Abstract
Pyruvate oxidase (PyOD) is a very useful enzyme for clinical diagnostic applications and environmental monitor. Optimization of the fermentation medium for maximization of PyOD constitutively, production by Escherichia coli DH5α/pSMLPyOD was carried out. Response surface methodology (RSM) was used to optimize the medium constituents. A 26–2 fractional factorial design (first order model) was carried out to identify the significant effect of medium components towards PyOD production. Statistical analysis of results shows that yeast extract, ammonium sulfate and composite phosphate were significant factors on PyOD production. The optimized values of these three factors were obtained by RSM based on the result of a 23 central composite rotatable design. Under these proposed optimized medium, the model predicted a PyOD activity of 610 U/L and via experimental rechecking the model, an activity of 670 U/L was attained.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pyruvate oxidase (PyOD, EC1.2.3.3) is a homo-tetrameric flavoenzyme. Each subunit contains one tightly and noncovalently bound FAD, thiamin diphosphate (ThDP), and Mg2+ for anchoring the diphosphate moiety of ThDP [19]. In the presence of phosphate and oxygen, PyOD catalyzes the oxidative decarboxylation of pyruvate, yielding hydrogen peroxide, carbon dioxide, and acetyl phosphate [20]. These characteristics make it a very useful enzyme for clinical diagnostic applications and environmental monitor. Combined with peroxidase, it has been used to determine the alanine transaminase (ALT) and aspartate transaminase (AST) in blood [18] and phosphate ion in river water [13] and wastewater [7]. Up to now, however, commercial product of PyOD is usually produced by conventional cultivation of the wild Lactobacillus lantarum or Aerococcus viridans strain and isolation of the secreted enzyme. These lead to the lower level of production (120 U/L) [4].
Escherichia coli is the most commonly used host for heterologous protein production. Because of the availability of well-established technologies for genetic manipulation and cultivation, a variety of therapeutic proteins have been successfully expressed in recombinant E. coli [5, 6, 10]. For pyruvate oxidase (PyOD), Lorquet et al. [11] had cloned the pyruvate oxidase gene (LP-PyOD) from L. plantarum Lp80 and investigated its expression characteristics by recombinant E. coli cell, but the expression level of PyOD was very low (0.23 U/mg). We have cloned the pyruvate oxidase gene from Aerococcus viridans ATCC10400 (AvPyOD) into the vector pSML104, then transformed the plasmid pSMLPyOD into E. coli DH5α. The recombinant pSMLPyOD/DH5α could express PyOD constitutively. Up to our knowledge, there are no reports on the mass production of PyOD from the recombinant E. coli.
The optimization of fermentation medium is an important problem in the development of economically feasible bioprocesses. Conventional practice of one-factor-at-a-time does not depict the combined effect of all the factors involved. It is also a time consuming process and requires a number of experiments [15]. Statistical methods through factorial experimental designs offer the simultaneous study of many factors and allow the study of interactive effects of many factors together and facilitate the prediction of the response for the values of factors not tested in the experiment. Response surface methodology (RSM) is a three factorial design that gives relationship between one or more measured dependent responses with a number of input (independent) factors. Response surface methodology (RSM) has some advantages that include less experiment numbers, suitability for multiple factor experiments, search for relativity between factors, and finding of the most suitable condition and forecast response [2]. Recent years, fractional factorial designs (FFD) and RSM, which are statistical techniques for designing experiments, building models, evaluating the effects of factors and searching for the optimum conditions, have successfully been employed for screening significant factors and optimization the medium composition in many bioprocess [1, 3, 8, 9, 12, 14, 16, 17].
The objective of the present study was to optimize the medium for PyOD production by recombinant E. coli using shake flask method. The first stage of this work involved a fractional factorial design for simultaneously screening up to six factors. Then RSM was used to optimize the significant factors screened by the first stage.
Materials and methods
Plasmid and expression system
The recombinant plasmid pSMLPyOD was constructed in our laboratory. Escherichia coli DH5α was used as the host. The pyruvate oxidase gene was isolated from A. viridans ATCC10400 (AvPyOD) and cloned into the expression vector pSML104 to generate a N-terminus His-tagged fusion expression plasmid pSMLPyOD, then transformed into E. coli DH5α. The recombinant DH5α/pSMLPyOD expressed heterogeneic pyruvate oxidase (PyOD) constitutively without the induction of IPTG.
Medium and culture conditions
The recombinant E. coli DH5α/pSMLPyOD cells kept in 20% glycerol (−80 °C) were transferred into a 500 mL Erlenmeyer flask with 100 mL LB broth consisting of 10 g/L peptone, 5 g/L yeast extract and 10 g/L NaCl with 50 mg/L tetracycline, and were grown overnight on an orbital shaker with 220 rpm agitation at 37 °C. This culture was used as seed culture.
For all experiments, batch cultures were carried out in 250 mL Erlenmeyer flask with 30 mL medium as per experiment design. These flasks were inoculated with 10% seed culture and grown 24 h on an orbital shaker with 220 rpm agitation at 37 °C. The pH of the medium was pre-adjusted to 7.0 before sterilization. For all experiments, culture mediums were added 50 mg/L tetracycline after sterilization.
Analytical methods
Preparation of crude enzyme
Crude cell extracts were prepared from 10-mL culture broth. About 20 mg lysozyme (chicken egg, 10,000 U/mg) was added to 10-mL culture broth, thoroughly mixed and stood 10 min at room temperature. The mixture was then frozen and thawed three times repeatedly. The lysate was centrifuged, and the pyruvate oxidase activity in supernatant was measured.
Pyruvate oxidase assay
Pyruvate oxidase was determined as described by Tao [18]. One unit is defined as the amount of enzyme which generates 1 μmol H2O2 per minute at 37 °C.
Fractional factorial designs
Experimental designs were carried out using Design Expert Software (Stat-Ease Inc., Minneapolis, MN, USA, Version 6.0.4). A 26–2 fractional factorial designs (FFD) was used to investigate the statistical significance of the factors of glycerol, peptone, yeast extract, ammonium sulfate, composite phosphate (K2HPO4:Na2HPO4:KH2PO4 = 1:2:1) and trace element solution (containing per liter: FeSO4·7H2O, 12.8 g; ZnSO4·7H2O, 2.84 g; CuSO4·5H2O, 1.6 g; MnSO4·H2O, 3.2 g; CaCl2, 0.28 g; CoCl2·6H2O, 1.6 g; H3BO4, 0.24 g; Na2MoO4, 12.8 g; KI, 0.16 g) on the pyruvate oxidase production by recombinant E. coli DH5α/pSMLPyOD. A total of 20 sets experiments containing four central points were employed (Table 1). In FFD, statistical designs are always expressed in coded values for convenience. Low and high level were coded as −1 and +1, the midpoint was coded as 0 [17]. The relation between the coded values and actual values were described as in the following equation:
where X i is the independent variable coded value, x i is the independent variable actual value, x 0 is the independent variable actual value on the center point and Δx i is the step change value. The range and the levels of the factors were shown in the form of both coded values and natural values (Table 2).
Settings of range and the levels for factors were based on the investigation of single factor, our previous laboratory experience, and information published in the scientific literature.
Central composite design
Based on the results of the fractional factorial design, the experiment was further expanded to a central composite design. The significant factors identified from FFD, Yeast extract (x 1), ammonium sulfate (x 2), composite phosphate (x 3) were chosen as major factors. Other factors, which did not influence PyOD production significantly, were kept at constant level (glycerol, 10 g/L, peptone, 15 g/L and trace element solution 1.25 mL/L, respectively). A 23-factorial rotatable central composite design (CCD) with six star points (α = 1.682) and six replicates at the center points (n 0 = 6) leading to a total number of 20 experiments was employed to optimize the medium composition for PyOD production. Design Expert software was applied to analyze the obtained results. The quadratic model for predicting the optimal point was express as follow:
where y is the response variables, X 1, X 2 and X 3 are coded level of independent variables, β 0 is the intercept term, β 1, β 2 and β 3 are linear coefficients, β 11, β 22 and β 33 were quadratic coefficients, β 12, β 13 and β 23 are interactive coefficient. The statistical significance of the second-order model equation was determined by F-value and the proportion of variance explained by the model obtained was given by the multiple coefficient of determination, R 2.
The optimal values of these factors were determined by response surface and point prediction with Design Expert software.
Results and discussion
Fractional factorial design
The significance of glycerol (x 1), peptone (x 2), yeast extract (x 3), ammonium sulfate (x 4), composite phosphate (x 5) and trace element solution (x 6) were determined using 26–2 fractional factorial design. Coded values of factors, design and results of were shown in Table 1. ANOVA was employed for the determination of significant variables. On the basis of these experimental values (Table 1), statistical testing was carried out using Fisher’s statistical test for ANOVA.
The regression models can be applied in screening crucial and critical medium components. A linear regression equation [Eq. (3)] was obtained from analysis of variance, and all terms regardless of their significance was included in the following equation:
Since the F-value and P-value of the model were 126.31 and 0.0001, respectively (Table 3), the estimated models fit the experimental data adequately. The coefficient of determination R 2 of the model was calculated to be 0.9970 indicating that the model able to comprehend a 99% of the data variability.
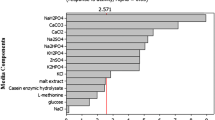
As seen from the ANOVA analysis results (Table 3), the F test values for factors of yeast extract (x3), ammonium sulfate (x 4), composite phosphate (x 5), interactions of x 1 x 3, x 1 x 5, x 2 x 4 were above the 1% level significant. Other factors in the medium such as glycerol, peptone, trace element and interactions of x 1 x 2, x 1 x 4, x 1 x 6, x 2 x 6 did not affect the PyOD production significantly. The significances of each term were also shown in the half normal plot (Fig. 1). Thus, the concentration of yeast extract (x3), ammonium sulfate (x 4) and composite phosphate (x 5) are significant factors affecting PyOD production of the recombinant DH5α/pSMLPyOD, especially the concentration of yeast extract (P-value <0.0001).
In addition, the “Curvature F-value” of 96.80 implies there is a significant curvature (as measured by difference between the average of the center points and the average of the factorial points) in the design space. So there is no need to do a steepest ascent experiment.
Central composite design
Based on the result of initial fractional factorial design, the concentration of yeast extract, ammonium sulfate and composite phosphate were further optimized using a rotatable central composite design. Experimental design and results are shown in Table 4.
Using Design Expert software, a full second-order polynomial model for the PyOD production was obtained from regression analysis of results of central composite design (X 1, X 2, X 3 represent yeast extract, ammonium sulfate and composite phosphate):
The model adequacy was checked by F test and the determination coefficient R 2. The high F-value (14.67) and a very low probability (P > F = 0.0001) indicated that the present model was in good prediction of the experimental results. Therefore, the obtained mathematical model was adequate. In addition, the goodness of fit of the model was expressed by the coefficient of determination R 2 (0.9296), indicating that 92.96% of the variability in the response could be explained by the model or only about 7.04% of the total variation were not explained by the model.
Effects of concentration of yeast extract, ammonium sulfate and composite phosphate on the PyOD production were further analyzed by the response surface plot (Fig. 2). All the response surfaces could be analyzed for determining the optimized value of the factors, but it was difficult to analyze all these simultaneously. Design Expert software could be used to determine the optimum values of these factors [16]. By the Design Expert software, the optimal concentration of yeast extract, ammonium sulfate and composite phosphate were calculated to be 12.67, 4.06, and 6.96% (w/v), respectively. The maximum response predicted from the model was 610 U/L. To confirm the predicted optimization medium, validated experiment was performed using the optimized medium, and an average 670 U/L of PyOD production was obtained. So the model was proven to be adequate. The final composition of medium optimized was (L): glycerol, 10 g; peptone, 15 g; yeast extract, 12.67 g; ammonium sulfate, 4.06 g; composite phosphate, 6.96 g; trace element solution, 1.25 mL; NaCl, 10 g.
Conclusion
The fractional factorial design and central composite design could be used to screen the significant factors and optimize the medium composite for pyruvate oxidase production by the recombinant E. coli DH5α/pSMLPyOD. Three factors, yeast extract, ammonium sulfate and composite phosphate were proved to be significant factors influencing the pyruvate oxidase production by the recombinant E. coli DH5α/pSMLPyOD. The optimized medium was obtained and the pyruvate oxidase production was increased to 670 U/L, which was 2.2 times as that before optimization.
References
Chakravarti R, Sahai V (2002) Optimization of compactin production in chemically defined production medium by Penicillium citrinum using statistical methods. Process Biochem 38:481–486
Chang C-Y, Lee C-L, Pan T-M (2006) Statistical optimization of medium components for the production of Antrodia cinnamomea AC0623 in submerged cultures. Appl Microbiol Biotechnol 72:654–661
Elibol M (2004) Optimization of medium composition for actinorhodin production by Streptomyces coelicolor A3(2) with response surface methodology. Process Biochem 39:1057–1062
Elstner E, Schleifer K, Gotz F (1987) Pyruvate oxidase. United States Patent 4,666,832
Jeong KJ, Choi JH, Yoo WM, Keum KC, Yoo NC, Lee SY, Sungf M-H (2004) Constitutive production of human leptin by fed-batch culture of recombinant rpoS Escherichia coli. Protein Expr Purif 36:150–156
Khalilzadeh R, Shojaosadati SA, Maghsoudi N, Mohammadian-Mosaabadi J, Mohammadi MR, Bahrami A, Maleksabet N, Nassiri-Khalilli MA, Ebrahimi M, Naderimanesh H (2004) Process development for production of recombinant human interferon-c expressed in Escherichia coli. J Ind Microbiol Biotechnol 31:63–69
Kwan RCH, Leung HF, Hon PYT, Barford JP, Renneberg R (2005) A screen-printed biosensor using pyruvate oxidase for rapid determination of phosphate in synthetic wastewater. Appl Microbiol Biotechnol 66:377–383
Lin Y, Zhang Z, Thibault J (2007) Aureobasidium pullulans batch cultivations based on a factorial design for improving the production and molecular weight of exopolysaccharides. Process Biochem 42:820–827
Lo PK, Hassan O, Ahmad A, Mahadi NM, Illias RM (2007) Excretory over-expression of Bacillus sp. G1 cyclodextrin glucanotransferase (CGTase) in Escherichia coli: optimization of the cultivation conditions by response surface methodology. Enzyme Microb Technol 40:1256–1263
Looser V, Hammes F, Keller M, Berney M, Kovar K, Egli T (2005) Flow-cytometric detection of changes in the physiological state of E. coli expressing a heterologous membrane protein during carbon-limited fedbatch cultivation. Biotechnol Bioeng 92(2):69–78
Lorquet F, Goffin P, Muscariello L, Baudry J.-B, Ladero V, Sacco M, Kleerebezem M, Hols P (2004) Characterization and functional analysis of the poxB gene, which encodes pyruvate oxidase in Lactobacillus plantarum. J Bacteriol 186(12):3739–3759
Mahat MK, Illias RM, Rahman RA, Rashid NAA, Mahmood NAN, Hassan O, Aziz SA, Kamaruddin K (2004) Production of cyclodextrin glucanotransferase (CGTase) from alkalophilic Bacillus sp. TS1-1: media optimization using experimental design. Enzyme Microb Technol 35:467–473
Nakamura H, Tanaka H, Hasegawa M, Masuda Y, Arikawa Y, Nomura Y, Ikebukuro K, Karube I (1999) An automatic flow-injection analysis system for determining phosphate ion in river water using pyruvate oxidase G (from Aerococcus viridans). Talanta 50:799–807
Nikerel IE, Toksoy E, Kirdar B., Yildirim R (2005) Optimizing medium composition for TaqI endonuclease production by recombinant Escherichia coli cells using response surface methodology. Process Biochem 40:1633–1639
Rao KJ, Kim C-H, Rhee S-K (2000) Statistical optimization of medium for the production of recombinant hirudin from Saccharomyces cerevisiae using response surface methodology. Process Biochem 35:639–647
Sayyad SA, Panda BP, Javed S, Ali M (2006) Optimization of nutrient parameters for lovastatin production by Monascus purpureus MTCC 369 under submerged fermentation using response surface methodology. Appl Microbiol Biotechnol 73:1054–1058
Tang X-J, He G-Q, Chen Q-H, Zhang X-Y, Ali MAM (2004) Medium optimizaiton for the production of thermal stable β-glucanase by Bacillus subtilis ZJF-1A5 using response surface methodology. Bioresour Technol 93:175–181
Tao JQ (2007). High yield leakage expression of recombinant pyruvate oxidase from Aerococcus viridans ATCC10400 in Escherichia coli and its purification. China. East China University of Science and Technology
Tittmann K, Golbik R, Ghisla S, Hübner G (2000) Mechanism of elementary catalytic steps of pyruvate oxidase from Lactobacillus plantarum. Biochemistry 39:10747–10754
Tittmann K, Proske D, Spinka M, Ghisla S, Rudolph R, bner GH, Kern G (1998) Activation of thiamin diphosphate and FAD in the phosphatedependent pyruvate oxidase from Lactobacillus lantarum. J Biol Chem 273(21):12929–12934
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhao, J., Wang, Y., Chu, J. et al. Statistical optimization of medium for the production of pyruvate oxidase by the recombinant Escherichia coli . J Ind Microbiol Biotechnol 35, 257–262 (2008). https://doi.org/10.1007/s10295-007-0301-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-007-0301-x