Abstract
Ethyl(R)-4-chloro-3-hydroxybutanoate ((R)-CHBE) are obtained by cetyltrimetylammonium bromide (CTAB) permeabilized fresh brewer’s yeast whole cells bioconversion of ethyl 4-chloro-3-oxobutanoate (COBE ) in the presence of allyl bromide. The results showed that the activities of alcohol dehydrogenase (ADH) and glucose-6-phosphate dehydrogenase (G6PDH) in CTAB permeabilized brewer’s yeast cells increased 525 and 7.9-fold, respectively, compared with that in the nonpermeabilized cells and had high enantioselectivity to convert COBE to (R)-CHBE. As one of co-substrates, glucose-6-phosphate was preprepared using glucose phosphorylation by hexokinase-catalyzed of CTAB permeabilized brewer’s yeast cells. In a two phase reaction system with n-butyl acetate as organic solvent and with 2-propanol and glucose-6-phosphate as co-substrates, the highest (R)-CHBE concentration of 447 mM was obtained with 110–130 g/l of the CTAB permeabilized cells at optimized pH, temperature, feeding rate and the shake speed of 125 r/min. The yield and enantiomeric excess (ee) of (R)-CHBE reached 99.5 and 99%, respectively, within 6 h.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pure chiral molecules are needed in the pharmaceutical and chemical industry as intermediates for the production of drugs or fine chemicals. (R)- or (S)-CHBE, which could be converted to l-carnitine or HMG-CoA reductase inhibitor and 1,4-dihydropyridine type β-blocker, respectively, is one of the important chiral building blocks [1]. Nevertheless, (R)-is in greater demand yet less readily available [2, 3].
Several synthetic procedures have recently been developed to obtain (R)-CHBE. For example, asymmetric reduction of COBE by a microbial aldehyde reductase in an organic solvent-water diphasic system [1, 4], or by Escherichia coli strains, which coexpress both the aldehyde reductase gene and the glucose dehydrogenase gene [5, 6]. Although all of these procedures are successful in raising the yield and ee of (R)-CHBE , typically also result in higher costs in much effort directed towards screening different microorganisms. The baker's yeast, Saccharomyces cerevisiae, is also generally used to reduce dicarbonyl compounds (in particular α- and β-diketones and keto esters) to chiral alcohols with high ee. However, products are formed at a low rate [7–9]. Here is an example of the use of baker’s yeast in stereoselective reduction in which the introduction of allyl bromide to baker’s yeast and interact with the enzymatic system of yeast shifts the stereoselectivity of the reduction of COBE toward the (R)-CHBE in 97% ee, but only had 3 mmol l−1 of COBE was transformed with a conversion of 98% to the corresponding (R)-CHBE [3]. The technique is apparently not suitable for the large-scale production required by industry.
CTAB has been used for permeabilization of baker’s yeast for enhanced activity of Saccharomyces cerevisiae ADH, hexokinase (HK) and G6PDH. the permeabilized baker’s yeast cells could be used for measuring total intracellular enzyme activity and an alternative biocatalyst for analytical and preparative purposes [10, 11].
Herein we report the results of microbiol reduction of COBE to the corresponding (R)-alcohol using permeabilization of fresh brewer’s yeast cells by CTAB in the presence of allyl bromide and using the ADH/2-propanol and G6PDH/glucose-6-phosphate system for NAD (P) H-regeneration (Fig. 1).
Materials and methods
Chemicals
NAD+, NADP+, ATP and ethyl 4-chloro-3-hydroxybutanoate [(R)- and (S)-enantiomers] were obtained from sigma-Aldrich. All other chemicals used were of reagent grade, except where noted.
Microorganism and permeabilization
Fresh brewer’s yeast whole cells was obtained from Chong Qing Beer Group Ltd., Chongqing, China. The yeast cells were harvested by centrifugation at 3,000 rpm for 10 min. The yeast pellet was again diluted 2–3 times with saline at 0–2°C, screened on a sieve shaker equipped with a 100 mesh screen and centrifuged at 5,000 rpm for 30 min, and then cells were examined after mixing equal amounts of yeast sample and a 0.01% methylene blue solution on a microscope slide and the number of living and dead cells was counted in a haemocytometer three times per sample. Dead cells appeared as stained when examined under a microscope while living ones were unstained. The percentage of living cells is represented as the average of these three determinations [12]. When the total number of living cells reached ≥ 95%, the cells were permeabilized with 0.2% w/v CTAB [11]. After centrifugation and wash, the permeabilized brewer’s yeast cells were used as biocatalyst in glucose phosphorylation and bioconversion of COBE.
Glucose phosphorylation by permeabilized brewer’s yeast cells
Glucose-6-phosphate used as one of co-substrates was prepared using glucose phosphorylation by hexokinase-catalyzed of permeabilized brewer’s yeast and using slight modifycations of the procedure described previously [13, 14]. Essentially, permeabilized brewer’s yeast (500 g) was suspended in a 1 l solution containing 0.2 M sodium phosphate buffer pH 6.6, 35 mM MgCl2, 0.50 M glucose and 0.35 M sodium dihydrogenphosphate, and then poured into a flask was kept in an rotary shaker equipped with a constant temperature water bath. The reaction was performed at 30°C for 2 h with continuous shaking at 125 rpm. The reaction mixture was centrifuged at 5,000 rpm for 30 min to remove cells and insoluble substrate, and the supernatant (640 ml, containing 0.31 M of glucose-6-phosphate) was used as one of co-substrates without further purification.
Optimum temperature and pH
To determine the optimum pH, the permeabilized brewer’s yeast cells were suspended uniformly in a set of different concentrations of phosphate buffer of pH ranging from 5.0 to 10.0 and incubation at 30°C for 30 min. The optimum temperature for maximum enzyme activity was determined by varying incubation temperature of the suspension from 20–45°C.
Bioconversion
After permeabilized brewer’s yeast cells suspended in 150 ml supernatant of glucose-6-phosphate prepared above containing 4 g allyl bromide in a flask was preincubated at 30°C for 1 h with continuous shaking at 80 rpm, pH was adjusted to 7.0 with 2.0 M NaoH, and then 15 mM MgCl2, 0.40 M glucose, 0.2 M sodium phosphate, 50 ml 2-propanol, 0.1 mM NAD+ and 0.1 mM NADP+, respectively, was added to the suspension and dilute to a final volume of 748 ml with distilled water. The reaction was started by 252 ml COBE/n-butyl acetate (COBE : n-butyl acetate was = 1:3 v/v) was continuously fed into the stirred suspension at a rate of 63 ml/h for 4 h. Flask were incubated at 30°C for 6 h with continuous shaking at 125 rpm, the concentration of COBE, (R)-CHBE and (S)-CHBE were measured by GC, as described in “Analysis of reactant and products”.
Enzyme assay
For measuring enzyme activity, permeabilized and nonpermeabilized yeast cells were respectively suspended in 0.1 M sodium phosphate buffer at a cell concentration of 0.2 g/ml, the suspension was used for the assay of the ADH, G6PDH and HK according to the published method [10]. In brief, the assay of HK is based upon the reduction of NADP+ through a coupled reaction with G6PDH and was determined spectrophotometrically by measuring the increase in absorbancy at 340 nm. The activity of G6PDH was determined by measuring the increase in absorbancy resulting from the reduction of NADP+. The activity of ADH was determined by measuring the increase in the rate of absorbancy at 340 nm resulting from the reduction of NAD+. For the three enzymes, one unit of enzyme activity (U) reduces 1 μmol of NAD(P)+ per min at 25°C and pH 7.0.
Analysis of reactant and products
After the reaction mixture was centrifuged at 5,000 rpm for 30 min , the pellet was washed with water (300 ml) and ethyl acetate (600 ml), the combine supernatant and filtrate were extracted subsequently with two portions of ethyl acetate (600 ml × 2).The combined organic layer was washed with saturated sodium bicarbonate (300 ml) and subsequently with saturated brine (300 ml × 2), dried over anhydrous magnesium sulfate and filtered. The concentrations of COBE, (R)-CHBE and (S)-CHBE were determined on a Varian 3800 GC equipped with FID detector (Varian, Darmstadt, Germany). Chiraldex-GTA capillary column was used : 20 m, 0.25-mm i.d; Advanced Separation Technologies Inc. (Astec), Whippany, New Jersey, United States. Acetophenone was used as an internal standard.
Results and discussion
The activities of the intracellular enzymes ADH, HK and G6PDH in permeabilized and nonpermeabilized brewer’s yeast cell
In bioconversion of COBE (450 mM) mediated by CTAB permeabilized brewer’s yeast cells, the intracellular enzymes ADH, HK and G6PDH were used. ADH used for catalytic reduction of COBE to (R)-CHBE in the presence of allyl bromide, HK used for phosphorylation of glucose to glucose-6-phosphate and ADH and G6PDH used for regeneration of the cofactor NADH and NADPH using both co-substrates (2-propanol and glucose-6- phosphate). The permeabilization treatments with 0.2% CTAB significantly enhanced the activities of brewer’s yeast cells. Especially, the activities of ADH and G6PDH were significantly increased 525 and 7.9-fold, respectively, compared with those in the control (nonpermeabilized) cells (Table 1).
In nonpermeabilized cells, enzymes ADH, G6PDH and HX are either poorly expressed or not measurable. This is mainly due to the permeability barrier of the cell membrane to substrate and/or products. In permeabilized cells, the increases in HK activity may enhance the rate and concentration of glucose phosphorylation into glucose-6-phosphate, thus forming a particulate co-substrate at the starting stage of bioreductions. The increases in ADH and G6PDH activity may enhance the bioconversion rate of COBE and the regeneration of the cofactor.
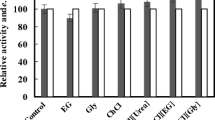
Effect of temperature and pH on enzyme activity
Permeabilized cells retained high activities at various temperatures and pH (Fig. 2). To determine the optimum temperature, the enzyme activity was measured at different temperatures ranging from 20 to 45°C, in phosphate buffer. The optimum temperature recorded was at 20–40°C for ADH activity. The enzyme activity gradually declined at temperatures beyond 40°C. A similar type of result was observed where a maximum temperature of 30–35°C was recorded for a G6PDH (Fig. 2a). When the cells was incubated in a set of different concentrations of phosphate buffers of pH ranging from 5.0 to 10.0 at 30°C, the maximal enzyme activities occurred at pH 7.0–9.0 and pH 6.0–9.0 for ADH and G6PDH, respectively (Fig. 2b).
Based on above results and the reduced nicotinamide cofactor, COBE, and (R)-CHBE are readily decomposed under acidic, alkaline or higher temperature conditions, the optimum temperature and pH for the bioconversion of COBE were identified at 30°C and pH 7.0, respectively
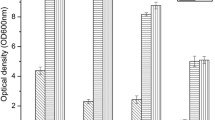
Effect of the permeabilized and nonpermeabilized brewer’s yeast cell concentration on bioconversion
Water-n-butyl acetate has been demonstrated to be the most suitable two-phase system for production of (R)-CHBE or (S)-CHBE [1, 15]. Thus, the bioconversion of COBE was carried out in aqueous-n-butyl acetate two-phase reaction system containing glucose-6- phosphate, MgCl2, glucose, NAD+, NADP+, 2-propanol, allyl bromide and fresh brewer’s yeast as the biocatalyst. As shown in Fig. 3, reactions with varied amounts of permeabilized brewer’s yeast cells were carried out to identify the dosage of yeast cells needed for a high ee and high yield of (R)-CHBE. The ee and yield of (R)- CHBE rapidly reached 92.5% and 85%, respectively, when the dosage of permeabilized brewer’s yeast was increased to 80 g/l. After the apparent plateau of both near 94–95% and 91–92%, respectively, ee and yield attained 99% and 99.5%, respectively, within 6 h, with further increase in the dosage of CTAB permeabilized brewer’s yeast cells to 110 g/l. Further increase of CTAB permeabilized brewer’s yeast to 130 g/l did not continuously improve ee and yield, Surprisingly, however, increasing the dosage of CTAB permeabilized brewer’s yeast up to 140 g/l progressively decreased ee and yield of (R)-CHBE. Therefore, the optimum dosage of CTAB permeabilized Brewer’s yeast cells was 110–130 g/l to obtain (R)-CHBE at high ee and high yield.
Effect of the concentration of CTAB permeabilized and nonpermeabilized brewer’s yeast cells on the bioconversion of CHBE . Symbols of closed circle and square represent respectively yield and ee of permeabilized brewer’s yeast cell group, symbols of up triangle and down triangle represent respectively ee and yield of nonpermeabilized brewer’s yeast cell group
This procedure allowed reconstitution of the bioconversion of COBE using nonpermeabilized brewer’s yeast cell as biocatalyst had only a yield of 34.4% and an optical purity of 96% ee after 6 h incubation when the dosage of the yeast cells was 110 g/l (Fig. 3). Further increase of nonpermeabilized cells to 130 g/l did not also significantly improve ee and yield.
To investigate the effect of permeabilization on (R)-CHBE production, time-course reactions of both permeabilized and nonpermeabilized yeast cell were investigated under optimal conditions (Fig. 4a, b). The permeabilized cells produced 447.2 mM l−1 (R)-CHBE with a productivity of 74.5 mM l−1 h−1 and 2.06 mM l−1 (S)-CHBE while nonpermeablized cells produced 155 mM l−1 (R)-CHBE with a productivity of 25.8 mM l−1 h−1 and 3.27 mM l−1 (S)-CHBE, and after 6 h incubation, COBE was not measurable in permeabilized cells group while a large quantities of COBE was observed in nonpermeabilized cell group. It is obvious that these differences were probably caused by the difference between the two groups of yeast cells in the enzyme ADH and G6PDH activity. The lower activity of the enzyme ADH and G6PDH in the nonpermeabilized yeast cell not only caused COBE accumulation but also led to a lower ee and lower yield.
Reaction time course of the bioconversion of COBE by brewer’s yeast cells in the presence of allyl bromide, pH = 7.0, 30 C, permeabilized or nonpermeabilized brewer’s yeast cells: 110 g/l, C0BE was fed for 4 h, shake speed 125 r/min. Symbols of square, up triangle and diamond represent respectively, C R-CHBE, CCOBE and C S-CHBE of permeabilized brewer’s yeast cell group, symbols of down triangle, closed circle and cross, represent respectively C R-CHBE, CCOBE and C S-CHB E of nonpermeabilized brewer’s yeast cell group
In conclusion, CTAB permeabilized brewer’s yeast cells in the presence of allyl bromide and the ADH/2-propanol and G6PDH/glucose-6-phosphate system for NAD(P)H-regeneration enable us to use a new method of obtaining high yield and high ee for the bioconversion of the β-ketoester to its corresponding (R)-alcohol in an n-butyl acetate-water two-phase system. The method is useful because the activities of ADH and G6PDH in the permeabilized cells are much greater than that in the nonpermeabilized cells and the stereoselectivity is high enough. Another attractive feature of this method is that it is readily available and very easy to handle.
References
Shimizu S, Kataoka M, Kita K (1998) Chiral alcohol synthesis with microbial carbonyl reductases in a water-organic solvent two-phase system. Ann NYAcad Sci 864:87–95
Wang G, Hollingsworth RI (1999) Synthetic routes to l-carnitine and l-gamma-amino-beta-hydroxybutyric acid from (S)-3-hydroxybutyrolactone by functional group priority switching. Tetrahedron. Asymmetry 10:1895–1901
Forni A, Caselli E, Prati F, Bucciarelli M, Torre G (2002) Highly enantioselective reduction of ethyl 4-chloro-3-oxobutanoate to l- and D-3-hydroxyesters with baker’s yeast. Arkivoc (xi):45–53
Shimizu S, Kataoka M, Katoh M, Morikawa T, Miyoshi T, Yamada H(1990) Stereoselective Reduction of ethyl 4-chloro-3-oxobutanoate by a microbial aldehyde reductase in an organic solvent-water diphasic system. Appl Environ Microbiol 56(8):2374–2377
Kataoka M, Yamamoto K, Kawabata H, Wada M, Kita K,Yanase H,Shimizu S (1999) Stereoselective reduction of ethyl 4-chloro-3-oxobutanoate by Escherichia coli transformant cells coexpressing the aldehyde reductase and glucose dehydrogenase genes. Appl Microbiol Biotechnol 51(4):486–490
Liu Y, Xu ZN, Jing KJ, Jiang XX, Lin JP, Wang F, Cen PL (2005) Asymmetric reduction of ethyl 4-chloro-3-oxobutanoate to ethyl (R)-4-chloro-3-hydroxybutanoate with two co-existing, recombinant Escherichia coli strains. Biotechnol Lett 27(2):119–125
Bertau M, Burli M (2000) Enantioselective microbial reduction with baker’s yeast on an industrial scale. Chimia 59:503–507
Chin-Joe I, Straathof AJJ, Pronk JK, Jongejan JA, Heijnen JJ (2001) Influence of the ethanol and glucose supply rate on the rate and enantioselectivity of 3-oxo ester reduction by baker’s yeast. Biotechnol Bioeng 75:29–38
Novak J, Zarevucka M, Wimmer Z, Tykva R (2001) A comparison of the enantioselective reduction potential of strains of Saccharomyces cerevisiae towards a selected ketone. Biotechnol Lett 23:1517–1522
Gowda LR, Joshi MS, Bhat SG (1988) In situ assay of intracellular enzyme of yeast (Kluyveromyces tragilis) by digitonin permeabilization of cell membrane. Anal Biochem 175:531–536
Gowda LR, Bachhawat N, Bhat SG (1991) Permeabilization of bakers’ yeast by cetyltrimethylammonium bromide for intracellular enzyme catalysis. Enzyme Microb Technol 13:154–157
Smart KA, Chambers KM, Lambert I, Jenkins C (1999) Use of methylene violet staining procedures to determine yeast viability and vitality. J Am Soc Brew Chem 57(1):18–23
Leisola M, Linko M (1991) Preparation and purification of fructose-1,6-diphosphate. Acta Chem Scand B28 (5):555–558
Colowick SP, Kaplan NO (1982) Glucose-6-phosphate: hexokinase-catalyzed phosphorylation of glucose. In: Wood WA (ed) Methods in enzymology. vol. 89, carbohydrate metabolism (Part D). Academic, New York, pp 112–113
Engelking H, Pfaller R, Wich G, Weuster-Botza D (2004) Stereoselective reduction of ethyl 4-chloro acetoacetate with recombinant Pichia pastoris. Tetrahedron Asymmetry 15:3591–3593
Acknowledgments
This work was supported by the special fund of three items of expenditure on science and technology department of Central District in Chongqing. We also thank the Chongqing University of Medical Sciences for partial financial support of this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yu, MA., Wei, YM., Zhao, L. et al. Bioconversion of ethyl 4-chloro-3-oxobutanoate by permeabilized fresh brewer’s yeast cells in the presence of allyl bromide. J Ind Microbiol Biotechnol 34, 151–156 (2007). https://doi.org/10.1007/s10295-006-0179-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-006-0179-z