Abstract
A very high level of alkalophilic and thermostable pectinase and xylanase has been produced from newly isolated strains of Bacillus subtilis and Bacillus pumilus respectively. Enzyme production for pectinase was carried out under SSF using combinations of cheap agricultural residues while xylanase was produced under submerged fermentation using wheat bran as substrate to minimize the cost of production of these enzymes Among the various substrates tested, the highest yield of pectinase production was observed by using combination of WB + CW (6592 U/g of dry substrate) supplemented with 4% yeast extract when incubated at 37 °C for 72 h using deionized water of pH 7.0 as moistening agent. The biobleaching effect of these cellulase free enzymes on kraft pulp was determined. Both xylanase and pectinase showed stability over a broad range of pH from 6 to 10 and temperature from 55 to 70 °C. The bleaching efficiency of the pectinase and xylanase on kraft pulp was maximum after 150 min at 60 °C using enzyme dosage of 5 IU/ml of each enzyme at 10% pulp consistency with about 16% reduction in kappa number and 84% reduction in permanganate number. Enzyme treated pulp when subjected to CDED1D2 steps, 25% reduction in chlorine consumption and upto 19% reduction in consumption of chlorine dioxide was observed for obtaining the same %ISO brightness. Also an increase of 22 and 84% in whiteness and fluorescence respectively and a decrease of approximately 19% in the yellowness of the biotreated pulp were observed by pretreatment of the pulp with our enzymatic mixture.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the past few years, the need for safer and “environmental friendly” technologies has become imminent. Efforts to save the environment and yet achieve the goals of chemical technology are gaining momentum in several frontier developments. One such area is the pulp and paper industry where the quantities of raw materials processed are huge, as well as the use of naturally hazardous chemicals are also large [32]. Only in the past two decades, however, the use of microbial enzymes is gaining momentum to make the technology eventually totally free from hazardous chemicals.
In the paper production process, pulping is a step during which cellulose fibres are broken apart and lignin is removed. Kraft pulping is the most common and while it removes most of the constituent lignin, the residual lignin covalently bound to carbohydrate moieties is one of the chief components that impart dark brown color to the kraft pulp [14]. This yellow/brownish kraft pulp must be bleached before paper production. A multistage bleaching process is used to remove the residual lignin using chlorine (C), chlorine dioxide (D), and NaOH treatments [15]. The chlorinated organic byproducts generated during this process by using these chemicals are toxic, mutagenic, highly persistent, bioaccumulating, and thus cause numerous harmful disturbances in the biological systems [3, 11, 14, 16, 30, 32]. This necessitates the use of microbial enzymes to reduce the use of chlorine and chlorine dioxide in the paper making process.
Biological bleaching of pulp till date has been approached mainly by the use of xylanolytic enzymes. Xylan is found mainly in the secondary cell wall and is considered to be forming an interphase between lignin and other polysaccharides such as pectin [32]. The first scientific report of pulp biobleaching using xylanase was published by Viikari et al. [34] whereas there have been very few reports so far on the efficacy of pectinase pretreatment for biobleaching. With the advancement of biotechnology and increased reliance of pulp and paper industries on the use of microorganisms and their enzymes for biobleaching and paper making, the use of enzymes other than xylanase and ligninase, such as mannanase, pectinase and galactosidase is increasing in the pulp and paper industries in many countries [3, 22]. It has been found that bleached or alkaline treated pulps contain a substantial amount of harmful pectins. By incorporating pectinase in the bleached or alkaline treated pulp such harmful pectins in the aqueous phase of the pulp are degraded and thus rendered harmless to papermaking process [3].
Xylanases and pectinases have been reported from bacteria, fungi, actinomycetes and yeasts [9, 24, 35]. The use of abundantly available and cost-effective agricultural residues to achieve higher xylanase and pectinase yields provide suitable means to reduce the manufacturing cost of biobleached paper. No report is published so far utilizing a combination of xylanase and pectinase for effective pulp biobleaching. The present study reports high level production of thermostable, cellulase-free pectinase and xylanase by newly isolated strains of Bacillus subtilis SS and Bacillus pumilus ASH respectively using cheap agricultural residues and their potential application in the pulp and paper industry.
Materials and methods
Microorganisms and growth conditions
Bacillus subtilis and B. pumilus were isolated from soil samples collected from sanitary landfill and were identified by the Institute of Microbial technology (IMTECH), Chandigarh, India on the basis of their morphological, physiological and biochemical characteristics and were maintained and stored at 4 °C on nutrient agar medium. Both the strains are alkalophilic being capable of growing at pH values up to 11.0. The isolated bacterial strains are Gram positive, moderate thermophile with minimum, optimum and maximum temperature for growth at 10, 37 and 60 °C, respectively. Bacillus subtilis was used to produce pectinase while xylanase was produced by B. pumilus. The qualitative assay of pectinase enzyme activity was carried out by culturing the microorganism on the nutrient agar plates containing pectin (2% w/v). The clear zones around the colonies indicated the pectinase activity. The qualitative estimation of xylanase production was performed by Congo red staining [5]. Both the cultures were maintained on nutrient agar slants (g/l): Peptone, 10.0; NaCl, 5; Yeast Extract, 3; and Agar, 2% at 4 °C.
Pectinase production in SSF
To 10 g of substrate in 250-ml Erlenmeyer flask, 20 ml mineral salt solution (g/l: MgSO4.7H2O, 0.2; K2HPO4, 0.5; pH 7.0) was added and sterilized by autoclaving at 121 °C for 20 min. The flasks were brought to room temperature and inoculated with 10% (v/w) inoculum of overnight culture of B. subtilis and incubated in a humidified incubator at 37 °C for 72 h. The flasks were tapped at regular intervals in order to mix the contents.
Enzyme extraction
The enzyme from each flask was extracted twice with 10 mM glycine phosphate buffer pH 9.5 (100 ml for 10 g of substrate) and the contents were squeezed through a wet muslin cloth. The enzyme extract was centrifuged at 10,000g for 30 min at 4 °C and the clear supernatant was used as crude enzyme.
Chemicals
Polygalacturonic acid, birchwood xylan, carboxymethyl cellulose, 3, 5-dinitrosalicylic acid were purchased from Sigma Chemical Co. (St. Louis, MO, USA). All other chemicals used were of the highest purity grade available commercially. Pulp used for biobleaching was hardwood pulp obtained from Ballarpur Industries Limited (BILT), Yamunanagar, Haryana, India. Agro residues were procured locally.
Pectinase, xylanase and cellulase assay
Polygalacturonic acid, birchwood xylan and carboxymethyl cellulose were used as the assay substrates for pectinase, xylanase and cellulase respectively. The reaction mixtures containing 400 μl of 1% polygalacturonic acid and carboxymethyl cellulose prepared in 0.1 M glycine phosphate buffer (pH 9.5) and 100 μl of appropriately diluted enzyme produced by B. subtilis were incubated at 65 °C for 10 min for pectinase and cellulase assay, respectively. The reaction mixtures for xylanase and cellulase assay contained 490 μl of 2% of respective substrates prepared in phosphate buffer, pH 7.0 and 10 μl of appropriately diluted enzyme produced by B. pumilus and were incubated at 60 °C for 10 min. The enzyme activity was determined by measuring the release of reducing sugars during the enzyme substrate reaction using Miller’s method [27]. One IU of activity towards the substrates mentioned above was defined as 1 μmol of galacturonic acid, xylose or glucose equivalent released per minute under the above assay conditions by referring to their standard curves. All the experiments were carried out independently in triplicates and the results presented here are the mean of the three. Pectinase activity in SSF was expressed as U/g of dry substrate.
Optimization of various parameters for pectinase production
For cost-effective production of polygalacturonase by SSF, the strain was grown with different combinations of maximum productive cheap agroresidues mixture (5 g each) as substrates: (1) wheat bran + cotton seed cake (WB + CSC) (2) wheat bran + citrus waste (WB + CW) (3) wheat bran + alpha-alpha leaves (WB + ααl) (4) cotton seed cake + citrus waste (CSC + CW) (5) cotton seed cake + alpha-alpha leaves (CSC + ααl) (6) citrus waste + alpha-alpha leaves (CW + ααl) and (7) mixture of all the four substrates (2.5 g each) (WB + CSC + CW + ααl) moistened with 20 ml of mineral salt solution for pectinase production by B. subtilis SS. After sterilization by autoclaving, the flasks were cooled and inoculated with 18 h grown inoculum. The enzyme was extracted and assayed.
Time course of enzyme production
The flasks were incubated at 37 °C after inoculation for different time periods ranging from 24 to 144 h.
Effect of different moistening agents
Different moisturizing agents were tried such as MA I (g/l): KH2PO4, 1.0; NaCl, 5.0; CaCl2, 0.1; MgSO4.7H2O, 0.4; pH 7.0; MA II (g/l): K2HPO4, 1.0; MgSO4.7H2O, 0.4; pH 7.0, prepared in distilled water for enzyme production in SSF. Besides these salt solutions, deionized water and distilled water were also used as moistening agents.
Effect of pH of moisturizing agent
SSF was carried out using suitable moistening agent with different pH ranging from 6.0 to 10.0. The flasks were incubated at 37 °C for 72 h.
Effect of moisture level
The effect of moisture level on the pectinase production was studied by varying the substrate-to-moisture ratio (w/v) ranging from 1:1.5 to 1:3.
Effect of temperature
The influence of temperature on the enzyme production was studied by incubating at different temperatures, ranging from 30 to 50 °C for 72 h.
Inoculum size
Flasks containing 10 g of substrate were inoculated with 5, 10, 15 and 20% of 18 h old inoculum. The inoculated flasks were incubated at 37 °C for 72 h. Thereafter the enzyme was extracted and assayed.
Effect of additives
The effect of different additives was studied by supplementing the moistened substrates with yeast extract, peptone and glucose at a concentration of 4% (w/w).
Production of xylanase under submerged fermentation
The xylanase production was studied in Erlenmeyer flask (250 ml) containing 50 ml of basal medium having (g/l): Yeast extract, 2.5; Peptone, 2.5; KNO3, 2.5; supplemented with Wheat bran, 20.0 and Olive oil, 0.2%(w/v); pH 8.0. The flasks were inoculated with 2.5% (O.D. ∼0.5) of the overnight grown inoculum and incubated at 37 °C under shaking conditions (200 rpm) for 26 h. The enzyme was harvested by centrifuging at 10,000g for 15 min. The supernatant was treated as enzyme and was assayed for xylanase activity.
Optimization of enzyme dose and other reaction parameters for biobleaching
For efficient biobleaching of the paper pulp different parameters such as pH, temperature and enzyme dosage were optimized by carrying out the enzymatic treatment at 10% (w/v) pulp consistency in transparent plastic bags. Experiments were conducted at different pH values ranging from 7 to 10 and at temperatures varying from 55 to 70 °C. The optimization of enzyme dose for biobleaching was carried out by treating moistened unbleached pulp with varying doses of pectinase and xylanase enzyme mixture, ranging between 2.5 and 5.0 IU/g of each enzyme keeping a constant retention time of 150 min.
Biobleaching of kraft pulps (ECDED1D2)
Kraft pulps used for bleaching were kindly provided by Ballarpur Industries Limited, Yamunanagar, India. Pulp samples were washed thoroughly with distilled water before and after each experiment till neutrality and were oven dried. The above, optimized parameters were used in each experiment. Prewashed kraft pulp (50 g) of 10% consistency was taken in plastic bag and treated with the optimized dose of enzymatic mixture consisting of pectinase from B. subtilis and xylanase produced from B. pumilus.
Then pectinase and xylanase treated pulp was exposed to CDED1D2 bleaching sequence and thereafter various experiments were conducted to measure the reduction in chlorine consumption to obtain the same amount of brightness in both enzyme-treated and control (untreated). In these experiments, all the parameters and treatment conditions were kept the same except for the dose of chlorine treatment given at the chlorine and chlorine dioxide (CD) stage. The percentage of chlorine used in the bleaching sequence was calculated from the brown pulp Kappa number, which represents the content of residual lignin in the brown pulp. All the experiments were carried out in triplicates. After the bleaching experiments, the pulp samples were washed with distilled water, filtered and suction-dried, and stored in plastic bags for the later determination of the Kappa number.
Pulp properties
The enzyme treated pulp was thoroughly washed and handsheets were prepared under standardized pressure and air-dried in a room with standardized light, humidity and temperature, following Technical Association of Pulp and Paper Industry (TAPPI) recommendations and the investigation of pulp properties was carried out according to TAPPI standard methods [33]. The Kappa number, which estimates the lignin content, was determined by the reaction of pulp samples with acidified potassium permanganate (Tappi method T236 cm-85). The brightness of the handsheets was measured as %ISO (International Organization of Standardization, ISO) by reflectance at 457 nm with ISO Colourtech, USA, according to Tappi protocol (T-452 om-87). The yellowness, whiteness and fluorescence of pretreated pulp were also evaluated by ISO Colourtech, USA at 457 nm (T 1216), and Permanganate No. (P. no.) was estimated by reaction of pretreated pulp with acidified permanganate solution by using starch and potassium iodide as an indicator.
Results and discussion
Pectinase production under SSF
The production of pectinase by B. Subtilis SS was tested in solid state fermentation using various agroresidues as substrates. Agroresidues were considered as the best substrates for SSF and enzyme production by microorganisms [13]. Among the various substrates tested, the highest yield of pectinase production was observed using combination of WB + CW (4,607 U/g per dry substrate) when incubated at 37 °C for 72 h (Fig. 1). Wheat bran is a complete nutritious feed for microorganisms having all the ingredients and remains loose even under moist conditions providing a large surface area [2, 9]. The high productivity of enzyme in WB + CW combination is due to very high nutrient concentration of wheat bran and high pectin content in citrus waste [26]. Wheat bran serves both as a source of carbon and nitrogen. There are reports related to the fungal and bacterial production of pectinase by utilizing various agro industrial residues like coconut oil cake, cotton seed oil cake, ground nut oil cake, sesame oil cake, rice bran [19]; wheat bran [19, 20]; lemon peel, sunflower head, sorghum stem [29] but no work has been reported till now by using these substrates in combination which can enhance the production many fold while minimizing the cost.
A low level of pectinase activity was detected in the earlier stages of incubation but gradually increased to reach the maximum at 72 h of incubation. Decrease in pectinase production after 72 h may be due to accumulation of end product which hampers pectinase production or due to scarcity of the nutrients. The period required for incubation depends on the enzyme production pattern and the growth rate of microorganism. Similar incubation period for enzyme production has been reported from Streptomyces lydicus [19] where as comparatively longer incubation periods were required by fungal systems.
The different mineral salt solutions used as moistening agents (MA) showed varying extent of pectinase production. The highest enzyme titer (5,128 U/gds) was obtained when mixture of WB + CW was moistened with deionized water followed by MA I (4,901 U/gds), MA II (4,731 U/gds), and distilled water (2,543 U/gds). Deionized water without any mineral supplementation had been used as moistening agent for xylanase production [5].
Deionized water with different pH ranging from 6.0 to 10.0 was used as moistening agent in SSF. Maximum production of pectinase enzyme (5,187 U/gds) was observed at pH 7 (Fig. 2). With increase or decrease in pH of moistening agent, the enzyme production decreased. The variations in pectinase production due to pH changes may be because of the availability of nutrients at that particular pH. An optimum pH of 7.5 for pectinase production by Bacillus sp.MG-cp-2 using wheat bran as substrate was reported by Kapoor et al. [20].
The maximum pectinase titer (5,710 U/gds) was found when WB + CW were moistened with deionized water in the ratio of 1:2.5. On increasing or decreasing the moisture level, the pectinase yield decreased (Fig. 3). In SSF, moisture content is a significant factor, which determines the success of a process [28]. On increasing or decreasing the moisture level pectinase production decreased. The reason may be that the moisture content lower than the optimum level leads to the poor solubility of solid substrate nutrients, high water tension and improper swelling whereas the higher moisture content decreases the porosity due to gummy texture of the substrate, alters the particle structure, leads to impaired oxygen transfer and decreases the diffusion [7, 17]. An optimum substrate-to-moisture ratio of 1:5 for pectinase production has been reported for Streptomyces sp. RCK-SC using wheat bran as substrate [23].
The SSF was carried out by incubating the flasks at different temperatures such as 30, 37, 40, 45 and 50 °C for 72 h. The maximum enzyme production was found at 37 °C (5768 U/gds) in a humidified incubator and decrease in the enzyme production was observed at 30 °C (2,143 U/gds) and 40 °C (5,634 U/gds) temperature. Maximum production of enzyme at 37 °C may be due to the optimum growth of bacterium and higher biomass production at this temperature.
Pectinase production was highest (6,086 U/gds) when inoculum was used at a level of 15% whereas with increase (20%, 5,683 U/gds) or decrease (10%, 5,711 U/gds) in the inoculum size from 15% resulted in a decrease in pectinase production. Earlier 10% (v/w) of inoculum was used for the production of polygalacturonase from Bacillus sp. MG-cp-2 [19] where as an optimum inoculum size of 15% has been reported from B. licheniformis A99 in case of xylanase production under SSF [1].
Among the different additives tested for pectinase production, yeast extract (6,592 U/gds) and peptone (6,298 U/gds) induced the enzyme production, while glucose (3,124 U/gds) notably repressed the pectinase production which may be due to catabolite repression. Similar results have been reported by many workers, such as synthesis of pectinase by A.niger on pectin supplemented with glucose/sucrose repressed the enzyme [31]. An alkali and thermotolerant pectinase produced by a mesophilic Bacillus sp. DT7 using SSF conditions repressed the enzyme production when supplemented with glucose [21]. Similarly in case of α-amylase production by B. coagulans under SSF, wheat bran supplemented with glucose repressed the enzyme production [2].
Optimization of various parameters for pulp biobleaching
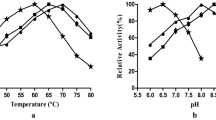
The pectinase produced by B. subtilis and xylanase produced by B. pumilus are cellulase-free as no endoglucanase activity could be detected with carboxymethylcellulose at different pH, which make them suitable for application in pulp biobleaching. Various parameters such as enzyme dosage, pH and temperature were optimized for the enzyme mixture in order to enhance the efficiency of enzymatic pulp bleaching [18]. These optimized conditions suitable for the enzymatic mixture were used to obtain the same %ISO brightness as it was achieved in chemical bleaching. From an industrial point of view, it is simple to adjust the pH but difficult and expensive to control temperature due to cost of cooling. The ideal solution therefore would be to use enzymes with higher pH and temperature stability, which will make the large-scale operations simpler and cost-effective [32]. Both xylanase and pectinase extracted from B. pumilus and B. subtilis respectively showed stability over a broad range of pH from 6 to 10 and temperature from 55 to 70 °C. However, the maximum efficiency of the enzymatic mixture in biobleaching was obtained at 60 °C and at pH 7.0. At a retention time of 150 min, an enzyme dosage of 5 IU/ml each of xylanase and pectinase was found suitable for biobleaching (Table 1). Higher enzyme doses did not lead to any significant enhancement in the biobleaching efficiency.
Biobleaching of kraft pulp
The bleaching efficiency of the cellulase-free enzyme mixture containing pectinase from B. subtilis and xylanase from B. pumilus on kraft pulp was maximum after 150 min at 60 °C using enzyme dosage of 5 IU/ml of each enzyme at 10% pulp consistency with about 16% reduction in kappa number and 84% reduction in permanganate number which is much significant as compared to pretreatment with xylanase decrease of only 83% in permanganate number [6]. After treatment with this enzyme mixture an increase of 7% in brightness was observed in case of unbleached pulp with respect to control. At each stage of chemical bleaching, the biobleaching effects of pectinase and xylanase treatment were observed on unbleached kraft pulp. In the ECDED1D2 process, nearly 53% increase in brightness by xylanase was achieved after chlorination, 36% after alkali treatment, 35% after D1 stage, 4% after D2 and only 1% after the final step. This indicates that the biobleaching effect of this enzyme mixture was maximum at the first stage where the maximum reduction in kappa number and P. no. was achieved that was significantly more as compared to the other stages of either biobleaching or chemical bleaching. Therefore, prebleaching by this enzyme mixture can be established as the most suitable step to facilitate bleach boosting of pulp. The application of xylanases for improvement in pulp bleaching has been reported by several workers by using xylanase from B. pumilus ASH [6], Streptomyces sp. QG-11-3 [9], S. cuspidosporus [25], B. licheniformis 77-2 [12], Chaetomium cellulolyticum [4], and Streptomyces sp. strain S38 [15]. An overall bleach-boosting of eucalyptus kraft pulp was obtained when alkaline pectinase from Streptomyces sp. QG-11-3 was used in combination with xylanase from the same organism for biobleaching [10].
Enzyme treated pulp when subjected to CDED1D2 steps, 25% reduction in chlorine consumption and upto 19% reduction in consumption of chlorine dioxide was observed for obtaining the same %ISO brightness (Table 2). Battan et al [6] have reported chlorine reduction of upto 20% by pretreatment of pulp using only xylanase produced by B. pumilus and a chlorine reduction of 8% by using xylanase from Streptomyces sp. QG-11-3 has been reported earlier. A combination of these two enzymes was very effective in leading to the percentage increase of 22 and 84% in whiteness and fluorescence respectively and a decrease of approximately 19% in the yellowness of the biotreated pulp was observed whereas pretreatment of pulp by xylanase alone caused increase of only 21% in whiteness and 28% in fluorescence [6]. Thus apart from making the bleaching process environmental-friendly, the use of these enzymes also makes the process economically feasible while improving the pulp properties.
The scanning electron microscopic (SEM) studies revealed that this enzymatic mixture of pectinase and xylanase introduced greater porosity, swelling up and separation of pulp microfibrils and pulp fibres compared to the smooth surfaces of untreated pulp. After enzymatic treatment, when the pulp fibres were subjected to addition of chlorine bleaching chemicals, swelling, separation and loss in compactness in the pulp fibres was observed (Figs. 4, 5). This indicates that the addition of pectinase and xylanase to the pulp fibres prior to the addition of bleaching chemicals renders the pulp fibres more accessible to the chemical bleaching agents, thereby, reducing the requirement of chlorine and chlorine compounds in the subsequent bleaching process [8].
In conclusion, the criterion for any process to be industrially favorable is its cost-effectiveness and eco-friendly nature. Therefore, the enzymes have been produced under optimized conditions to achieve a very high yield. Also, in the paper industry, xylanase and pectinase free of cellulase activity are required for selective removal of only the hemicellulose component and pectins respectively with minimal damage to the cellulose pulp. Both these enzymes are cellulase-free and their wide range of thermal and pH stability make them suitable for their application in pulp and paper industry. This is the first report of using pectinase and xylanase mixture for effective bleaching of paper pulp and the results indicate that this enzymatic mixture is more efficient than using pectinase and xylanase alone.
References
Archana A, Sathyanarayana T (1999) Xylanase production by thermophilic Bacillus lichieniformis A99 in solid state fermentation. Enzyme Microb Technol 21:12–17
Babu KR, Satyanarayana T (1995) α-Amylase production by thermophilic Bacillus coagulance in solid-state fermentation. Proc Biochem 30:5–9
Bajpai P (1999) Application of enzymes in the pulp and paper industry. Biotechnol Progress 15:147–157
Baraznenok VA, Becker EG, Ankudimova NV, Okunev NN (1999) Characterization of neutral xylanase from Chaetomium cellulolyticum and their biobleaching effect on eucalyptus pulp. Enzyme Microb Technol 25:651–659
Battan B, Sharma JK, Kuhad RC (2006) High-level xylanase production by alkalophilic Bacillus pumilus ASH under solid-state fermentation. World J Microbiol Biotechnol 22:1281–1287
Battan B, Sharma JK, Dhiman SS, Kuhad RC (2007) Enhanced production of cellulase-free thermostable xylanase by Bacillus pumilus ASH and its potential application in paper industry. Enzyme Microb Technol DOI: 10.1016/j.enzmictec.2007.06.006
Beckord LD, Kneen E, Lewis KH (1945) Bacterial amylase production on wheat bran. Ind Eng Chem 37:692–696
Beg QK, Bhushan B, Kapoor M, Hoondal GS (2000) Enhanced production of a thermostable xylanase from Streptomyces sp. QG-11-3 and its application in biobleaching of eucalyptus kraft pulp. Enzyme Microb Technol 27:459–466
Beg QK, Kapoor M, Mahajan L, Hoondal GS (2001) Microbial xylanases and their industrial applications: a review. Appl Microbiol Biotechnol 56:326–338
Beg QK, Kapoor M, Mahajan L, Hoondal GS (2001) Bleach-boosting of eucalyptus kraft pulp using combination of xylanase and pectinase from Streptomyces sp. QG-11-3. Res Bull Panjab Univ Sci 51:71–78
Christov LP, Szakacs G, Balakrishnan H (1999) Production, partial characterization, and use of fungal cellulase-free Xylanase in pulp bleaching. Process Biochem 34:511–517
Damiano VB, Bocchini DA, Gomes E, Da Siva R (2003) Application of crude xylanase from Bacillus licheniformis 77-2 to the bleaching of eucalyptus Kraft pulp. World J Microbiol Biotechnol 19:139–144
Feniksova RV, Tikhomrova AS, Rakhleeva BE (1960) Conditions for forming amylase and proteinase insurface culture of Bacillus subtilis. Microbiologica 29:745–748
Garg AP, Roberts JC, McCarthy AJ (1998) Bleach boosting effect of cellulase-free xylanase of Streptomyces thermoviolaceus and its comparison with two commercial enzyme preparations on birchwood kraft pulp. Enzyme Microb Technol 22:594–598
Georis J, Giannotta F, De Buyl E, Granier B, Frere JM (2000) Purification and properties of three endo-β-1,4-xylanase produced by Streptomyces sp. strain S38 which differ in their ability to enhance the bleaching of kraft pulps. Enzyme Microb Technol 26:178–186
Gupta S, Bhushan B, Hoondal GS (2000) Isolation, purification and characterization of xylanase from Staphylococcus sp. SG-13 and its application in biobleaching of kraft pulp. J Appl Microbiol 88:325–334
Hasseltine CW (1972) Solid state fermentations. Biotechnol Bioeng 14:517–532
Hong Q, Shin NH, Chang H (1989) Effect of oxygen extraction on organic chlorine contents in bleach plant effluents. Tappi J 72:157–161
Jacob N, Prema N (2006) Influence of mode of fermentation on production of polygalacturonase by a novel strain of Streptomyces lydicus. Food Technol Biotechnol 44:263–267
Kapoor M, Beg QK, Bhushan B, Dadhich KS, Hoondal GS (2000) Production and partial purification and characterization of a thermo-alkali stable polygalacturonase from Bacillus sp. MG-cp-2. Process Biochem 36:467–473
Kashyap DR, Soni SK, Tewari R (2003) Enhanced production of pectinase by Bacillus sp. DT7 using solid state fermentation. Biores Technol 88:251–254
Kirk TK, Jefferies TW (1996) Role of microbial enzymes in pulp and paper processing. In: Jeffereies TW, Viikari L (eds) Enzymes for pulp and paper processing. ACS symposium series. American Chemical Society, Washington DC, pp 1–14
Kuhad RC, Kapoor M, Rustagi R (2004) Enhanced production of alkaline pectinase from Streptomyces sp. RCK-SC by whole-cell immobilization and solid-sate cultivation. World J Microbiol Biotechnol 20:257–263
Kuhad RC, Singh A, Eriksson KEL (1997) Microorganisms and enzymes involved in the degradation of plant fibre cell walls. Adv Biochem Eng Biotechnol 57:47–125
Maheshwari MU, Chandra TS (2000) Production and potential applications of a new xylanase from a new strain of Streptomyces cuspidosporus. World J Microbiol Biotechnol 16:257–263
Martin N, de Souza SR, da Silva R, Gomes E (2004) Pectinase production by fungal strains in solid-state fermentation using agro-industrial bioproduct. Brazilian Arch Biol Technol 47:813–819
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428
Pandey A, Soccol CR, Mitchell D (2000) New development in solid state fermentation: I - bioprocesses and products. Proc Biochem 35:1153–1169
Patil SR, Dayanand A (2006) Exploration of Regional Agrowastes for the production of Pectinase by Aspergillus niger. Food Technol Biotechnol 44:289–292
Ragaukas AJ, Poll KN, Cesternino A (1994) Effects of xylanase pretreatment procedures on non chlorine bleaching. Enzyme Microb Technol 16:492–495
Solis-Pereira S, Favela-Torres E, Viniegra-Gonzelez G, Gutierres-Rojas M (1993) Effect of different carbon sources on the synthesis of pectinases by Aspergillus niger in submerged and solid state fermentations. Appl Microbiol Biotechnol 39:36–41
Srinivasan MC, Rele MV (1999) Micobial Xylanase for pulp industry. Curr Sci 77:137–142
TAPPI test methods (1996) Technical association of the pulp and paper industry. TAPPI Press, Atlanta
Viikari L, Ranua M, Kantelinen A, Sundquist J, Linko M (1986) Bleaching with enzymes. In: Proceedings of the third international conference on biotechnology pulp and paper industry, Stockholm, 16–19 June, pp 67–69
Wong KKY, Tan LUL, Saddler JN (1988) Multiplicity of β-1, 4-xylanase in microorganisms: functions and applications. Microb Rev 52:305–17
Acknowledgment
The authors thank Ballarpur Industries Pvt. Ltd. (BILT), Haryana, India for providing kraft pulp and laboratory facilities. Sonia Ahlawat and Saurabh Sudha Dhiman wishes to thank Kurukshetra University, Kurukshetra for University Research Scholarship during the course of investigation. Bindu Battan greatly acknowledges the financial assistance provided by Council of Industrial Research, India in the form of senior research fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ahlawat, S., Battan, B., Dhiman, S.S. et al. Production of thermostable pectinase and xylanase for their potential application in bleaching of kraft pulp. J Ind Microbiol Biotechnol 34, 763–770 (2007). https://doi.org/10.1007/s10295-007-0251-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-007-0251-3