Abstract
A field experiment was performed to monitor changes in exogenous bacteria and to investigate the diversity of indigenous bacteria during a field trial of microbial enhanced oil recovery (MEOR). Two wells (26–195 and 27–221) were injected with three exogenous strains and then closed to allow for microbial growth and metabolism. After a waiting period, the pumps were restarted and the samples were collected. The bacterial populations of these samples were analyzed by denaturing gradient gel electrophoresis (DGGE) with PCR-amplified 16S rRNA fragments. DGGE profiles indicated that the exogenous strains were retrieved in the production water samples and indigenous strains could also be detected. After the pumps were restarted, average oil yield increased to 1.58 and 4.52 tons per day in wells 26–195 and 27–221, respectively, compared with almost no oil output before the injection of exogenous bacteria. Exogenous bacteria and indigenous bacteria contributed together to the increased oil output. Sequence analysis of the DGGE bands revealed that Proteobacteria were a major component of the predominant bacteria in both wells. Changes in the bacteria population in the reservoirs during MEOR process were monitored by molecular analysis of the 16S rRNA gene sequence. DGGE analysis was a successful approach to investigate the changes in microorganisms used for enhancing oil recovery. The feasibility of MEOR technology in the petroleum industry was also demonstrated.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Microbial enhanced oil recovery (MEOR) processes employ microbial metabolites to achieve several aims, including lowering interfacial tension between brine and oil, reducing oil viscosity and enhancing permeability in limestone reservoirs [25]. Compared to other EOR processes, MEOR processes have several advantages: lower energy consumption, no thermal processes, and no chemical processes [1, 15, 16]. However, the technical performances in many field trials have been inconsistent [36]. It is difficult for microbiologists to monitor and control the conditions for optimal performance during the process of MEOR. First, the injected exogenous strains are hard to track. Many studies have been done in oil reservoirs [32], however, few report the monitoring the changes of injected strains for MEOR [36]. The relationship between the microbial operation and oil yields is still very vague. Therefore, microbial processes are generally viewed with considerable skepticism.
Fingerprinting techniques, such as denaturing gradient gel electrophoresis (DGGE) or terminal restriction fragment length polymorphism (T-RFLP) analysis, offer the best compromise between the number of samples processed and the information obtained. DGGE, in particular, provides both rapid comparison data for many communities and specific phylogenetic information derived from excised bands [5]. DGGE has recently become a common technique to detect bacterial population ecology and dynamics in environmental samples, especially multiple samples [12, 17–19, 27]. As for oil reservoirs, there have been studies mainly on microbial diversity in oil wells and bacterial changes during their exploitation [7, 8, 13, 21].
The aim of this work was to assess the efficacy of MEOR field trials via exogenous bacterial injection, monitor bacterial population changes in the oil wells and prepare a time course of the diversity profile using molecular fingerprinting to demonstrate if the exogenous bacteria and their indigenous counterparts effect oil recovery in the Jin45 zone at the Liaohe Oil Field, Liaoning Province, China [11]. The Jin45 zone has been in primary production since November 1984. Steam stimulation was used from January 1986 to November 1991. Then, filling pattern technology was adopted twice. From May 2006, we employed an MEOR approach to replace steam stimulation.
Materials and methods
Strains and media
Three strains, LJ-1, LJ-2 and LJ-3, were isolated from an oil-polluted soil sample in the Liaohe Oil Field. The mineral medium for oil utilization contained (g l−1): Na2HPO4 1.5, KH2PO4 3.48, (NH4)2SO4 4, MgSO4 · 7H2O 0.7, yeast extract 0.01 and 0.5% (w/v) crude oil, pH 7.2. The medium was incubated at 37 °C [35]. Surface tension was measured with an interfacial tensiometer (JK99B, Powereach, Shanghai) at room temperature. The crude oil-degrading ability was measured by infrared spectrophotometry (M-22A, Midwest group, Beijing). Experiments were repeated at least twice, and consistent results were obtained.
Preparation of the inocula
Three bacteria, LJ-1, LJ-2 and LJ-3, were used as the inoculum. They were separately cultivated in 200 m l of mineral medium with 2% (v/v) liquid paraffin as the sole carbon source. When the culture reached the late exponential growth phase (7.2 × 108 cells ml−1), it was used to inoculate a 10-l carboy of the same medium, which was incubated at 37 °C for 48 h with agitation and aeration until the culture reached the late exponential phase of growth. One liter of cell concentrate was used to inoculate a 132-l tank. The medium was prepared aseptically due to the lack of facilities on site, and tanks were incubated for approximately 48 h at ambient temperature.
Field experiment
A field expedition was conducted on two oil production wells 26–195 and 27–221 in the Jin45 zone of Liaohe Oil Field. First, 5,000 l of formation water was pumped out from each well (flow rate, 500 l min−1) before injecting exogenous bacteria. Then, the two wells each received two separate inoculations with the three strains (consortium LJ) on 16 June and 26 July 2006, including 374 l of strain LJ-1 (6.7 × 107 cells ml−1), 293 l of strain LJ-2 (7.8 × 108 cells ml−1) and 333 l of strain LJ-3 (7.7 × 108 cells ml−1). After inoculation, 15,000 l of mineral medium (as nutrient) and 5,000 l of formation water were injected successively (flow rate, 500 l min−1). Finally, production was stopped for 10 days to allow time for bacterial growth and metabolism. After this incubation period, the pumps were restarted and water samples were collected over time. Flow meters were attached to the tubing of the wells to measure the volume of fluids produced. The total volume produced was recorded when each sample was collected.
Sampling
Production water samples were collected every week from the two oil production wells. Samples were colleted in bottles that had previously been autoclaved and rinsed with water from each sample. All samples were stored at 4 °C and then analyzed within 48 h .
DNA extraction
DNA was extracted from water samples using a modified method [33]. Each water sample (1,000 m l) was centrifuged at 10,000×g for 20 min. The integrity of the extracted DNA was checked by agarose gel electrophoresis and the concentration was measured by the absorbance at 260 nm. DNA was stored at −20 °C until analyzed.
PCR amplification of 16S rRNA V9 region
PCR amplification was performed in a 50 μl PCR reaction mixture containing 1.5 U Taq DNA polymerase, 10× buffer, and 50 pmol of each primer, 200 μmol l−1 dNTP and 100 ng of purified DNA extract. PCR was conducted with primers 1,055F and 1,406R. A 40 bp GC-clamp was attached to the 5′ end of the 1,406R primer [6, 17]. Theoretically, these primers should amplify a 323-bp product, including the highly variable V9 region. A modified Touch-down PCR program was employed. DNA was first incubated at 94 °C for 10 min, followed by cycles consisting of 1 min at 94 °C, 1 min at X °C, 3 min at 72 °C (X represents annealing temperature which was decreased by 2 °C from 66 °C to 52 °C and 3 cycles were run for each annealing temperature), then 15 cycles comprising 1 min at 94 °C, 1 min at 55 °C, 3 min at 72 °C, and finally 10 m in of extension at 72 °C. For each set of reactions, a negative control, in which the template was replaced by an equivalent volume of sterile deionized water, was included. The PCR products were validated by agarose gel electrophoresis and then purified to eliminate the ssDNA and heteroduplexes [30, 37].
DGGE
DGGE analysis of the amplified sequences was performed on 16 × 16 cm 6% polyacrylamid gels with a denaturing gradient of 40 to 60%. The PCR amplicon (200 ng) was loaded in each well and electrophoresis was performed at a constant voltage of 160 V for 200 min at 60 °C in the DGGE tank (Biorad, USA). Gels were stained with ethidium- bromide and photographed under UV light by a Bio-Rad gel Doc XR system [17].
Cloning of DGGE bands sequences
DGGE bands were cut from the gels and DNA was recovered using a Biospin Polyacrylamide Gel Extraction Kit (Japan). The fragments were amplified with the V9 region primers without GC clamp. The fragments were separated by electrophoresis with 1.0% agarose gels, recovered by a DNA Recovery Kit, ligated to pGEM-T cloning vector and transformed into E. coli DH5α.
Sequences analysis
Three positive clones from each DGGE band were selected randomly for sequence analysis. If the sequence of the three clones was not identical, more clones from the same band were sequenced to estimate the number of sequences co-migrating on the same DGGE band. The clones were sequenced on an automated ABI 377 sequencer using a T7 primer. DNA sequences were submitted to NCBI to determine their closest phylogenetic relatives. Sequences that differed by less than 3% were considered to be the same phylotype [28], and each phylotype was represented by a sequence type. Sequence alignments were performed by Clustal X [29]. A phylogenetic tree was constructed by Mega 3.1 based on Kimura Two-parameter model [10] and neighbor-joining algorithm [25].
Nucleotide sequence accession numbers
The nucleotide sequences reported in this paper have been deposited in the NCBI nucleotide sequence databases under accession numbers EU009936 to EU009951 and EU333951 to EU333966.
Results
Characteristics of the injected strains
Three strains were isolated from an oil-polluted soil sample in the Liaohe Oil Field. According to the physiological-biochemical characterizations and 16S rRNA gene sequences analysis, strains LJ-1, LJ-2 and LJ-3 were identified as belonging to the genera Brevibacillus, Rhizobium and Nocardia, respectively. Strain LJ-1 grew well with strains LJ-2 and LJ-3, and it accounted for 40% of the total cell concentration in the consortium. The LJ-2 and LJ-3 strains degraded 65.1 and 46.7% of the crude oil in 100 ml mineral medium with 0.5 g crude oil after being cultivated at 37 °C for 5 d. Strain LJ-3 was also able to produce a biosurfactant and reduce the surface tension to 34.7 mN m−1, about half of the control 70.2 mN m−1. The consortium LJ, which contained all three of these strains, degraded 75.0% of the crude oil and reduced the surface tension to 28.8 mN m−1.
Chemical characteristics of the Liaohe Oil Field
According to analyses by atomic absorption spectrophotometry, the formation water was classified as a bicarbonated sodic type with an NaCl concentration of approximately 0.52 g l−1, and a pH of 7.2 (Table 1). The average gross chemical composition (in weight %) of the crude oil was around 33.12% saturates, 22.42% aromatics, 35.97% resins and 8.49% asphaltenes. Low saturate and high resin and asphaltene contents are characteristic of biodegraded heavy oils.
Oil and liquid production
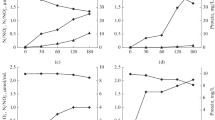
Yields of oil and liquid production from wells 26–195 and 27–221 were measured by the staff at the Liaohe Oil Field and the data are shown in Fig. 1. In well 26–195 (Fig. 1a), there was almost no crude oil output by water flooding before injection with bacteria on June 16. From June 17 to June 25, the well was stopped to allow for microbial growth and metabolism. On June 26, pumping was resumed. The liquid and oil production increased. The average oil production was 1.57 tons per day. In the well 27–221 (Fig. 1b), the yields were higher. After pumping on August 5, the liquid and oil production increased during 1 week reaching 14.6 and 5.7 tons per day, respectively, at which point they leveled off until October 29. Oil production was maintained at a relatively high yield (averaging 4.52 tons per day). The data suggested that the MEOR approach played an active role in increasing oil production in the oil wells.
Monitoring changes in injected strains
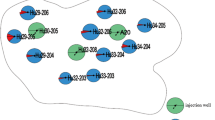
Twenty water samples were collected from the two oil wells. Total DNA was extracted from each sample and amplified by the V9 region primers. V9 regions of three injected strains were also amplified. To monitor the changes in the injected strains and to compare bacterial populations during MEOR, PCR-DGGE analysis was conducted using the injected strains LJ-1, LJ-2 and LJ-3 as markers. The banding patterns were shown in Fig. 2.
DGGE patterns of liquid samples DNA from two wells. 26–195 (a) Lane 1 Before injection (June 15), lane 2 July 26, lane 3 August 10, lane 4 August 26, lane 5 September 4, lane 6 September 11, lane 7 September 18, lane 8 September 25, lane 9 October 3, lane 10 October 9, lane 11 LJ-1, lane 12 LJ-2, lane 13 LJ-3, 27–221 (b) lane 1 start of the exploitation (August 28), lane 2 September 4, lane 3 September 11, lane 4 September 18, lane 5 September 25, lane 6 October 3, lane 7 October 9, lane 8 October 16, lane 9 October 24, lane 10 October 30, lane 11 LJ-1, lane 12 LJ-3, lane 13 LJ-2
In both wells, there were obvious bands with identical migration to those of the injected strains LJ-1, LJ-2 and LJ-3. In samples from 26–195 (Fig. 2a), band n was identical to LJ-1 and band f was identical to LJ-2, and they were the major band in all samples. There was no band that corresponded to LJ-3. In samples from well 27–221 (Fig. 2b), bands A, E and LJ-1 had the same migration pattern, as did bands B, F and LJ-2, and bands N and LJ-3. With DGGE, the relative intensity of each band most likely represents the relative abundance of a particular species in the population [17]. Our results showed that the presence of exogenous strains in the oil wells could be detected using PCR-DGGE analysis. Meanwhile, three injected bacteria were isolated by agar plate dilution method from these water samples. Morphological and 16S rRNA gene sequence analysis further proved their existence in the formation.
Sequence analyses of the DGGE bands
In order to determine the microbial community structure and analyze changes in the microbial community in the wells during oil recovery, major DNA bands marked in Fig. 2 were excised from the DGGE gels and sequenced. Thirty-two DNA sequences were compared with those in the NCBI database and related sequences are listed in Tables 2 and 3. Phylogenetic trees are shown in Figs. 3 and 4.
Phylogenetic analysis of DGGE bands from the 26–195 water samples. Bootstrap values (expressed as percentages of 1,000 replications) greater than 50% are shown at branching points. The scale bar represents the number of changes per nucleotide position. Accession numbers of the sequences retrieved from the database are shown in parentheses
Phylogenetic analysis of DGGE bands from the liquid samples of the well 27–221. Bootstrap values (expressed as percentages of 1,000 replications) greater than 50% are shown at branching points. The scale bar represents the number of changes per nucleotide position. Accession numbers of the sequences retrieved from the database are shown in parentheses
In well 26–195, 5 bands (a–e) present in the water sample before injecting extraneous bacteria on June 15 were sequenced. All five sequences belonged to the group Proteobacteria, including Betaproteobacteria (a, b, d and e) and Gammaproteobacteria (c). During the MEOR process, ten new bands appeared. Bands m and b had the same sequence and were related to Brachymonas petroleovorans (100%, similarity). This strain was simulated by the injection of nutrients at the end of the trial. Band n was identical with the injected strain LJ-1, and was very similar to Brevibacillus sp. (99.0%, similarity). Band f was the same as the strain LJ-2, and was clearly related to Rhizobium sp. (97%, similarity). Band g was similar to Dictyoglomus thermophilum (94%, similarity) and it became the major band during the experiment. Ten novel bands from the samples represented three genera. Bands d, h and p were related to Comamonas sp. (98–100%, similarity) and band h to Dictyoglomus thermophilum (94%, similarity). The remaining bands (i j, k, l and o) were most closely related to uncultured bacteria at similarity levels in the 87–100% rage. Strain LJ-3 was not detected in the water samples.
All stimulated indigenous strains found in well 27–221 belonged to the group Proteobacteria, including Alphaproteobacteria, Betaproteobacteria, Gammaproteobacteria and Epsilonproteobacteria. Bands A and E had the same sequences as injected strain LJ-1 and were most similar to Brevibacillus sp. (99.0%, similarity). Bands B and F were identical with strain LJ-2 and appeared related to Rhizobium sp. (97%, similarity). Band G was most closely related to Pseudomonas sp. (88% similarity), band I to Acinetobacter sp. (84%, similarity), band K to Comamonas sp. (90%, similarity), band L to Mesorhizobium plurifarium (100%, similarity), and band M to Agrobacterium tumefaciens (100%, similarity). The sequence of band N was the same as that of strain LJ-3 and both were related to Nocardia sp. (99.0%, similarity). The rest of the bands (C, H, J, O and P) were similar to uncultured bacteria. Percent similarities ranged from 86 to 99%.
Discussion
In our trial, the efficacy of an MEOR approach was tested at Liaohe Oil Field, which was at the end of the secondary phase of oil production. For injection, we used a bacterial mixture, “LJ”, containing three microorganisms. Two oil wells, 26–195 and 27–221, in the Jin45 zone of the Liaohe Oil Field were inoculated with the strains and nutrients. PCR products were analyzed by PCR-DGGE analysis. On the DGGE patterns, bands of the three injected strains were detected and it appeared that these strains had become the predominant population in the oil well during the process of recovery.
Indigenous microbes were also stimulated in the oil wells during the test. The most related species identified by DNA sequencing of DGGE bands are listed in Tables 2 and 3. In well 26–195, band b was detected in the production water sample before injection; during the MEOR process, it became the major band. DNA sequence analysis showed that this strain was similar to Brachymonas petroleovorans (similarity 100%). Brachymonas petroleovorans is a cyclohexane-degrading bacterium isolated from a wastewater plant of petroleum refinery [22]. The Pseudomonas genus represented by bands c and o is also a group that contains many oil-degrading bacteria [26, 31]. Bands d and p were identified as Comamonas sp. which is a genus that contains many bacteria with the ability to degrade organic compounds [2]. Band g was simulated during the MEOR process. It was similar to Dictyoglomus thermophilum (similarity 94%) which is a chemoorganotrophic, anaerobic, extremely thermophilic bacterium [24]. Other bands were not predominant and frequent in the water samples, such as i, k and l, which were matched to Hydrogenophaga sp. a9 (similarity 99%), Acinetobacter radioresistens (similarity 100%) and Bacteriovorax sp. EPC3 (similarity 97%), respectively. These genera are also important environmental bacterial groups [9, 14, 20].
In well 27–221, all the detected indigenous bacteria belonged to the group Proteobacteria including Alphaproteobacteria, Betaproteobacteria, Gammaproteobacteria and Epsilonproteobacteria. On the DGGE pattern, band D was novel and present at a high concentration in the production water samples. The sequence analysis of this band indicated that it was related to an uncultured gamma Proteobacterium (similarity 86%). The results suggested that it might represent a new species. Band O emerged at the later stage of treatment and became a major band. DNA sequence analysis showed that it was similar to an uncultured strain and the closest relative was Burkholderia sp. N1US7 (similarity 99%). Species of the Burkholderia genus, including B. cepacia and B. fungorum, have been isolated from different environmental samples [4, 23] and are well-known degraders of chlorinated pesticides and polychlorinated biphenyls. Bands C, G, H and P belonged to the genus Pseudomonas and shared more than 97% similarity, with the exception of band G which was only 88% similar. DNA sequences of bands L and M showed 100% similarity with Mesorhizobium plurifarium and Agrobacterium tumefaciens, respectively, both of which belong to Rhizobiales. Bacteria represented on the DGGE pattern were well-known environmental microorganisms and some of them were related to oil-utilizing bacteria. However, our investigation of the existing diversity was certainly limited and biased. First, PCR-DGGE profiles could only detect the predominant population in the environmental samples [3]. Second, the reported sensitivity of DGGE was 1% of the template DNA [17]. Third, many problems might arise during the sample collection, DNA extraction, PCR amplification or DGGE steps (gel resolution, gel staining) [34]. Only microorganism populations with high concentrations could be detected by DGGE fingerprinting.
Combining the time courses shown in Figs. 1 and 2, we conclude that the oil yield was related to the quantity and density of the bacterial bands. For example, in well 26–195, the oil yield was low on September 18 and the DGGE pattern bands were fewer and lower in density. On September 25, more bands were detected and the oil yield had increased correspondingly. The same result was observed in the well 27–221. After October 29, the oil yield began to drop and the DGGE bands had decreased correspondingly; particularly the LJ-3 band was missing. The exogenous bacterial group, LJ, and the indigenous strains may have all contributed to the oil yields. In a subsequent experiment, we plan to inject the bacterial group LJ with lower and higher proportions of LJ-3 in the wells 26–195 and 27–221, respectively.
Many factors influence oil recovery during the MEOR process, such as formation conditions and microbial community structures. However, our results confirm the feasibility of the MEOR technique. After injection of exogenous bacteria and nutrients, the indigenous bacteria were simulated and together with the injected exogenous bacteria formed a new microbial community that contributed to the increased oil yield. PCR-DGGE fingerprints monitored the changes in the exogenous and indigenous microorganism populations. This technology was informative for detection of the injected strains and provided guidance for isolation of useful strains from the production water samples.
References
Banat IM (1995) Biosurfactants production and possible uses in microbial enhanced oil recovery and oil pollution remediation: a review. Bioresour Technol 51:1–12
Boon N, Goris J, De Vos P, Verstraete W, Top EM (2000) Bioaugmentation of activated sludge by an indigenous 3-chloroaniline-degrading Comamonas testosterone strain, I2gfp. Appl Environ Microbiol 66:2906–2913
Casamayor EO, Schäfer H, Bañeras L, Pedrós-Aliós C, Muyzer G (2000) Identification of spatio-temporal differences between microbial assemblages from two neighboring sulfurous lakes: comparison by microscopy and denaturing gradient gel electrophoresis. Appl Environ Microbiol 66:499–508
Coenye T, Laevens S, Willems A, Ohlen M, Hannant W, Govan JRW, Falsen E, Vandamme P (2001) Burkholderia fungorum sp. nov and Burkholderia caledonica sp. nov, two new species isolated from the environment, animals and human clinical samples. Int J Syst Evol Microbiol 51:1099–1107
Díez B, Pedrós-Alió C, Marsh TL, Massana R (2001) Application of denaturing gradient gel electrophoresis (DGGE) to study the diversity of marine picoeukaryotic assemblages and comparison of DGGE with other molecular techniques. Appl Environ Microbiol 67:2942–2951
Ferris MJ, Muyzer G, Ward DM (1996) Denaturing gradient gel electrophoresis profiles of 16S rRNA-defined populations inhabiting a hot spring microbial mat community. Appl Environ Microbiol 62:340–346
Grabowski A, Nercessian O, Fayolle F, Blanchet D, Jeanthon C (2005) Microbial diversity in production waters of a low-temperature biodegraded oil reservoir. FEMS Microbiol Ecol 54:427–443
Gerdes B, Brinkmeyer R, Dieckmann G, Helmke E (2005) Influence of crude oil on changes of bacterial communities in Arctic sea-ice. FEMS Microbiol Ecol 53:129–139
Kämpfer P, Schulze R, Jäckel Udo, Malik KA, Amann R, Spring S (2005) Hydrogenophaga defluvii sp. nov. and Hydrogenophaga atypica sp. nov., isolated from activated sludge. Int J Syst Evol Microbiol 55:341–344
Kimura M (1980) A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16:111–120
Koopmans MP, Larter SR, Zhang CM, Mei BW, Wu TS, Chen YX (2002) Biodegradation and mixing of crude oils in Eocene Es3 reservoirs of the Liaohe basin, northeastern China. AAPG Bull 86:1833–1843
Lebaron P, Servais P, Troussellier M, Courties C, Muyzer G, Bernard L, Schäfer H, Pukall R, Stackebrandt E, Guindulain T, Vives-Rego J (2001) Microbial community dynamics in Mediterranean nutrient enriched seawater mesocosms: changes in abundances, bacterial cell-size, activity and composition. FEMS Microbiol Ecol 34:255–266
Li H, Yang SZ, Mu BZ, Rong ZF, Zhang J (2006) Molecular analysis of the bacterial community ina continental high-temperature and water-flooded petroleum reservoir. FEMS Microbiol Lett 257:92–98
Lösekann T, Knittel K, Nadalig T, Fuchs B, Niemann H, Boetius A, Amann R (2007) Diversity and abundance of aerobic and anaerobic methane oxidizers at the Haakon Mosby Mud Volcano, Barents Sea. Appl Environ Microbiol 73:3348–3362
McInerney MJ, Maudgalya S, Nagle DP, Knapp RM (2002) Critical assessment of the use of microorganisms for oil recovery. Recent Res Dev Microbiol 6:269–284
McInerney MJ, Nagle DP, Knapp RM (2005) Microbially enhanced oil recovery: past, present, and future. In: Ollivier B, Magot M (eds) Petroleum microbiology. ASM Press, Washington DC, pp 215–237
Muyzer G, de Waa EC, Uitterlinden AG (1993) Profiling of complex microbial population by denatuturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl Environ Microbiol 59:695–700
Muyzer G, Smalla K (1998) Application of denaturing gradient gel electrophoresis (DGGE) and temperature gradient gel electrophoresis (TGGE) in microbial ecology. Antonie van Leeuwenhoek 73:127–141
Morris CE, Bardin M, Berge O, Frey-Klett P, Fromin N, Girardin H, Guinebretière MH, Lebaron P, Thièry JM, Troussellier M (2002) Microbial biodiversity: approaches to experimental design and hypothesis testing in primary scientific literature from 1975 to 1999. Microbiol Mol Biol Rev 66:592–616
Navon-Venezia S, Zosim Z, Gottlieb A, Legmann R, Carmeli S, Ron EZ, Rosenberg E (1995) Alasan, a new bioemulsifier from Acinetobacter radioresistens. Appl Environ Microbiol 61:3240–3244
Orphan VJ, Taylor LT, Hafenbradl D, Delong EF (2000) Culture-dependent and culture-independent characterization of microbial assemblages associated with high-temperature petroleum reservoirs. Appl Environ Microbiol 66:700–711
Pierre ER, Mario WC (2003) Isolation of Brachymonas petroleovorans CHX, a novel cyclohexane-degrading L-proteobacterium. FEMS Microbiol Lett 227:101–106
Ramette A, LiPuma JJ, Tiedje JM (2005) Species Abundance and Diversity of Burkholderia cepacia Complex in the Environment. Appl Environ Microbiol 71:1193–1201
Saiki T, Kobayashi Y, Kawagoe K, Beppu T (1985) Dictyoglomus thermophilum gen. nov., sp. nov., a chemoorganotrophic, anaerobic, thermophilic bacterium. Int J Syst Bacteriol 35:253–259
Saitou N, Nei M (1987) The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Setti L, Mazzieri S, Pifferi PG (1999) Enhanced degradation of heavy oil in aqueous system by a Pseudomonas sp in the presence of natural and synthetic sorbents. Bioresour Technol 67:191–199
Smit E, Leeflang P, Gommans S, van den Broek J, van Mil S, Wernars K (2001) Diversity and seasonal fluctuations of the dominant members of the bacterial soil community in a wheat field as determined by cultivation and molecular methods. Appl Environ Microbiol 67:2284–2291
Stackebrandt E, Goebel BM (1994) Taxonomic note: A place for DNA-DNA reassociation and 16S rRNA sequence analysis in the present species definition in bacteriology. Int J Syst Bacteriol 44:846–849
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The ClustalX windows interface:flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 24:4876–4882
Thompson JR, Marcelino LA, Polz MF (2002) Heteroduplexes in mixed-template amplifications: formation, consequence and elimination by dreconditioning PCRT. Nucleic Acids Res 30:2083–2088
van Beilen JB, Li Z, Duetz WA, Smits THM, Witholt B (2003) Diversity of alkane hydroxylase systems in the environment. Oil Gas Sci Technol-Rev IFP 58:427–440
van Hamme JD, Singh A, Ward OP (2003) Recent advances in petroleum microbiology. Microbiol Mol Biol Rev 67(4):532–549
Watanabe K, Watanabe K, Kodama Y, Syutsubo K, Harayama S (2000) Molecular characterization of bacterial populations in petroleum-contaminated groundwater discharged from underground crude oil storage cavities. Appl Environ Microbiol 66:4803–4809
Wintzingerode FV, Goebel UB, Stackebrandt E (1997) Determination of microbial diversity in environmental samples: pitfalls of PCR-based rRNA analysis. FEMS Microbiol Rev 21:213–229
Xu EJ, LI M, Zhang H, Zhao B, Yang H, Qi XB, Zhang YZ, Tian H (2006) Multicyclic microbes Huff-Puff for improving heavy oil recovery in Liaohe oil fields. Oilfield Chem 23(3):263–268
Youssef N, Simpson DR, Duncan KE, McInerney MJ, Folmsbee M, Fincher T, Knapp RM (2007) In situ biosurfactant production by Bacillus strains injected into a limestone petroleum reservoir. Appl Environ Microbiol 73:1239–1247
Zhang XL, Yan X, Gao PP, Wang LH, Zhou ZH, Zhao LP (2005) Optimized sequence retrieval from single bands of TGGE (temperature gradient gel electrophoresis) profiles of the amplified 16S rDNA fragments from an activated sludge system. J Microbiol Method 60(1):1–11
Acknowledgments
This work was supported by Tianjin Natural Scientific Fund (No. 07ZDJCZD03200) and The Innovation Fund of Nankai University (No. Z06008-B1).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, J., Ma, T., Zhao, L. et al. Monitoring exogenous and indigenous bacteria by PCR-DGGE technology during the process of microbial enhanced oil recovery. J Ind Microbiol Biotechnol 35, 619–628 (2008). https://doi.org/10.1007/s10295-008-0326-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-008-0326-9