Abstract
The enzyme glucosyltransferase is an industrially important enzyme since it produces non-cariogenic isomaltulose (6-o-α-d-glucopyronosyl-1-6-d-fructofuranose) from sucrose by intramolecular transglucosylation. The experimental designs and response surface methodology (RSM) were applied for the optimisation of the nutrient concentrations in the culture medium for the production of glucosyltransferase by Erwinia sp. D12 in shaken flasks at 200 rpm and 30°C. A statistical analysis of the results showed that, in the range studied, the factors had a significant effect (P < 0.05) on glucosyltransferase production and the highest enzyme activity (10.84 U/ml) was observed in culture medium containing sugar cane molasses (150 g l−1), corn steep liquor (20 g l−1), yeast extract Prodex Lac SD® (15 g l−1) and K2HPO4 (0.5 g l−1) after 8 h at 30°C. The production of cell biomass by the strain of Erwinia sp. D12 was carried out in a 6.6-l fermenter with a mixing rate of 200 rpm and an aeration rate of 1 vvm. Fermentation time, cellular growth, medium pH and glucosyltransferase production were observed. The greatest glucosyltransferase activity was 22.49 U/ml, obtained after 8 h of fermentation. The isomaltulose production from sucrose was performed using free Erwinia sp. D12 cells in a batch process using an orbital shaker. The influence of the parameters sucrose concentration, temperature, pH, and cell concentration on the conversion of sucrose into isomaltulose was studied. The free cells showed a high conversion rate of sucrose into isomaltulose using batch fermentation, obtaining an isomaltulose yield of 72.11% from sucrose solution 35% at 35°C.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Isomaltulose is a sucrose isomer obtained commercially by the enzymatic conversion of sucrose catalysed by microbial glucosyltransferase and can be found naturally in honey and sugar cane extracts in very small quantities. It has been suggested as a non-cariogenic alternative to sucrose, and as such is currently in wide use as a sugar substitute in foods. It can also be converted into a sugar–alcohol mixture known as Isomalt®, that has low caloric value and non-cariogenic properties. In experimental studies with rats [15, 17] and in human studies [24], the production of insoluble glucan and acids from isomaltulose by strains of Streptococcus mutans was very low when compared to sucrose. Isomaltulose is perceived to be about 50% as sweet as sucrose [3, 4, 6, 15, 17, 20, 22, 23, 24] and shows very similar physical and organoleptic properties [2, 6]. In the organism, isomaltulose is hydrolysed by isomaltase and absorbed as glucose and fructose [23]. Unlike sucrose, the ingestion of isomaltulose has only a minor effect on glycemia, indicating its potential as a parenteral nutrient acceptable to both diabetics and non-diabetics [7, 8]. Teratogenic or mutagenic effects and toxicity are not associated with the consumption of isomaltulose by rats, it thus being safe for use in foods [11].
The conversion of sucrose into isomaltulose can be carried out using free cells [5, 19] or immobilized cells [1, 2, 4, 10, 18, 20, 25]. Response surface methodology was used to evaluate the effect of the culture medium components on glucosyltransferase activity, with the purpose of producing a low cost culture media. A two level fractional factorial design (25–1-FFD) was used to select the significant factors, and a two level rotatory central composite design (23-RCCD) used to optimise the culture medium. This paper reports the production of isomaltulose from sucrose using free Erwinia sp. D12 cells in repeated-batch processes.
Materials and methods
Microorganism and maintenance
Erwinia sp. D12, a producer of glucosyltransferase, was obtained from the Laboratory of Biochemistry (Faculty of Food Engineering/UNICAMP). The microorganism was maintained in a culture medium containing the following ingredients per litre of sterile water: 6 g sucrose, 4 g peptone, 0.4 g beef extract and 2 g agar. The cultures were kept at 5°C.
Optimisation of a culture medium containing agricultural wastes, using response surface methodology.
The in vitro study for the optimisation of the culture medium (in shaken flasks) was carried out using an experimental design and response surface methodology (RSM). All data were treated with the aid of STATISTICA® 5.0 from Statsoft Inc. (2325 East 13th Street, Tulsa, OK, 74104, USA).
Cultivation and enzyme production
The seed medium had the same composition as the production medium as shown in Tables 1, 2, 3 and 4. A loop full of cells was inoculated into 50 ml of seed medium contained in 250 ml Erlenmeyer flasks and incubated in an orbital shaker (New Brunswick Scientific, Edison, NJ, USA) at 30°C and 200 rpm for 15 h. This seed culture was inoculated into the production medium at a rate of 10% (v/v). After 8 h of fermentation, the culture was centrifuged at 10,070×g for 15 min and the glucosyltranferase activity determined.
Glucosyltransferase assay
The glucosyltransferase activity was determined from the increase in reducing power of a solution containing sucrose as described by Park et al. [16], with modifications. For the extraction of the intracellular enzyme, the cell mass was washed twice with distilled water and then suspended in 50 ml 0.05 M citrate–phosphate buffer, pH 6.0. The cell suspension was cooled to 5°C and disrupted by ultrasonic vibration (180 W for 20 s). After cell wall disruption, the samples were centrifuged at 12,300×g for 15 min at 5°C.
The enzyme activity of the supernatant was then determined. A mixture of 450 μl of a 4.0% (w/v) sucrose solution in 0.05 M citrate–phosphate buffer pH 6.0, and 50 μl of enzyme solution, were incubated for 20 min at 35°C. Reducing sugars were measured by Somogyi’s method [21] using glucose as the standard. One unit of glucosyltransferase activity (U) was defined as the amount of enzyme that liberates 1 μmol of reducing sugar (isomaltulose)/minute from the sucrose solution, under standard assay conditions.
Evaluation of the effect of the variables on glucosyltransferase activity
A two level fractional factorial design (25−1-FFD) was employed to evaluate the variables that significantly affected glucosyltransferase activity. Table 1 shows the 25−1-FFD matrix covering the five variables, to evaluate their effect on glucosyltranferase activity. The variables considered for the design were sugar cane molasses (x 1/SCM), corn steep liquor (x 2/CSL), yeast extract Prodex Lac SD® (x 3/YEP), K2HPO4 (x 4) and pH (x 5). These factors and their levels are listed in Table 2. The sugar cane molasses, corn steep liquor (Milhocina®) and yeast extract Prodex Lac SD® were obtained from the Companhia Energética Santa Elisa; Corn Products Brazil and Prodesa Produtos Especiais para Alimentos Ltda, respectively.
Each independent variable was investigated at a high (+1) and a low (−1) level. Centre point runs (three points) were included in the matrix and a statistical analysis was used to identify the effect of each variable on the response. The significance was determined by applying the Student’s t test, P value and significance levels.
Optimisation using rotatory central composite design and response surface methodology
Response surface methodology was used to optimise the levels of the significant variables as identified by the two level fractional factorial design. A two level rotatory central composite design (23-RCCD) with six axial points (α) and three replicates at the central point, giving a total of 17 assays per experiment as per Table 3, was used to optimise the components for glucosyltransferase production by the strain Erwinia sp. D12, to obtain maximum enzymatic activity and reduce costs in the fermentation process.
Growth determination and glucosyltransferase production in the optimal culture medium in a 6.6-l fermenter
Bacterial growth and glucosyltransferase activity were determined in the following optimal culture medium: sugar cane molasses (150 g l−1), yeast extract Prodex® (15 g l−1), corn steep liquor-Milhocina® (20 g l−1) and pH 7.5 in a New Brunswick Bioflo IIc bioreactor 6.6-l fermenter (New Brunswick Scientific, Edison, NJ, USA). A loop full of culture were inoculated into each of six 250 ml Erlenmeyer flasks containing 50 ml of optimised culture medium and incubated in an orbital shaker at 200 rpm and 30°C for 15 h. A 300 ml aliquot of inoculum was transferred into 2,700 ml of optimised culture medium contained in a 6.6-l fermenter, and incubated under the following conditions: temperature 27°C, initial pH 7.5, aeration rate 1 vvm and agitation speed of 200 rpm. Samples were collected at time-defined intervals and submitted to analysis.
Growth of the microorganism, glucosyltransferase activity and variation of the culture medium pH
Aliquots (20 ml) of the culture broth were centrifuged at 12,300×g for 15 min at 5°C. The cell mass was washed twice with 20 ml of distilled water and re-suspended into 20 ml of distilled water. A Beckman DU 70 spectrophotometer (Beckman-Coulter, Inc., Fullerton, CA, USA) was used to monitor cell growth by measuring the optical density at 660 nm (OD660). Glucosyltransferase activity was estimated as described previously. The pH of the culture medium was measured with an Orion model 710A potentiometer (Orion Research Inc, Boston, MA, USA).
Influence of temperature, pH and substrate and cell concentrations on the conversion of sucrose into isomaltulose
The experiments were conducted in a batch mode using an orbital shaker at 200 rpm. The assays to evaluate the influence of temperature (30, 35 and 38°C), pH (5.0, 5.5, 6.0, 6.5 and 7.0), sucrose concentration (35, 37, 40, 42 and 45% w/v) and cell mass concentration (10, 15 and 20% w/v) on the conversion of sucrose into isomaltulose, were carried out in Erlenmeyer flasks containing the reaction medium. Aliquots of the reaction medium were collected after different incubation times (5, 15, 25, 35, 45 and 60 min). The carbohydrate analyses were carried out using a Dionex DX-600 High Performance Liquid Chromatograph.
HPLC-PAD analysis
The sugar analyses were performed using an HPLC system consisting of a DIONEX DX-600 (Dionex Corporation, 1228 Titan Way, Sunnyvale, CA, USA) chromatograph equipped with an Electrochemical Detector ED50, a CarboPac™ PA 1 column (4 × 270 mm) and a CarboPac™ PA 1 guard column (4 × 500 mm), with a mobile phase of 200 mM NaOH at a flow rate of 1 ml min−1 and a temperature of 20°C. The carbohydrates were identified from their retention times as compared to those of the fructose, glucose, sucrose and isomaltulose standards (Sigma Chemical Co., St Louis, MO, USA).
Performance of the repeated batch operations using free cells
After studying the influence of temperature, pH and substrate and cell concentrations on the conversion of sucrose into isomaltulose, repeated-batch conversion runs were carried out in 250 ml Erlenmeyer flaks containing 50 ml of 35% (w/v) sucrose solution and 10% free cells of Erwinia sp. D12 (w of wet cell/v) at pH 6.5. The flasks were maintained in an orbital shaker at 150 rpm and 35°C for 15 min. At the end of each batch, samples were collected and submitted to analysis. The reaction medium was then centrifuged at 10,070×g for 20 min at 25°C and the free cells used for the next batch conversion of sucrose into isomaltulose with fresh substrate. This process was repeated several times. The carbohydrate analyses were carried out using the Dionex DX-600 High Performance Liquid Chromatograph as described previously.
Results and discussion
Evaluation of the effect of the significant variables on glucosyltransferase activity
The experimental design with 19 experiments and 5 factors or independent variables and their corresponding glucosyltransferase activity are indicated in Table 1. The variables listed contained different concentrations of medium components, as shown in Table 2: x1, sugar cane molasses (SCM) at a low concentration of 20 g l−1 and a high concentration of 180 g l−1; x 2, corn steep liquor (Milhocina®-CSL) at a low concentration of 5 g l−1 and a high concentration of 35 g l−1; x 3, yeast extract Prodex® at a low concentration of 5 g l−1 and a high concentration of 15 g l−1; x 4, K2HPO4 at a low concentration of 0.5 g l−1 and a high concentration of 1.5 g l−1; and x 5, pH at a low value of 5.0 and a high value of 9.0. The responses obtained were evaluated statistically and those variables with a confidence level of 95% were regarded as having a significant effect on glucosyltransferase activity. The estimates of the main effects are shown in Table 5.
It is possible to observe that variations in SCM, YEP and pH significantly (P < 0.05) affected glucosyltransferase activity and that the main effects were positive, suggesting that the increase in their values or ranges in the medium resulted in the promotion of enzyme production. The estimated effect of CSL was positive, suggesting that an increase in its concentration could improve the enzyme activity, however the effect was not significant (P > 0.05) so its concentration was fixed at 20 g l−1. The main effect of K2HPO4 was negative, however the variations in concentration of this medium component did not significantly (P > 0.05) affect glucosyltransferase activity, suggesting that its lower (−1) concentration was plentiful. Thus, using the two level fractional factorial designs the variables that could significantly affect glucosyltransferase activity were identified.
Optimisation using a rotatory central composite design and response surface methodology
The variables sugar cane molasses (SCM), yeast extract Prodex® (YEP) and pH were the most effective factors promoting glucosyltransferase activity, and their values were further optimised using a two level rotatory central composite design (23-RCCD). The experimental results for glucosyltransferase activity using the complete 23-RCCD with four replicates of the central points, six assays corresponding to the axial points and eight assays corresponding to the factorial points, are shown in Table 3. The levels studied with the decoded values are shown in Table 4.
The correlation measurements used in the estimation of the regression equation were the multiple correlation coefficients (R) and the determination coefficient (R 2). The closer the value of R was to 1, the better the correlation between the observed and predicted values. Table 6 shows the analysis of variance (ANOVA/F test) for this experiment and the coefficient of determination (R 2). The goodness of fit of the model was checked using the coefficient of determination, which was shown to be 0.97, indicating that only 3% of the total variation was not explained by the model. This indicated that the accuracy and general ability of the quadratic model was good, and the analysis of the response trends using the model was considered to be reasonable. The value for R was 0.98, showing excellent correlation between the predicted and experimental values. The F value of 38.33 for glucosyltransferase activity implied that the model was statistically significant (F model exceeded the tabulated value of F 0.95,7,9 by 11.7 times). The lack of fit test compares the residual error to the pure error from replicates central points. The lack of fit F value of 22.31 implied that the lack of this factor was significant. There is only a 0.05% chance that a lack of fit F value this large could occur due to noise. Thus the estimated model adequately fitted the experimental data.
The regression coefficients and the corresponding P values for the model of glucosyltransferase activity are presented in Table 7. The P values were used as a tool to check the significance of each coefficient, and also indicated the strength of the interaction between each independent variable. The larger the magnitude of the T value and the smaller the value of P, the more significant is the corresponding coefficient. It can be seen that almost the whole regression was significant except for one linear coefficient (SCM) and one interaction coefficient (YEP and pH). These results were good enough indicators for a model representing the real relationship between glucosyltransferase activity and the independent variables. Using the 23-RCCD, the polynomial model (Eq. 1) for glucosyltransferase activity was regressed by only considering the significant terms, as shown below:
where y was the predicted response for glucosyltransferase activity and x 1, x 3 and x 5 were the coded values for SCM, YEP and pH, respectively. The response surfaces and contour curves (Figs. 1, 2) representing the predicted model indicated the levels of the variables necessary for an optimal process. The components SCM, YEP and pH affected the production of glucosyltransferase by Erwinia sp. D12.
The experimental design and response surface methodology indicated that the following amounts of SCM (150 g l−1), YEP (15 g l−1), CSL (20 g l−1) and K2HPO4 (0.5 g l−1) and a pH of 7.5, increased the glucosyltransferase activity, obtaining a maximum glucosyltransferase activity of 10.84 U/ml. The glucosyltransferase activity obtained using agricultural wastes (corn steep liquor and sugar cane molasses) was 3.7 times higher than that produced by the strain Klebsiella sp., when 2.95 U/ml was obtained using cells incubated in a culture medium composed of beef extract and sucrose [16]. The glucosyltransferase purified from Pseudomonas mesoacidophila MX-45, isolated by Nagai et al. [14], presented 13.89 U/mg protein. However, the authors used a media composed of beef extract, peptone, NaCl and Na2HPO4 to obtain the cells. Huang et al. [6] obtained glucosyltransferase activity of 5.2–11.08 U/ml from the strain Klebsiella planticola CCRC 19112 in the early stationary phase, using a variety of carbon sources (sucrose and fructose were the most effective), inorganic salts (NaCl, KH2PO4 and NaH2PO4) and supplemental nitrogen sources (bacto-tryptone was the most effective). The strain Klebsiella planticola MX-10 showed maximum glucosyltransferase activity of 4.3 U/ml [25]. Using a fermentation medium composed of peptone (4%) and yeast extract (0.4%); and the strain Klebsiella sp. LX3, 15.12 U/ml of enzyme activity was obtained [12]. In this study, sugar cane molasses, corn steep liquor Milhocina® (agricultural wastes) and commercial yeast extract (Prodex Lac SD®) were used with the purpose of producing a low cost culture medium. The results were satisfactory and the components sugar cane molasses and corn steep liquor were, respectively, very useful carbon and nitrogen sources for enzyme production.
Growth determination and glucosyltransferase production using the optimised culture medium in a 6.6-l fermenter
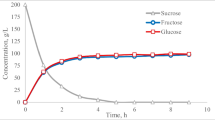
Glucosyltransferase production and the growth characteristics of Erwinia sp. D12 using the optimised culture medium are illustrated in Fig. 3. The highest enzyme activity was obtained after 8 h of fermentation (22.49 U/ml), then decreasing to 16.75 U/ml after 10 h and remaining constant, between 14 and 17.5 U/ml, up to 26 h of fermentation time. The pH of the culture medium was between 5.9 and 6.1 during fermentation, suggesting little production of acid as a by-product. Moraes et al. [13] obtained 15.6 U/ml of glucosyltranferase activity from Erwinia sp. cells incubated in a 3.0-l fermenter containing culture medium composed of sugar cane molasses, bacteriological peptone and beef extract, at 30°C, 200 rpm and 1 vvm. The cell mass obtained from the bioreactor fermentation at 27°C was used in further studies to verify the conversion of sucrose to isomaltulose from free cells in repeated-batch processes.
Influence of temperature, pH and substrate and cell concentrations on the conversion of sucrose to isomaltulose
The influences of temperature, pH and substrate and cell concentrations on the conversion of sucrose into isomaltulose were evaluated using an orbital shaker, and the time courses for isomaltulose formation by free cells are shown in Figs. 4 and 5. Figure 4a, b and c illustrates the conversion of sucrose into isomaltulose at 30, 35 and 38°C, respectively. The maximum isomaltulose yield (63.33%) at 38°C was obtained at pH 5.0 after 35 min reaction. At 30°C the highest conversion (63.83%) was obtained at pH 6.5 after 60 min reaction. However, at 35° the highest isomaltulose yields were obtained: 64.16 and 64.94% after 35 and 45 min, respectively.
Time course for isomaltulose production from sucrose by Erwinia sp. D12 free cells varying wet cell mass and sucrose concentration: a 10% wet cell × sucrose; b 15% wet cell × sucrose; c 20% wet cell × sucrose. Filled diamond 35%sucrose, filled square 37% sucrose, asterisk 40% sucrose, cross 42% sucrose, filled triangle 45% sucrose
Figure 5a, b and c illustrate the effect of wet cell mass and sucrose concentration on sucrose conversion into isomaltulose. The highest isomaltulose yields were verified using 10% wet cell mass with a 35% sucrose solution (Fig. 5d) and 15% wet cell mass with a 37% sucrose solution (Fig. 5e), obtaining, respectively, 69.45% of isomaltulose after 15 min, and 69.53% after 25 min. The productivity using the 10% wet cell mass and 35% sucrose solution (0.0463 g isomaltulose/g sucrose/min) was higher than with the 15% wet cell mass and 37% sucrose solution (0.0278 g isomaltulose/g sucrose/min).
Performance of repeated batch operations using free cells
The stability of the glucosyltransferase from Erwinia sp. D12 during the conversion of sucrose into isomaltulose was evaluated by using the free cells repeatedly during 16 batch operations (Fig. 6). The conversion of sucrose into isomaltulose after eight batches was higher than 60% and the highest conversion of sucrose into isomaltulose (72.11%) was verified in batch number one. In the first batch, traces of sucrose, glucose and fructose were detected and the conversion rate was 97%. After eight batches, the cells were centrifuged at 10.070×g for 20 min and washed once with distilled water. The cell mass was incubated with a fresh culture medium overnight to observe the effect on the viability of the microorganism. However, it was verified that the cells had no effect on the isomaltulose yield after the treatment with fresh culture medium, and the conversion rate decreased to 34.78% of isomaltulose in batch number 16. The conversion of sucrose into isomaltulose using whole cells of Klebsiella planticola CCRC 10112 was 76% [6] and using the purified enzyme from the strain Klebsiella planticola MX-10, it was 63.9%(18). Véronèse and Perlot [26] obtained 72.6% isomaltulose using a purified glucosyltransferase from Serratia plymuthica ATCC 15928. The isomaltulose yields obtained from Erwinia sp. [13] and Klebsiella sp. LX3 [12] cells immobilized in calcium alginate were 50 and 87%, respectively. The isomaltulose yield using Serratia plymuthica cells immobilized in chitosan was 80% [9].
Conclusions
Using response surface methodology (RSM), statistically optimal values for the independent variables are acquired from the central points of the contour along the major and minor axes. Using RSM, the optimal concentrations for the sugar cane molasses (SCM), corn steep liquor (Milhocina®-CSL), yeast extract (Prodex Lac SD®-YEP) and K2HPO4 in the production of glucosyltransferase by Erwinia sp. D12 were found to be 150, 20, 15 and 0.5 g l−1, respectively, and the optimal pH was 7.5. After 8 h of fermentation in this media at 30°C, 10.84 U/ml of glucosyltransferase were obtained. When Erwinia sp. D12 was grown aerobically in a 6.6-l fermenter, the greatest production of glucosyltransferase (22.49 U/ml) was obtained after 8 h of fermentation. The free cells of Erwinia sp. D12 converted a 35% sucrose solution into isomaltulose in a batch process, obtaining 72.11% isomaltulose after 15 min at 35°C. High reaction speeds and yields were obtained using Erwinia sp D12 cells in a batch process. New investigations are necessary to optimise the conversion of sucrose into isomaltulose.
References
Bucke C, Cheetham PSJ (1982) Production of isomaltulose. US patent 201,462
Cheethan PSJ, Imber CE, Isherwood J (1982) The formation of isomaltulose by immobilized Erwinia rhapontici. Nature 299:628–631
Godshall MA (1997) How carbohydrates influence food flavors. Food Technol 51:63–66
Hashimoto H, Yamada K, Yoshimura J (1987) Glucosylation of methyl β-d-arabinofuranoside with 6′-chloro-6′-deoxysucrose and immobilized Protaminobacter rubrum. Biotechnol Lett 9:849–854
Heikkila H, Sarkki M, Lindroos M, Ojala P, Ravanko V, Tylli M (2000) Process of the production of isomaltulose and other products. US patent 082555
Huang JH, Hsu LH, Su YC (1998) Conversion of sucrose to isomaltulose by Klebsiella planticola CCRC 19112. J Ind Microbiol Biotechnol 21:22–27
Kawai K, Okuda Y, Yamashita K (1985) Changes in blood glucose and insulin after an oral palatinose administration in normal subjects. Endocrinol Jpn 32:933–936
Kawai K, Yoshikawa H, Murayama Y, Okuda Y, Yamashita K (1989) Usefulness of palatinose as a caloric sweetener for diabetic patients. Horm Metab Res 21:338–340
Krastanov A, Yoshida A (2003) Production of palatinose using Serratia plymuthica cells immobilized in chitosan. J Indus Microbiol Biotechnol 30:593–598
Lantero OJ (1983) Immobilization of the sucrose mutase in whole cells of Protaminobacter rubrum. E. patent 82109404.2
Lina BAR, Smits-van prooije AE, Waalkens-berendsen DH (1997) Embryotoxicity/ teratogenicity study with isomaltulose (palatinose) in rats. Food Chem Toxicol 35:309–314
LiX, Zhao C, Na Q, Zhang D (2003) Substrate induction isomaltulose synthase in a newly isolated Klebsiella sp. LX 3. J Appl Microbiol 95:2131–2136
Moraes ALL, Steckelberg C, Sato HH, Pinheiro A (2005) Produção de isomaltulose a partir da transformação enzimática da sacarose, utilizando-se Erwinia sp D12 imobilizada com alginato de cálcio. Ciênc Tecnol Aliment 25:95–102
Nagai Y, Sugitani T, Tsuyuki KI (1994) Characterization of alpha-glucosyltransferase from Pseudomonas mesoacidophila MX-45. Biosci Biotechnol Biochem 58:1789–1793
Ooshima T, Izumitani A, Sobue S, Okahashi N, Hamada S (1983) Non-cariogenicity of the disaccharide palatinose in experimental dental caries of rats. Infect Immun 39:43–49
Park YK, Uekane RT, Sato HH (1996) Biochemical characterization of a microbial glucosyltransferase that converts sucrose to palatinose. Rev Microbiol 27:131–136
Sasaki N, Topitsoglou V, Takazoe I, Frostell G (1985) Cariogenicity of isomaltulose (palatinose), sucrose and mixture of these sugars in rats infected with Streptococcus mutans E- 49. Swed Dent J 9:149–155
Sarkki M, Heikkila H, Viljava T (1999) Immobilization of microorganisms on weakly basic anion exchange substance for producing isomaltulose. US patent 857808
Schiweck H, Steinle G, Muller L, Gau W, Munir M (1980) Glucopyranosido-1,6-mannitol, a process for producing the same and its use as a sugar substitute. US patent 21,252
Shimizu J, Suzuki K, Nakajima Y (1982) Method of producing an immobilized alpha-glucosyltransferase useful in the production of palatinose from sucrose. UK patent 8124779
Somogyi M (1945) A new reagent for the determination of sugars. J Biol Chem 160:61–68
Takazoe I (1985) New trends on sweeteners in Japan. Int Dent J 35:58–65
Takazoe I, Frostell G, Ohta K, Topitsoglou V, Sasaki N (1985) Palatinose—a sucrose substitute. Swed Dent J 9:81–87
Topitsoglou V, Sasaki N, Frostell G (1984) Effect of frequent rinses with isomaltulose (palatinose) solution on acid production in human dental plaque. Caries Res 18:47–51
Tsuyuki K, Sugitani Y, Miyata Y, Ebashi T, Nakajima Y (1992) Isolation and characterization of trealulose-producing bacteria from Thailand soil. J Gen Appl Microbiol 38:483–490
Véronèse T, Perlot P (1999) Mechanism of sucrose conversion by the sucrose isomerase of Serratia plymuthica ATCC 15928. Enzyme Microb Technol 24:263–269
Acknowledgments
We are grateful to CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior), FINEP (Financiadora de Estudos e Projetos) and GETEC Guanabara Química Industrial S/A, for their financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kawaguti, H.Y., Buzzato, M.F. & Sato, H.H. Isomaltulose production using free cells: optimisation of a culture medium containing agricultural wastes and conversion in repeated-batch processes. J Ind Microbiol Biotechnol 34, 261–269 (2007). https://doi.org/10.1007/s10295-006-0194-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-006-0194-0