Abstract
A mixture of human-derived probiotic strains of Lactobacillus acidophilus, L. agilis and L. rhamnosus was used as a probiotic culture in ice cream manufacture. Viability and survival of these probiotic cultures were investigated in two different ice cream formulations. Ice cream with sucrose and ice cream with aspartame were prepared and each of these was divided into two subgroups: one with direct addition of the probiotic culture and one with milk fermented by the same probiotic culture. Ice cream samples were stored at −20°C for 6 months and the survival rate of cultures were determined monthly. Probiotic cultures underwent tests for resistance to bile salts, antibiotics, acidic conditions; they were found to be highly resistant to such challenges. Chemical analysis of ice cream samples, such as determination of acidity, pH and solid matter, was also performed. The probiotic cultures remained unchanged in ice cream stored for up to 6 months regardless of the sweeteners used. Using probiotic cultures in ice cream mixes did not alter the characteristics of the product.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Certain species of lactobacilli, when included in the diet, can have beneficial effects for humans [25, 26]. In order to provide a beneficial effect on the health of the host, it is necessary that the probiotic strain is able to survive in the gastro-intestinal tract [11]. It is therefore necessary to determine the number of lactobacilli in faeces and, if possible, to identify the different species directly [15]. Recently, a number of novel fermented dairy products have been developed and are being marketed as probiotic products in Germany and in other European countries [23]. Probiotic bacteria are frequently used as the active ingredient in functional foods such as bio-yoghurts, dietary adjuncts and health-related products [4]. Dairy food products incorporated with probiotic bacteria are gaining popularity and are experiencing an increasing market growth [12]. Health aspects attributed to the consumption of fermented dairy products supplemented with probiotic bacteria include improved lactose utilization, anticarcinogenic activity, control of intestinal infections and improved flavour and nutritional quality [19]. The utilization of lactose during fermentation can make the product more easily digested by lactose intolerant people [7].
Ice cream is a valuable food with significant nutritive qualities, and is well assimilated by the body. Ice cream seems therefore suitable for delivering probiotics in the human diet. Some studies [6, 14, 16, 17] have demonstrated that it is possible to produce ice cream-type frozen yogurt using different ratios of fermented mixes. In order to ensure that the product provides an adequate content of microorganisms, cells must survive freezing and refrigerated storage [1].
The aim of this study was to investigate the survival of probiotic bacteria in ice cream during 6 months of storage. The effect of artificial sweetener on these microorganisms was also included in the scope of this study. Ice cream made with non-nutritive sweetener is a useful way to deliver beneficial bacteria to people with diabetes and to people suffering obesity.
Materials and methods
Source of probiotic strains
Lactobacillus strains having probiotic properties used in this study were obtained from a previous study (G. Basyigit, unpublished). Resistance to low pH, bile salts and various antibiotics of the strains were also determined previously (G. Basyigit, unpublished). The strains were isolated from 12 adult males and females hospitalized in the University Research Hospital. A 1 g (wet weight) faeces sample from each was suspended in 9 ml physiological saline solution, homogenized (LG-1064 Tissue Grinder, Glass Pestle, USA) and tenfold dilutions were prepared (from 10−1 to 10−7). One hundred microlitres of each dilution was plated on MRS agar and incubated aerobically at 37°C for 72 h [20, 24, 26].
From MRS agar plates of each sample, colonies with different morphologies were selected and picked. Typical LAB colonies grown on MRS agar were examined microscopically. Then the catalase test was applied. The colonies that did not show catalase activity were selected for further analysis. All nonspore-forming straight rods were finally tested with API-50 CHL kits (Bio Mérieux, France), according to the manufacturer’s instructions.
Acidity, bile salts and antibiotics resistance test
The acidity resistance test was performed in MRS broth at pH 3.5. The initial number of LAB was determined at the beginning of the experiment by plating on MRS agar plates. A 0.5 ml aliquot of bacterial suspension was inoculated into 5 ml sterile MRS broth at pH 3.5 (adjusted using 0.1 N HCl). The tubes were incubated at 37°C. After exposure for 3 h to low pH, the number of viable bacteria was counted on MRS agar (pH 5.6) incubated for 48 h at 37°C [22, 27].
In order to determine bile salts resistance of the strains, a 0.5 ml aliquot of bacterial suspension was inoculated into 5 ml sterile MRS broth supplemented with 0.30% ox bile (Oxoid). The number of viable cells was determined as described above in the Acidity, bile salts and antibiotics resistance test. The tubes were incubated at 37°C. After exposure to ox bile for 3 h, the number of viable bacteria was counted on MRS agar (pH 5.6) plates incubated for 48 h at 37°C [9].
Antibiotic resistance of the isolates was tested against 12 selected antibiotics, including amoxicillin (10 μg), amoxicillin–clavulanic acid (30 μg), bacitracin (10 IU), carbenicillin (100 μg), cephalothin (30 μg), chloramphenicol (30 μg), clindamycin (2 μg), Nystatin (100 IU), ofloxacin (5 μg), polymyxin (300 IU), streptomycin (10 μg) and vancomycin (30 μg). Strains were grown in MRS broth (Oxoid) at 37°C for 24 h. The optical density of the cultures was adjusted to 0.5 Mcfarland using uninoculated MRS broth as blank. The cultures were inoculated (0.1%) into MRS agar kept at 45°C and poured into petri plates to solidify. After solidification of the inoculated agar plates, antibiotic discs (Oxoid) were placed on the surface of the plates. Following a 24-h incubation at 37°C, the diameters of inhibition zones around the discs were measured.
Ice cream manufacture
Two different ice cream recipes were utilized. One of the ice cream mixes included 15% sucrose and other one manufactured with 4% aspartam (Sanpa Brand Aspartame Sweetener Bilim Pharmaceuticals Co., Istanbul, Turkey). Furthermore, both of ice cream mixes contained 6% milk fat, 10% nonfat milk solids, 0.3% stabilizer, 0.3% natural flavour (vanillin).
The cream, milk, condensed skim milk, sucrose and stabilizer were provided by the University Dairy Plant (SDU, Isparta, Turkey) and ice cream manufacture was carried out at the Department of Food Engineering, SDU.
All ingredients were mixed with milk and the mixes were pasteurized at 95°C for 20 min. The mixes were then homogenized, cooled and finally stored at 4°C. After 24 h aging at 4°C, each mix was divided into two parts, one with sucrose and the other with aspartame and each of these were then divided into three additional parts. The first part was used as control group (sample 1) without any culture. In the second part, (sample 2 with sucrose and sample 5 with aspartame) probiotic cultures were directly mixed with the ice cream to obtain a high initial cell count between 106 and 108 cfu/g. Fifty percent fermented milk which contained probiotic cultures was added into the ice cream mix for the third part (sample 3 with sucrose and 6 with aspartame). Fermented milk was made from milk that was homogenized in an Odmez (Ukraine) homogenizer with a 140 kg/cm2 force at 65°C, then pasteurized at 82°C for 30 s in a pasteurizer (GEA-VT 10, Germany). The milk was then cooled down to 37°C and inoculated with probiotic cultures and mixed thoroughly to yield an initial population of 107 cfu/g. The mixture was then incubated at 37°C for 3 h. After incubation the fermented milk was added into the ice cream mix.
The same manufacturing procedure was employed for ice cream with sucrose and aspartame. All ice cream samples were incubated at 37°C for 2 h, and then dispensed into 200 ml sterile cups and stored at −20°C for 6 months. The samples were analysed monthly for cell viability during 180 days.
Ice cream analyses
In order to determine the changes in the chemical composition of ice cream samples during storage, all samples were tested for pH, acidity, nonfat solids, fat content on the first day of manufacture. All analyses were repeated at 30-day intervals. Chemical analysis and bacterial counts were repeated twice for all samples.
The pH of the samples was measured with a pH-meter (InoLab WTW-537, Germany). The average of two measurements was recorded. Titratable acidity of ice cream samples was determined by titration with 0.1 N NaOH, using phenolphthalein as an indicator. Results were expressed as milli equivalent of NaOH per gram of product.
Fat content of ice cream mixtures was determined by Gerber’s method [2]. Nonfat solid material in the ice cream samples was determined with automated equipment (Scaltec SMO-01, Heiligenstadt, Germany).
The preparation of the samples and dilutions for microbiological examinations was performed according to IDF standard 122C:1996 [3]. The number of LAB in the samples was determined by plating appropriate dilutions on MRS agar plates and incubated at 37°C for 48 h. Cell counts of ice cream samples were recorded at the beginning and during 180 days of storage.
Statistical design
The complete experiment was repeated twice. Data were analysed with one-way variance analysis of the values using a general linear model procedure. Ice cream samples were analysed for chemical and microbiological changes during storage. Also, the effects of sucrose and aspartame on the survival of probiotic cultures were compared by Tukey’s new multiple-range test. All statistical analyses were performed using the MINITAB® Version 13, program (MINITAB Inc., USA).
Results and discussion
Isolation and identification of Lactobacillus
The results of the low pH, bile salts and antibiotic resistance tests indicated that five of the isolated strains were suitable for probiotic ice cream manufacture. These five isolates were determined to be nonspore-forming straight rods by microscopic examination. All strains were also tested using API-50 CHL kits (Bio Mérieux, France) for identification at the species level. The strains that showed probiotic properties were presumptively identified as L. acidophilus AB5-18, L. agilis AA17-73, L. agilis AC18-88, L. rhamnosus AB20-100, L. acidophilus AK4-14. An equal mixture of these strains were prepared and used in probiotic ice cream manufacture.
Resistance to low pH, bile salts and antibiotics
Resistance of the strains to acidic conditions was examined at pH 3.5; the results indicated that all of the strains were resistant to low pH (Table 1). Similar results were obtained after the bile salt resistance tests. After 3 h of incubation in MRS broth with pH 3.5 or MRS broth supplemented with bile salts, the number of viable cells remained the same or showed a slight increase (Table 1).
Antibiotic resistance is an important criterion for the selection of cultures to be used as probiotics [18]. The antibiotics used in the experiment are the most frequently applied antibiotics to determine the antibiotic resistance of Lactobacillus strains to be selected as probiotics. According to the results (Table 2), strains were resistant to most antibiotics. The antibiotics most frequently used in the selection of an LAB as a probiotic organism are Nystatin, ofloxacin, polymyxin, streptomycin and vancomycin [5]. Table 2 shows that the strains were resistant to these important antibiotics.
Survival of probiotic cultures
The survival of the probiotic cultures was determined during the storage of the ice cream. As can be seen in Fig. 1, the numbers of bacteria during storage did not decrease in any of the ice cream samples. The type of sugar had no effect on the survival of the probiotic cultures (P > 0.05). However, the manufacturing process had a significant effect (P < 0.05) on the initial number of LAB for two ice cream samples. The initial number of LAB was 6.0 × 108 cfu/ml (sample 3) and 8.1 × 108 cfu/ml (sample 6) for the ice cream samples made by the addition of fermented milk. After 180 days of storage, the number of bacteria decreased to 4.2 × 108 and 3.9 × 108 cfu/ml, respectively. The initial number of LAB in the samples made by the direct addition of pregrown cultures was 5.3 × 107 cfu/ml (sample 2) and 2.5 × 108 cfu/ml (sample 5). LAB counts of these two samples after storage were 3.5 × 107 and 4.9 × 107, respectively. The number of LAB in ice cream samples made by the fermentation method was high in samples containing sucrose and aspartame (samples 3 and 6 in Fig. 1). Although the initial numbers of LAB were high in fermented ice cream samples compared to the samples made by the direct addition of pregrown cultures, the decrease was the same for both types during the 180 days of storage. Various studies have been done on ice cream or frozen yogurt using different sugar and fat concentrations [1, 13, 16, 21]. Most of these studies showed that probiotic LAB can survive in ice cream and frozen yogurt for up to 6 months in frozen conditions (−18 to −28°C). However, it was shown that in regular yogurt stored at refrigeration temperatures (2–5°C), LAB cultures cannot survive that long. In the study done by Nighswonger et al. [21], the viability of L. acidophilus and L. casei was investigated in fermented milk products after storage. In the same study, pregrown cultures were also added directly in the mixture and similar results were obtained.
Viable number of probiotic cells in ice cream samples made with sucrose and aspartame during storage. Circle fermented sample with aspartame SE ± 0.157, square fermented sample with sucrose SE ± 0.181, triangle nonfermented sample with aspartame SE ± 0.352, diamond nonfermented sample with sucrose SE ± 0.186 (SE standard error)
Ice cream analyses
Total solids
The initial amounts of total solids were different among groups depending on the type of sweetener used. Total solids did not change in any of the ice cream samples during storage. Total solids of the samples made with sucrose and aspartame were 38 and 30%, respectively. Although some researchers have stated that solids in frozen yogurt samples assist the survival of LAB [12], solid material had no effect on the survival rate of these bacteria in our study. The survival of probiotic cultures was the same in both the ice cream samples made with sucrose and with aspartame.
Acidity and pH
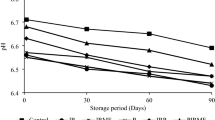
The acidity of the samples was determined by two different methods. NaOH titrations and pH measurements were repeated every 30 days during the 180-day storage. Acidity of the ice cream samples made by fermentation was higher than those made by the direct addition of cultures. This can be explained by conversion of lactose to lactic acid during fermentation. The pH of fermented samples was between 5.0 and 5.5, and 5.7 and 6.6 in the samples manufactured by the direct addition of pregrown cultures without fermentation. Although Nighswonger et al. [21] reported that the initial pH of frozen yogurt samples was 4.5 to 5.0 and was reduced to 4.2 to 4.4 after 28 days of storage at 7°C, the acidity of all ice cream samples in this study remained unchanged during the 6 months, presumably because of the low storage temperature (−20°C). Probiotic cultures are expected to be resistant to high acid conditions in order to pass through the digestion process [8, 10, 18]. Results showed that the probiotic strains used in this study were not affected by high acidity.
Conclusion
The aim of this study was to investigate the survival rate of added human-derived probiotic cultures during storage of ice cream manufactured with sucrose and aspartame. Neither frozen conditions during the storage period nor the type of sweeteners used had any undesired effect on the survival of probiotic cultures.
Ice cream is a dairy product which can be stored for a long time without any change in its chemical composition. Also, in this study, the number of viable probiotic cells in ice cream was unaltered during storage, demonstrating their stability. In some products that are kept at higher temperatures (1–4°C) like yoghurt, viability of cultures can be a problem. Thus ice cream can serve as an excellent environment to deliver probiotics without a loss in their beneficial effects. In this regard, ice cream has an advantage as a probiotic carrier over any other food. It can be successfully used commercially to deliver probiotic bacteria to children and other consumers from different age groups.
References
Alamprese C, Foschino R, Rossi M, Pompei C, Savani L (2002) Survival of Lactobacillus johnsonii and La1 and influence of its addition in retail-manufactured ice cream produced with different sugar and fat concentrations. Int Dairy J 12:201–208
Anonymous (1976) ISO 2446 Milk-determination of fat content: Gerber method determination of fat in ice cream (ice cream mix) according to the Gerber method. Technical Memorandum TM 214-1e. Grindsted Products, Edwin Rahrs Vej 38, DK-8220, Brabrand, Denmark
Anonymous (1996) FIL-IDF. Preparation of samples and dilutions for microbiological examination. International IDF Standard 122C:1996. Brussels, Belgium
Brassart D, Schiffirin E (1997) The use of probiotics to reinforce mucosal defence mechanisms. Trends Food Sci Technol 8:321–326
Charteris WP, Kelly PM, Morelli L, Collins JK (1998) Antibiotic susceptibility of potentially probiotic Lactobacillus species. J Food Prot 61:1636–1696
Christiansen PS, Edelsten D, Kristiansen JR, Nielsen EW (1996) Some properties of ice cream containing Bifidobacterium bifidum and Lactobacillus acidophilus. Milchwissenschaft 51:502–504
Davidson RH, Duncan SE, Hackney CR, Eigel WN, Boling JW (2000) Probiotic culture survival and implications in fermented frozen yogurt characteristics. J Dairy Sci 83:666–673
Dunne C, Murphy L, Flynn S, O’Mahony L, O’Halloran S, Feeney M, Morrissey D, Thornton G, Fitzgerald G, Daly C, Kiely B, Quigley EMM, O’Sullivan GC, Shanahan F, Collins JK (1999) Probiotics: from myth to reality. Demonstration of functionality in animal models of disease and in human clinical trials. Antonie van Leeuwenhoek 76:279–292
Erkkilä S, Petäjä E (2000) Screening of commercial meat starter cultures at low pH and in the presence of bile salts for potential probiotic use. Meat Sci 55:297–300
Fernández MF, Boris S, Barbés C (2003). Probiotic properties of human lactobacilli strains to be used in the gastrointestinal tract. J Appl Microbiol 94:449–455
Fuller R (1992) History and development of probiotics. In: Fuller R (ed) Probiotics: the scientific basis. Chapman and Hall, London
Godward G, Kailasapathy K (2003) Viability and survival of free, encapsulated and co-encapsulated probiotic bacteria in ice cream. Milchwissenschaft 58:161–164
Guinard JX, Little C, Marty C, Palchak TR (1994) Effect of sugar and acid on the acceptability of frozen yogurt to a student population. J Dairy Sci 77:1232–1238
Hagen M, Narvhus A (1999) Production of ice cream containing probiotic bacteria. Milchwissenschaft 54:265–268
Hartemink R, Domenech VR, Rombouts FM (1997) LAMVAB-A new selective medium for the isolation of Lactobacilli from faeces. J Microbiol Methods 29:77–84
Hekmat S, McMahon J (1992) Survival of Lactobacillus acidophilus and Bifidobacterium bifidum in ice cream for use as a probiotic food. J Dairy Sci 75:1415–1422
Inoue K, Ito T, Shiota K (1998) Preparation and properties of ice-cream type frozen yogurt. Int J Dairy Technol 51:44–51
Kailasapathy K, Chin J (2000) Survival of therapeutical potential of probiotic organisms with reference to Lactobacillus acidophilus and Bifidobacterium spp. Immunol Cell Biol 78:80–88
Klaenhammer TR (1998) Functional activities of Lactobacillus probiotics: genetic mandate. Int Dairy J 8:497–505
Mättö J, Fondén R, Tolvanen T, Von Wright A, Vilpponen-Salmela T, Satokari R, Saarela M (2005) Intestinal survival and persistence of probiotic Lactobacillus and Bifidobacterium strains administered in triple-strain yoghurt. Int Dairy J (in press) doi:10.1016/j.idairyj.2005.10.007
Nighswonger BD, Brashears MM, Gilliland SE (1996) Viability of Lactobacillus acidophilus and Lactobacillus casei in fermented milk products during refrigerated storage. J Dairy Sci 79:212–219
Papamanoli E, Tzanetakis N, Litopoulou-Tzanetaki E, Kotzekidou P (2003) Characterization of lactic acid bacteria isolated from a Greek dry-fermented sausage of their technological and probiotic properties. Meat Sci 65:859–867
Schillinger U (1999) Isolation and identification of lactobacilli from novel-type probiotic and mild yoghurts and their stability during refrigerated storage. Int J Food Microbiol 47:79–87
Silvi S, Verdenelli MC, Orpianesi C, Cresci A (2003) EU project Crownalife: functional foods, gut microflora and healthy ageing isolation and identification of Lactobacillus and Bifidobacterium strains from faecal samples of elderly subjects for a possible probiotic use in functional foods. J Food Eng 56:195–200
Stanton C, Gardiner G, Lynch PB, Collins JK, Fitzgerald G, Ross RP (1998) Probiotic cheese. Int Dairy J 8:491–496
Vinderola CG, Reinhaimer JA (2000) Enumeration of Lactobacillus casei in the presence of L. acidophilus, bifidobacteria and lactic starter bacteria in fermented dairy products. Int Dairy J 10:271–275
Xanthopoulos V, Litopoulou-Tzanetaki E, Tzanetakis N (2000) Characterization of Lactobacillus isolates from infant faeces as dietary adjuncts. Food Microbiol 17:205–215
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Başyiğit, G., Kuleaşan, H. & Karahan, A.G. Viability of human-derived probiotic lactobacilli in ice cream produced with sucrose and aspartame. J IND MICROBIOL BIOTECHNOL 33, 796–800 (2006). https://doi.org/10.1007/s10295-006-0128-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-006-0128-x