106–3113
Santamarta I, Pérez-Redondo R, Lorenzana LM, Martín JF, Liras P (2005) Different proteins bind to the butyrolactone receptor protein ARE sequence located upstream of the regulatory ccaR gene of Streptomyces clavuligerus. Mol Microbiol 56:824–835
Santamarta I, López-García MT, Pérez-Redondo R, Koekman B, Martín JF, Liras P (2007) Connecting primary and secondary metabolism: AreB, an IclR-like protein, binds the ARE ccaR sequence of S. clavuligerus and modulates leucine biosynthesis and cephamycin C and clavulanic acid production. Mol Microbiol 66:511–524
Sato K, Nihira T, Sakuda S, Yamagimoto M, Yamada Y (1989) Isolation and structure of a new butyrolactone autoregulator from Streptomyces sp. FRI-5. J Ferment Bioeng 68:170–173
Stutzman-Engwall KJ, Otten SL, Hutchinson CR (1992) Regulation of secondary metabolism in Streptomyces spp. and overproduction of daunorubicin in Streptomyces peucetius. J Bacteriol 174:144–154
Tahlan K, Anders C, Jensen SE (2004a) The paralogous pairs of genes involved in clavulanic acid and clavam metabolite biosynthesis are differently regulated in Streptomyces clavuligerus. J Bacteriol 186:6286–6297
Tahlan K, Park HU, Wong A, Beatty PH, Jensen SE (2004b) Two sets of paralogous genes encode the enzymes involved in the early stages of clavulanic acid and clavam metabolite biosynthesis in Streptomyces clavuligerus. Antimicrob Agents Chemother 48:930–939
Tahlan K, Anders C, Wong A, Mosher RH, Beatty PH, Brumlik MJ, Griffin A, Hughes C, Griffin J, Barton B, Jensen SE (2007) 5S clavam biosynthetic genes are located in both the clavam and paralog gene clusters in Streptomyces clavuligerus. Chem Biol 14:131–142
Takano E, Tao M, Long F, Bibb MJ, Wang L, Li W, Buttner MJ, Bibb MJ, Deng ZX, Chater KF (2003) A rare leucine codon in adpA is implicated in the morphological defect of bldA mutants of Streptomyces coelicolor. Mol Microbiol 50:475–486
Takano E (2006) γ-butyrolactones: Streptomyces signalling molecules regulating antibiotic production and differentiation. Curr Opin Microbiol 9:287–294
Tang L, Grimm A, Zhang YX, Hutchinson CR (1996) Purification and characterization of the DNA-binding protein DnrI, a transcriptional factor of daunorubicin biosynthesis in Streptomyces peucetius. Mol Microbiol 22:801–813
Trepanier NK, Jensen SE, Alexander DC, Leskiw BK (2002) The positive activator of cephamycin C and clavulanic acid production in Streptomyces clavuligerus is mistranslated in a bldA mutant. Microbiology 148:643–656
Wang L, Tahlan K, Kaziuk TL, Alexander DC, Jensen SE (2004) Transcriptional and translational analysis of the ccaR gene from Streptomyces clavuligerus. Microbiology 150:4137–4145
White J, Bibb M (1997) bldA dependence of undecylprodigiosin production in Streptomyces coelicolor A3(2) involves a pathway-specific regulatory cascade. J Bacteriol 179:627–633
Wietzorrek A, Bibb M (1997) A novel family of proteins that regulates antibiotic production in streptomycetes appears to contain an OmpR-like DNA-binding fold. Mol Microbiol 25:1181–1184
Aknowledgments
This research was supported by grants from the CICYT (Madrid) (Proyecto Bio2006-14853) and by the European Proyect LSHM-CT-2004-005224. We thank Prof. A. L. Demain for correcting the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liras, P., Gomez-Escribano, J.P. & Santamarta, I. Regulatory mechanisms controlling antibiotic production in Streptomyces clavuligerus . J Ind Microbiol Biotechnol 35, 667–676 (2008). https://doi.org/10.1007/s10295-008-0351-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-008-0351-8