Abstract
Silver products have been used for thousands of years for their beneficial effects, often for hygiene and in more recent years as antimicrobials on wounds from burns, trauma, and diabetic ulcers. Silver sulfadiazine creams (Silvazine and Flamazine) are topical ointments that are marketed globally. In recent years, a range of wound dressings with slow-release Ag compounds have been introduced, including Acticoat, Actisorb Silver, Silverlon, and others. While these are generally accepted as useful for control of bacterial infections (and also against fungi and viruses), key issues remain, including importantly the relative efficacy of different silver products for wound and burn uses and the existence of microbes that are resistant to Ag+. These are beneficial products needing further study, although each has drawbacks. The genes (and proteins) involved in bacterial resistance to Ag have been defined and studied in recent years.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Silver products have been used for their supposed beneficial effects for thousands of years, often for hygiene and more recently as antimicrobials. Silver-containing creams are favored topical ointments for large burns and marketed as Flamazine (10 mg/ml silver sulfadiazine) and Silvazine (10 mg/ml silver sulfadiazine plus 2 mg/ml chlorhexidine digluconate) [22, 24, 45–47]. In recent years, a range of wound dressings have been marketed containing slow-release Ag compounds [33, 44, 47]. These include Silverlon (Argentum Medical), Actisorb Silver (Johnson and Johnson), Acticoat (Smith and Nephew), and others (Table 1).
Among nonclinical hygienic uses of silver, slow-release “nanosilver” linings of washing machines (Fig. 1), dishwashers, refrigerators, and toilet seats are marketed and advertised, along with silver-coated domestic water filters, and Microdyn, an Ag-gelatin aggregate available in supermarkets to kill bacteria and viruses in salad ingredients (e.g. lettuce and tomatoes) and also used to paint the inside of village water filters in Central America. Silver-treated camping and exercise clothing have also been produced. In Japan, Ag-containing products include Hitachi dishwashing machines, Sharp washing machines, Toto toilet seats, home washing machine detergent, Shiseido underarm body deodorants (http://www.shiseido.co.jp/ag; an entertaining on-line short film) and Simplicity shoe spray to reduce the smell of wet boots. Samsung Electronics USA is marketing similar washing machines with slow electrical current release of Ag(I) cations as a biocide for warm water treatment of dirty clothes (http://www.hwhpr.com/pr/samsung/silvercare/). It is clear that we are exposed to a wide range of mostly unfamiliar uses of silver-containing products intended to function as antimicrobial biocides.
Silver-containing biocide products for food and water use including a Microdyn, a silver-protein colloid for washing salad vegetables such as lettuce and tomatoes to kill bacteria and viruses (Mexico City supermarket; http://www.silverinstitute.org/news/5b01.html), b Brita water filter containing silver-coated ion-exchange and activated carbon columns for domestic water purification (Chicago supermarket; http://www.brita.com.au/help/frequently_asked_questions) and c Nanosilver coating of Daewoo Electronics washing machine (sign above a Santiago de Chile subway; www.daewooelectronics.cl)
History of biocidal uses of silver products in clinical applications
For perhaps as long as 7000 years, the properties of silver in preventing diseases have been recognized, generally without knowing the basis (http://www.silvermedicine.org/history.html). For example, Alexander the Great (356–323 b.c.) was said to drink only from silver vessels, and this is attributed (more recently) to the antimicrobial activities of the released Ag+ cations [8]. The Romans used silver nitrate therapeutically and silver was entered in the official Roman book of medicines (http://www.silentsoundcentre.com/id35.html). The alchemical writings of Paracelsus (1493–1541 a.d.) speak of the virtues of silver as a healing substance (http://www.auroville.com/vijnana/silver/history.htm, http://www.silverlon.com/history.html) [34].
The antibacterial qualities of silver were recognized as soon as bacteria were identified as disease-producing agents and silver was used in infectious disease medicine [34]. Silver nitrate was introduced for treatment of skin ulcers, bone fractures, and suppurating wounds. K. S. F. Crede, a German obstetrician, introduced in 1884 the placing of AgNO3 solution in the eyes of newborn children to prevent gonorrheal infection [8, 34].
C. von Nägeli, also toward the end of the nineteenth century, recognized that silver functioned as an antibacterial agent at very low levels of Ag+ and coined the word “oligodynamic” (an unfortunate term) to mean that a small amount of silver is released [8] from the metallic surface when placed in contact with liquids. This term has been used widely in the published literature and medical brochures (http://www.discovervancouver.com/forum/topic.asp?TOPIC_ID=20888). The effect of low voltage direct current (DC) electricity in accelerating Ag+ release was recognized (e.g. [9, 11, 12, 35]), but definitive studies on the benefits of electrical stimulation are lacking. It is also difficult, sometimes, to generate DC potential at the bandage site.
At the beginning of the twentieth century, Albert C. Barnes in Philadelphia invented Argyrol (a silver protein solution; argyros is Greek for silver; http://www.neh.gov/news/humanities/2004-09/barnes.html) as a local antiseptic, especially to prevent eye infections. This product was the basis for Barnes’ personal fortune that he used to amass a notable French impressionist art collection and museum that is equally known for continuing litigation since his death, half-a-century ago. Barnes recognized that silver nitrate eye-drops often were caustic to human tissues and that a more benign and effective silver product could be produced by absorption of Ag+ on the surface of colloidal proteins such as gelatin. Some US states had laws requiring the specific product Argyrol (rather than AgNO3) for treatment of eyes of newborns.
Also early in the twentieth century, the surgeon William S. Halstead introduced the use of silver foil wound dressings (http://www.auroville.com/vijnana/silver/history.html), a use that continued until just after World War II, when antibiotics largely replaced silver. Nevertheless, use of silver foil dressings for wounds was listed in the Physician’s Desk Reference until 1955.
The most familiar human exposure to Ag is from dental amalgams that contain 35% Ag(0) and 50% Hg(0) [18]. The slow release of Hg from amalgams is known to select for Hg-resistant bacteria in the gut [59]. Ag is also released [42] and Ag-resistant oral bacteria were recently reported [14]. Furthermore, it appears that Ag release from film processing, mining sites, and dental amalgams are large sources of Ag in human wastewaters where selection for Ag resistance might also occur (e.g. [5, 29]). As stated above, human exposure to Ag compounds generally has no serious adverse health effect [12, 50], although prolonged high-level use of silver preparations (often as “health-supplements”) can rarely result in problems.
A brief statement is appropriate about the misuses of silver compounds in personal hygiene and environmental applications. An Internet search for Argyria (from the Greek argyros for silver) will find many examples (http://www.silvermedicine.org/, http://www.colloidalsilver101.com/colloidalsilverliquid.html). Argyria occurs when subdermal Ag deposits results in an irreversible gray to blue-black coloring of the skin. It is the rare result of ingesting large amounts of silver preparations, usually as health stimulants [50]. Argyria is permanent, but not physically harmful; it is an inherent serious cosmetic problem. Rosemary Jacobs (http://www.homepages.together.net/∼rjstan) is an opponent of silver-containing health products, who developed argyria as a teenager, as the result of long-term use of Ag-containing nasal drops. She has spoken against these products for nearly 50 years. Jacobs states that colloidal silver diet supplements and related products for human health and hygiene generally lack claimed values. That, however, does not affect the usefulness of Ag products on bandages and in wound treatment, and the possible (less clearly shown) benefits of Ag of treating domestic water and food sources.
Current bio-medical uses of silver products
Silver products are used in medicine in an expanding range of products. The most important current use is undoubtedly as a biocide to prevent infections of long-term problem sites including burns, traumatic wounds, and diabetic ulcers. Additional uses include coating of catheters and other devices implanted on or within the body. The hygienic uses including disinfecting water supplies expand the potential range.
Burns continue to be serious clinical problems. However, most deaths of burn patients today result from infections rather than the burns themselves. Modak et al. [45, 46] combined two useful antibacterial agents, silver nitrate and sulfadiazine, to form an extremely useful agent Silvazine. It is thought that Silvazine functions with the slow release of Ag+ as the primary biocide while sulfadiazine serves mostly to keep Ag+ in solution and to prevent the light-sensitive formation of black colloidal Ag0 on the skin surface, again a serious cosmetic problem with AgNO3-based products, since patients object to skin blackening [34]. Silver sulfadiazine burn ointment [35] was licensed to Marion Laboratories (in 1969), with the US patent issued in 1973. The company producing Silvazine became Hoechst Marion Roussel (1995) after Hoechst acquired Marion Merrell Dow (formed by the merger of Marion Laboratories and Merrell Dow Pharmaceuticals) and silver sulfadiazine is now available from on-line catalogs, such as that from Sigma Chemical Co. (http://www.sigmaaldrich.com/catalog/search/ProductDetail/ALDRICH/481181). Of course, Hoechst was responsible for the first metalloid-based antimicrobial drug, the organoarsenic compound Salvarsan, almost 100 years earlier (in 1910). That marked the birth of chemotherapy with the first antimicrobial treatment for syphilis. Paul Ehrlich (1854–1915) found Salvarsan in his search for a “magic bullet” against disease-causing microbes. Silver sulfadiazine quickly became the drug of choice for burns, and is widely used to controlling bacterial infection. It is available commercially as a water-soluble ointment and was widely employed during the Vietnam War and since. However, patient discomfort from laborious application and cleaning (pain and lack of patient acceptance) remain with Silvazine, which as a cream-based product that must be spread on and removed from the burn surface repeatedly [8].
A range of Ag-containing bandages have recently come in to commercial use (Table 1), including Silverlon (Argentum Medical; with which the third author is associated), Actisorb Silver (Johnson and Johnson), and Acticoat (Smith and Nephew) as primary alternatives at this time. These products and the primary conclusions concerning them in 2006 are briefly:
-
(a)
These products are useful for major medical problems including wide-body burns, sepsis in traumatic wounds, and chronic diabetic ulcers (e.g. http://www.fda.gov/cdrh/pdf4/k041316.pdf). Figure 2 shows a Silverlon bandage opened from its sterile foil wrapping and a roll of the same product being applied to a leg skin graft.
-
(b)
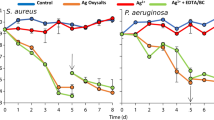
With the goal for products useful for human wounds in vivo in clinical settings, both in vivo testing on experimental animal wounds, e.g. [10, 36], and in vitro laboratory tests (such as disk zone inhibition assays; Fig. 3), and liquid bacterial killing curves [23, 60] are frequently used as substitutes and continue to be needed. At this time, most studies reported (e.g. [32, 48, 49, 58, 60]) have been preliminary and mostly not adequately controlled. Although it has long been recognized that chloride anions and serum proteins effectively remove free Ag+ from the wound environment [35] and therefore in vitro measurements cannot substitute for direct in vivo animal and human studies. Nevertheless, in vitro studies are useful as they are more rapid, cheaper and more carefully controlled. Further listing of the complexities and limitations of available published studies (and frequently unpublished studies available on corporate Internet sites) will not resolve these questions. What is needed today are new measurements comparing in vitro [60] and in vivo [36] tests based on better understanding of the complexities and realistic comparisons of available commercial products—all of which we anticipate will be beneficial in clinical settings.
-
(c)
As a result, conclusions as to one product being more effective than another during in vitro tests [49, 58, 60] have little bearing on the efficacy of these products in human medicine.
-
(d)
The time of application (from minutes and hours for in vitro tests to days, weeks, and months for in vivo applications) and the complex relationship between slow Ag release from a bandage to its distant attack on infecting microbes remain problems differing between in vitro and in vivo tests and which need attention.
While it is accepted that silver in the bandage either as nanocrystals of Ag(0) or as bound cationic Ag(I) must be released as Ag+ in order to leave the bandage surface, the complexities of Ag+ movement in a serum plasma environment with approximately 60 mg/ml serum proteins and 0.16 M NaCl have not been studied. Ag+ is precipitated out from solution by moderate Cl− anions and at higher Cl− concentrations solubilized as complex anions such as [AgCl2]−, which affects the sensitivity of bacterial cells to Ag+ and the relative difference between susceptability of sensitive and resistant bacteria [26]. Proteins are known to bind Ag, which is used as a “silver stain” for protein gel electrophoresis and to quantitate overall protein concentrations (e.g. http://www.sigmaaldrich.com/sigma/general%20information/vol4%20issue1%20proteosilver.pdf) and proteins such as gelatin are used to maintain silver in a biocidal available form. Not surprisingly, added blood serum affects the in vitro measurements of Ag+ sensitivity (L. T. Phung, in preparation).
Microbial growth inhibition zones on Petri dishes containing agar with a LB (Luria Bertani broth without NaCl; see [26]), b LB + 10% v/v Fetal Bovine Serum (FBS), c Mueller Hinton medium, or d M9 phosphate-based minimal medium with glucose, around 6.5 mm diameter paper disks with added 0 (left), 0.5 (top and bottom) or 1 (right) μmol AgNO3 or a 6.5 mm Silverlon bandage disk. Plates spread with 2 × 106 log phase cells of E. coli sensitive strain J53 (top half-plates) or resistant strain J53(pMG101) [27,43] (bottom half-plates)
Starting in the early 1970s, Becker and coworkers [4, 57] in Syracuse, New York began the use of silver-coated fabrics for the treatment of complex bone infections. A. B. Flick and R. O. Becker developed broader clinical applications for silver nylon fabrics, which evolved into the current Silverlon product line (http://www.silverlon.com/). Chu et al. [9–11] studied the wound healing properties of silver-plated fabrics and in addition the benefit of direct DC current. O. M. Alvarez (http://www.juzousa.com/juzosilverstudies.html) also studied the effect of electrically activated silver-coated fabrics, on partial thickness skin wounds of the pig as an animal model, and Deitch et al. [15, 16, 35] studied the efficacy of these products on chronic human bone infections.
While the products listed in Table 1 are promising for control of bacterial, viral, and fungal infections, many key issues are unresolved. These include the differing efficacy of the various available silver products for different uses and the problems arising from Ag+-resistant microbes. In general, different products all share slow and biocidal release of silver from bandages. However, there is no perfect biocide; and each has its drawbacks (reviewed by [25, 52–54]).
Silver-treated catheters (e.g. [13]) are used to prevent bacterial colonization and associated infections. These include Algid Ag IV Patch, a silver alginate-containing disk with a slit in the middle to accommodate catheters (http://www.deroyal.com/Literature_Live%5Cderoyal%5Cnews%5Cmaintenance%5CPDF_files%5C354_algidex_patch_literature.pdf). Without biocides, catheters develop slime-containing biofilms that enhance further bacterial infection. There are no available clinical studies for the Algid product for the application reducing intravascular related sepsis. Silver-coated urinary tract catheters reduce frequencies of urinary track infection [13].
Microbial silver resistance
Many metal cations (Cd2+, Hg2+, Pb2+, and Tl+ are examples in addition to Ag+) are toxic and nonessential; and bacterial cells have genetically-determined resistance systems to each [55, 56]. Silver ions are highly toxic to all microorganisms, probably due to poisoning of membrane respiratory electron transport chains and components of DNA replication [6, 17, 19, 31, 37, 45, 50]. Bacterial Ag+ resistance has been reported repeatedly [7, 14, 30, 39, 43] but the genetic basis was not understood until recently [27, 28, 52–54]. Bacterial silver resistance, like that to other toxic metal ions, is frequently encoded by genes located on plasmids [14, 28], but also sometimes found encoded on the chromosome (reviewed by [55, 56]). For example, the determinant studied in most detail was originally found on Salmonella plasmid pMG101 [43] and encodes resistances to Ag+, Hg2+, and tellurite, as well as to several antibiotics [27, 43]. Salmonella and Escherichia coli have in addition a related chromosomal Ag+ resistance determinant [20, 28, 52]. Metal ion resistances (such as that to Ag+) are frequently selected without awareness when antibiotics and metal salts are used as antiseptics. For example, in a random collection of enteric bacteria from a Chicago hospital, more than 10% had genes for Ag+ resistance [52].
Silver-resistant bacteria have been reported from other sources where silver exposure might be expected to select for resistance [1, 7, 14, 30]. Ag+-resistant E. coli mutants were step by step selected in the laboratory [39] and shown to have active Ag+ efflux, presumably due to a chromosomally encoded system, perhaps the CusCBFA system [20, 28], which had not been identified at that time. In addition, the E. coli mutant silver-resistant strains were deficient in outer membrane porin proteins [39].
The silver resistance determinant from plasmid pMG101 contains nine genes [27, 52] and the functions for eight named genes and their corresponding protein products (Fig. 4) have been assigned primarily on the basis of homologies to known proteins for other metal resistances. The SilE protein is a small periplasmic metal-binding protein (Fig. 4) [27, 52], homologous to the PcoE protein of E. coli copper resistance [38, 52]. SilCBA constitute a three-polypeptide membrane potential-driven cation/proton exchange complex (Fig. 4) that is a member of the resistance, nodulation, and cell division (RND) superfamily of cation efflux pumps [52]. SilA is a large (over 1,000 amino acids in length) inner membrane cation pump protein; SilB is a periplasmic “membrane fusion protein” that contacts both the SilA inner membrane protein and the SilC outer membrane protein (Fig. 4). Between the silC and silB genes, a small gene was initially not assigned a function as it lacked homologs [27, 52] but its protein product is now called SilF (Fig. 4), because it is about 50% identical in sequence to the chromosomal gene product CusF, which is also involved in Ag+ resistance [21]. CusF is a periplasmic Cu+/Ag+-binding protein that probably functions as a chaperone to carry Cu+ or Ag+ to the equivalent CusCBA Ag+/Cu+ efflux pump (Figs. 4, 5; [21, 41]).
Protein products of bacterial plasmid silver resistance genes (modified from [52])
The product of the last gene of the silver resistance determinant, SilP, is predicted to be a P-type ATPase, a member of another large family of homologous heavy-metal cation resistance efflux ATPases [55, 56]. Structural data are available on the related Ca2+ P-type efflux ATPase (reviewed recently by [56]). These ATPases contain a membrane component (Fig. 4) and three intracellular domains referred to as the activator (A), nucleotide binding (N) and phosphorylation (with aspartate, D) domains. There are several important canonical features in the SilP P-type ATPase sequence, starting from the N-terminus proximal poly-histidine (H5DH2) presumed Ag+-binding domain in the cytoplasm that is considered equivalent to but different from the Cu2+-binding motif which includes a cysteine-X2-cysteine sequence found in related ATPases from bacterial to human sources (e.g. [55]), including the closely related E. coli CopA copper P-type ATPase. The SilP and CopA sequences are unrelated for the N-terminal cation recognition domains of about 275 amino acids and then closely similar for the remaining regions, including eight predicted trans-membrane alpha-helical regions (Fig. 4; [2, 55]) with a CPC tripeptide that is considered part of the transport pathway in the fourth of these trans-membrane sequences. This proline is conserved in all P-type ATPases regardless of substrate and the CPC is characteristic of a class of presumed Cu+ and Ag+ trans-locating ATPases [2].
The silver resistance determinant is unique among resistance systems in encoding two energetically different efflux pumps. The SilF periplasmic chaperone protein that is thought to carry Ag+ from its periplasmic site of release by SilP to the periplasmic uptake site of SilA, as part of the SilCBA complex (Fig. 4). With two periplasmic Ag+-binding proteins ascribed different functions in Fig. 4, one can ask about their relationships. SilE and SilF are basically unrelated, with SilE thought to form a largely alpha-helical secondary structure (Fig. 5a) with five Ag+ cations bound by ten histidine imidazole N atoms coordinating the cation binding [52] while the SilF homolog CusF forms a basically beta sheet structure with the cation bound by a single histidine (His58) N atom and two methionine (Met69 and Met71) S atoms (Fig. 5b) [3, 41]. The structures in Fig. 5 are preliminary as the structure of SilE is derived from circular dichroism and imidazole proton NMR analysis [52] and the structure for SilF/CusF available only for the CusF variant without bound cation [41]. Nevertheless, these are unrelated periplasmic polypeptides with dissimilar monovalent cation-amino acid coordination. Sequence relationship “trees” for SilE and SilF proteins show that both families of polypeptides have tight clusters for known sequences of SilE and SilF (greater than 90% amino acid identities) plus secondary clusters of homologous protein sequences presumably for binding of other cations (PcoE for SilE and CusF for SilF are involved in copper resistance and approximately 50% identical in amino acid primary sequences). It seems likely that all four proteins, SilE, PcoE, SilF, and CusF, bind both monovalent cations Ag+ and Cu+, but not divalent cations such as Cu2+. Careful experimental measurements of cation preference and the sequence basis for binding are needed.
The sil genes known to date occur only on IncH incompatibility group plasmids, which are large, multiple antibiotic resistance plasmids [28]. The plasmids with sil genes were originally isolated based on antibiotic resistances and from enteric bacteria from varying geographic locations. These initial findings suggest that Ag+ resistance might exist widely but is not known in the absence of a ready means of testing. A wide distribution of sil-homologous determinants localized on plasmids and on the bacterial chromosome might pose a threat toward effective use of silver compounds as biocides, analogous to the development of antibiotic-resistant bacteria when antibiotic usage increases indiscriminately [40, 51].
References
Annear DI, Mee BJ, Bailey M (1976) Instability and linkage of silver resistance, lactose fermentation and colony structure in Enterobacter cloacae. J Clin Path 29:441–443
Arguello JM (2003) Identification of ion-selectivity determinants in heavy-metal transport P1B-type ATPases. J Membr Biol 195:93–108
Astashkin AV, Raitsimring AM, Walker FA, Rensing C, McEvoy MM (2005) Characterization of the copper(II) binding site in the pink copper binding protein CusF by electron paramagnetic resonance spectroscopy. J Biol Inorg Chem 10:221–230
Becker RO (1999) Silver ions in the treatment of local infections. Metal-Based Drugs 6:311–314
Belly RT, Kydd GC (1982) Silver resistance in microorganisms. Dev Indust Microbiol 23:567–577
Bragg PD, Rainne DG (1974) The effect of silver ions on the respiratory chain of Escherichia coli. Can J Microbiol 20:883–889
Bridges K, Kidson A, Lowbury EJL, Wilkins MD (1979) Gentamicin- and silver-resistant Pseudomonas. Br Med J 1:446–449
Burrell RE (2003) A scientific perspective on the use of topical silver preparations. Ostomy Wound Manage 49(5A Suppl.):19–24. www.owm.com
Chu CS, Matylevitch NP, McManus AT, Goodwin CW, Pruitt BA Jr (2000) Accelerated healing with a mesh autograft/allodermal composite skin graft treated with silver nylon dressings with and without direct current in rats. J Trauma 49:115–125
Chu CS, McManus AT, Mason AD, Pruitt BA Jr (2005) Topical silver treatment after escharectomy of infected full thickness burn wounds in rats. J Trauma 58:1040–1046
Chu CS, McManus AT, Matylevich NP, Mason AD Jr, Pruitt BA Jr (1995) Enhanced survival of autoepidermal-allodermal composite grafts in allosensitized animals by use of silver-nylon dressings and direct current. J Trauma 39:273–278
Clement JL, Jarrett PS (1994) Antibacterial silver. Metal-Based Drugs 1:467–482
Darouiche RO 1999. Anti-infective efficacy of silver-coated medical prostheses. Clin Infect Dis 29:1371–1377
Davis IJ, Richards H, Mullany P (2005) Isolation of silver- and antibiotic-resistant Enterobacter cloacae from teeth. Oral Microbiol Immunol 20:191–194
Deitch EA, Marino AA, Gillespie TE, Albright JA (1983) Silver-nylon: a new antimicrobial agent. Antimicrob Agents Chemother 23:356–359
Deitch EA, Marino AA, Malakanok V, Albright JA (1987) Silver nylon cloth: in vitro and in vivo evaluation of antimicrobial activity. J Trauma 27:301–304
Dibrov P, Dzioba J, Gosink KK, Hase CC (2002) Chemiosmotic mechanism of antimicrobial activity of Ag+ in Vibrio cholerae. Antimicrob Agents Chemother 46:2668–2670
Dunne SM, Gainsford ID, Wilson NH (1997) Current materials and techniques for direct restorations in posterior teeth. Part 1: silver amalgam. Internat Dental J 47:123–136
Feng QL, Wu J, Chen GQ, Cui FZ, Kim TN, Kim JO (2000) A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J Biomed Mater Res 52:662–668
Franke S, Grass G, Nies DH (2001) The product of the ybdE gene of the Escherichia coli chromosome is involved in detoxification of silver ions. Microbiology 147:965–972
Franke S, Grass G, Rensing C, Nies DH (2003) Molecular analysis of the copper-transporting efflux system CusCFBA of Escherichia coli. J Bacteriol 185:3804–3812
Fraser JF, Bodman J, Sturgess R, Faoagali J, Kimble RM (2004) An in vitro study of the anti-microbial efficacy of a 1% silver sulphadiazine and 0.2% chlorhexidine digluconate cream, 1% silver sulphadiazine cream and a silver coated dressing. Burns 30:35–41
Gallant-Behm CL, Yin HQ, Liu S, Heggers JP, Langford RE, Olson ME, Hart DA, Burrell RE (2005) Comparison of in vitro disc diffusion and time kill-kinetic assays for the evaluation of antimicrobial wound dressing efficacy. Wound Repair Regen 13:412–421
George N, Faoagali J, Muller M (1997) Silvazine (silver sulfadiazine and chlorhexidine) activity against 200 clinical isolates. Burns 23:493–495
Gibbs RJ (1999) Silver colloids: do they work? Paperback, published by R. Gibbs, 40 pp, ISBN: 0967699207
Gupta A, Maynes M, Silver S (1998) The effects of halides on plasmid silver resistance in Escherichia coli. Appl Environ Microbiol 64:5042–5045
Gupta A, Matsui K, Lo JF, Silver S (1999) Molecular basis for resistance to silver cations in Salmonella. Nat Med 5:183–188
Gupta A, Phung LT, Taylor DE, Silver S (2001) Silver resistance genes in plasmids of the IncHII incompatibility group and on the Escherichia coli chromosome. Microbiology 147:3393–3402
Haefeli C, Franklin C, Hardy K (1984) Plasmid-determined silver resistance in Pseudomonas stutzeri isolated from a silver mine. J Bacteriol 158:389–392
Hendry AT, Stewart IO (1979) Silver-resistant Enterobacteriaceae from hospital patients. Can J Microbiol 25:915–921
Holt KB, Bard AJ (2005) Interaction of silver (I) ions with the respiratory chain of Escherichia coli: an electrochemical and scanning electrochemical microscopy study of the antimicrobial mechanism of micromolar Ag+. Biochemistry 44:13214–13223
Innes ME, Umraw N, Fish JS, Gomez M, Cartotto RC (2001) The use of silver coated dressings on donor site wounds: a prospective, controlled matched pair study. Burns 27:621–627
Ip M, Lui SL, Poon VKM, Lung I, Burd A (2006) Antimicrobial activities of silver dressings: an in vitro comparison. J Med Microbiol 55: 59–63
Klasen HJ (2000) Historical review of the use of silver in the treatment of burns. I. Early uses. Burns 26:117–130
Klasen HJ (2000) A historical review of the use of silver in the treatment of burns. II. Renewed interest for silver. Burns 26:131–138
Lansdown ABG, Williams A, Chandler S, Benfield S (2005) Silver absorption and antibacterial efficacy of silver dressings. J Wound Care 14:155–160
Lansdown ABG (2002) Silver: 1. its antibacterial properties and mechanism of action. J Wound Care 11:125–130
Lee SM, Grass G, Rensing C, Barrett SR, Yates CJ, Stoyanov JV, Brown NL (2002) The Pco proteins are involved in periplasmic copper handling in Escherichia coli. Biochem Biophys Res Commun 295:616–620
Li X-Z, Nikaido H, Williams KE (1997) Silver-resistant mutants of Escherichia coli display active efflux of Ag+ and are deficient in porins. J Bacteriol 179:6127–6132
Liu HH (1999) Antibiotic resistance in bacteria: a current and future problem. Adv Exp Med Biol 455:387–396
Loftin IR, Franke S, Roberts SA, Weichsel A, Heroux A, Montfort WR, Rensing C, McEvoy MM (2005) A novel copper-binding fold for the periplasmic copper resistance protein CusF. Biochemistry 44:10533–10540
Lygre GB, Hol PJ, Eide R, Isrenn R, Gjerdet NR (1999) Mercury and silver in saliva from subjects with symptoms self-related to amalgam fillings. Clin Oral Investig 3:216–218
McHugh SL, Moellering RC, Hopkins CC, Swartz MN (1975) Salmonella typhimurium resistant to silver nitrate, chloramphenicol, and ampicillin. Lancet 1: 235–240
Melaiye A, Youngs WJ (2005) Silver and its applications as an antimicrobial agent. Expert Opin Ther Pat 15:125–130 http://www.ashley-pub.com
Modak SM, Fox CL Jr (1974) Mechanism of silver sulfadiazine action on burn wound infections. Antimicrob Agents Chemother 5:582–588
Modak SM, Sampath L; Fox CL Jr 1988 Combined topical use of silver sulfadiazine and antibiotics as a possible solution to bacterial resistance in burn wounds. J Burn Care Rehabil 9:359–363
Monafo WW, West MA (1990) Current treatment recommendations for topical burn therapy. Drugs 40:364–373
Pruitt BA Jr, McManus AT, Kim SH, Goodwin CW (1998) Burn wound infections: current status. World J Surg 22:135–145
Richard JW III, Spencer BA, McCoy LF, Carino E, Washington J, Edgar P, Rosenblatt J, Goodheart R, Heggers JP ((2002) Acticoat™ versus Silverlon®: the truth. J Burns 1:11–19
Russell AD, Hugo WB (1994) Antimicrobial activity and action of silver. Progress Med Chem 31:351–370
Salyers AA, Amabile-Cuevas CF (1997) Why are antibiotic resistance genes so resistant to elimination? Antimicrob Agents Chemother 41:2321–2325
Silver S (2003) Bacterial silver resistance: molecular biology and uses and misuses of silver compounds. FEMS Microbiol Rev 27:341–354
Silver S, Gupta A, Matsui K, Lo JF (1999) Resistance to Ag(I) cations in bacteria: environments, genes and proteins. Metal-Based Drugs 6:315–320
Silver S, Lo J-F, Gupta A (1999) Silver cations as an antimicrobial agent: clinical uses and bacterial resistance. APUA Newslett 17:1–3
Silver S, Phung LT (1996) Bacterial heavy metal resistance: new surprises. Annu Rev Microbiol 50:753–789
Silver S, Phung LT (2005) A bacterial view of the periodic table: genes and proteins for toxic inorganic ions. J Ind Microbiol Biotechnol 32:587–605
Spadaro JA, Berger TJ, Barranco SD, Chapin SE, Becker RO (1974) Antibacterial effects of silver electrodes with weak direct current. Antimicrob Agents Chemother 6:637–642
Thomas S, McCubbin P (2003) A comparison of the antimicrobial effects of four silver-containing dressings on three organisms. J Wound Care 12:101–107
Vimy MJ, Hooper DE, King WW, Lorscheider FL (1997) Mercury from maternal “silver” tooth fillings in sheep and human breast milk: a source of neonatal exposure. Biol Trace Elem Res 56:143–152
Yin HQ, Langford R, Burrell RE (1999) Comparative evaluation of the antimicrobial activity of ACTICOAT antimicrobial barrier dressing. J Burn Care Rehabil 20:195–200
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Silver, S., Phung, L.T. & Silver, G. Silver as biocides in burn and wound dressings and bacterial resistance to silver compounds. J IND MICROBIOL BIOTECHNOL 33, 627–634 (2006). https://doi.org/10.1007/s10295-006-0139-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-006-0139-7