Abstract
Sequential batch and continuous operation of a rotating biological contacting (RBC) reactor and the effects of dissolved oxygen on the decoloration of amaranth by Trametes versicolor were evaluated. Amaranth belongs to the group of azo dyes which are potential carcinogens and/or mutagens that can be transformed into toxic aryl amines under anaerobic conditions. Cultivation of T. versicolor in a stirred tank reactor was found to be unsuitable for amaranth decoloration due to significant biomass fouling and increase in medium viscosity. Assuming that decoloration follows first-order kinetics, amaranth was decolorized more rapidly when T. versicolor was immobilized on jute twine in a RBC reactor operated either in a sequential batch (k=0.25 h–1) or in a continuous (0.051 h−1) mode compared to a stirred tank reactor (0.015 h−1). Oxygen was found to be essential for decoloration with the highest decoloration rates occurring at oxygen saturation. Although longer retention times resulted in more decoloration when the RBC was operated in the continuous mode (about 33% amaranth decoloration), sequential batch operation gave better results (>95%) under similar nutrient conditions. Our data indicate that the fastest decoloration should occur in the RBC using nitrogen-free Kirk’s medium with 1 g/l glucose in sequential batch operation at rotational speeds and/or aeration rates which maintain oxygen saturation in the liquid phase.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In Canada, most (96%) wet processing mills discharge their effluent to the municipal sewer [1]. However, some municipalities forbid the discharge of dyes or any material which imparts color to the effluent entering their system since traditional biological wastewater treatment has low dye removal efficiency [6, 11]. Color is not aesthetically pleasing and it also affects light penetration and gas solubility in an aquatic environment. Furthermore, some synthetic dyes such as azo dyes may be carcinogens or mutagens [20, 25] and, under anaerobic conditions, can be transformed into aryl amines which are potentially more toxic than the parent compounds [5, 6]. Although white rot fungi (WRF) can effectively decolorize a wide range of dyes, few studies have examined WRF reactor systems to treat these effluents.
Rotating biological contacting (RBC) reactors are a likely choice to treat dye effluents as they allow surface immobilization of WRF such as Trametes versicolor [16]. Immobilized cultures tend to have a higher level of activity and are more resilient to environmental perturbations such as pH, or exposure to toxic concentrations than suspension cultures. RBCs have been used to treat domestic wastes for small communities [17], effluents from a meat packing plant [13], food processing [12], leather tanneries [24], and bioremediation of organopollutants such as nitro- and chloro-phenols [22], and trichloroethylene [2]. Currently, there are approximately 1,800 wastewater treatment facilities using RBCs worldwide, employing about 11,000 individual units [1].
There have been few studies of WRF immobilized in RBCs to decolorize effluents or dyes. Phanerochaete chrysosporium immobilized on discs of roughened plastic [3, 9] and Coriolus versicolor SCC63 and Rhizomucor pusillus RM7 immobilized on fine plastic mesh [23] have decolorized bleach plant effluent in RBCs. C. versicolor MUCL immobilized on plastic discs decolorized a maximum of 80% of a phthalocyanine dye, Eversol Turquoise Blue G, [10] and T. versicolor ATCC 20869 immobilized on jute twine decolorized 70–80% of a carpet dye effluent containing two anthraquinone dyes [16] in a RBC operated in a repeated batch mode. Apart from the last two references, there are no other reports in the literature on using WRF immobilized in a RBC to decolorize dyes and none which relate dissolved oxygen to decoloration efficiency by these fungi. WRF are strictly aerobic organisms and the oxygen transfer rate is known to limit the performance of RBCs [18]. Hence, the objectives of this study were to investigate the effects of dissolved oxygen on the decoloration of amaranth, as a model azo dye, by T. versicolor and to evaluate sequential batch and continuous modes of operation of a RBC.
Materials and methods
Culture maintenance and inoculum preparation
Trametes versicolor ATCC 20869 was maintained at 4°C on malt agar plates and was used to inoculate 500 ml Erlenmeyer shake flasks containing 200 ml of sterilized, modified Kirk’s medium [19]. The inoculum was incubated for 5 days at 30°C and 200 rpm on a rotary shaker (Innova 2000, New Brunswick Scientific, NJ). The pellets of mycelial biomass were allowed to settle to the bottom of the flask by gravity and the supernatant discarded. A 10% (volume of biomass/volume of liquid medium) inoculum was aseptically added to the stirred tank reactor or the RBC. All materials and culture media were autoclaved for 20 min at 121°C before use.
Decoloration in a stirred tank reactor
Trametes versicolor was grown as a suspension culture in a 2.3 l stirred tank reactor (Bioflo IIC, New Brunswick Scientific) with a working volume of 1.8 l in Kirk’s modified medium and an initial amaranth (Sigma-Aldrich, Canada) concentration of 15 mg/l. The aeration rate was set at 1 l/min, mixing was 200 rpm and reactor temperature was 26°C. Aliquots of a concentrated amaranth solution were added to the reactor at 100 and 300 h to achieve final concentrations of 20 and 28 mg/l, respectively. At 325 h, aeration was stopped and the reactor sparged with N2 for about 50 h.
Decoloration in RBC
A 5.3 l RBC pyrex glass reactor (150 mm diameter × 300 mm length) containing 2.5 l of modified Kirk’s medium with about 45% submergence had four rectangular (10 cm × 25 cm) stainless steel frames attached to the central shaft at the longest side and 90o from each other. The stainless steel shaft was coupled to a variable speed peristaltic pump and was fitted through a bearing assembly in the center of stainless steel plates at either end of the RBC. The dissolved O2 concentration was measured in a recirculation loop using a sterilizable, polarographic dissolved oxygen probe (Ingold Inpro 6000 series) and pH was measured off-line. Prior to decoloration, T. versicolor was colonized on jute twine wrapped around the rectangular frames for 5 days in modified Kirk’s medium at an aeration rate of 1 vvm supplied to the aqueous phase and a shaft rotational speed of 20 rpm. The spent growth medium and any suspended biomass were then removed and the reactor rinsed with 2.5 l of sterile distilled water. A fresh volume of decoloration medium was added for sequential batch decoloration or for continuous operation. The decoloration medium consisted of 25–100 mg amaranth (Sigma-Aldrich, Canada) and 1 g /l glucose, unless otherwise indicated. Batch decoloration was assessed at increasing rotational speeds (18–42 rpm) or aeration rates (0.25 and 2.5 l/min) and sequential batch decoloration was compared to continuous operation at two hydraulic retention times (9.5 and 12.3 h) and two nutrient levels (modified Kirk’s medium and a decoloration medium consisting only of dye and glucose).
Decoloration assay
Amaranth concentration was measured spectrophotometrically at 523 nm (λ max of amaranth) [19]. Decoloration rates were compared using the first-order decoloration rate constant, k, [21] such that \( {\text{ln}}{( {\frac{c} {{c_{0} }}} )} = - kt, \) where c 0 and c are the initial and final dye concentrations over the time interval, t, respectively. There was no decrease of color with uninoculated controls.
Results and discussion
Growth and decoloration in a stirred tank reactor
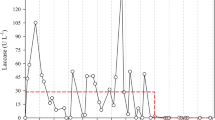
Trametes versicolor was grown as a suspension culture in a stirred tank reactor with an initial amaranth concentration of 15 mg/l. Although the fungal mycelium formed fairly homogenous pellets in shake flasks, there was mostly filamentous growth in the stirred tank reactor resulting in an obvious increase in broth viscosity and significant amounts of biomass attached to the inner surfaces of the reactor: the glass walls, the impellers, baffles, pH probe, etc. It was impossible to achieve a homogenous suspension. These mixing difficulties probably resulted in the low initial rate of amaranth decoloration observed, 0.2 mg/l per h (Fig. 1). When the dye concentration decreased to less than 5 mg/l, aliquots of a concentrated amaranth solution were added to the reactor at 100 and 300 h to achieve final concentrations of 20 and 28 mg/l, respectively. Since amaranth decoloration by T. versicolor is known to follow first-order kinetics [21] if there is no mass transfer limitation, decoloration rates were compared using initial rate data to determine the first-order reaction rate constant, k. The k values were similar for the first and second decolorations, 0.018 and 0.017 h−1, respectively. During the third decoloration, beginning at 325 h, aeration was replaced with N2 sparging for about 50 h. Once N2 sparging began, decoloration ceased and resumed when air sparging was restarted. The reaction rate constant before (0.016 h−1) and after (0.011 h−1) air sparing was similar (Fig. 1), demonstrating that the decolorizing ability of the culture was not significantly affected by being deprived of O2 for up to 50 h. Similar results were obtained in independent experiments in which decoloration did not occur in the absence of O2.
Trametes versicolor is a strict aerobe which requires O2 for growth, secondary metabolism and for its ligninolytic enzymes to function. Of these enzymes, laccase is a multicopper glycoprotein which performs one-electron oxidation of aromatic compounds such as dye molecules and, at the same time, reduces O2 to H2O. Peroxidases such as MnP need H2O2 to complete their catalytic cycle. Although lignin-degrading cultures can produce H2O2 in several ways, the underlying reaction involves the reduction of O2 to H2O2 [4]. Our results clearly show that decoloration by T. versicolor requires O2, most likely for the ligninolytic enzymes. Since culture of this filamentous fungus in a stirred tank reactor resulted in serious mass transfer and operational difficulties, and since immobilization of the biomass would result in less need to divert carbon and energy toward growth, immobilization of T. versicolor in a RBC for dye decoloration was evaluated.
Sequential batch operation
Using a RBC reactor can avoid many of the problems observed in suspension culture. Since the biomass is immobilized, there would be no viscosity problems and the higher biomass concentration per unit volume compared to stirred tanks would result in higher treatment efficiencies as well as better recovery from feed perturbations, and better performance at low influent concentrations. Furthermore, industrial RBCs operate at low rotational speeds hence they have lower energy costs, and they generally have lower maintenance requirements.
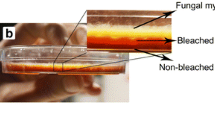
After the establishment of T. versiolor on jute twine, which had been previously found to be a good immobilization support [16, 19], the sequential batch decoloration of amaranth in the RBC was evaluated. After a fresh volume of decoloration medium (i.e., 50 mg/l amaranth and 1 g/l glucose in water alone) replaced the spent growth medium, there was a lag of over 20 h with amaranth decolorized at an overall rate of 1.04 mg/l per h (Fig. 2). At 80 h, the decolorized medium was replenished with fresh modified Kirk’s medium to allow biomass growth. When the spent growth medium was replaced with a second volume of decoloration medium (50 mg/l amaranth and 1 g/l glucose), there was no lag phase before decoloration resumed with k of 0.25 h−1. When the decoloration medium was changed a third time at 160 h to 100 mg/l amaranth and 1 g/l glucose, there was also no lag phase and a similar k (0.22 h−1) was achieved. During the three decolorations, the dissolved oxygen ranged around 50–80% saturation and the pH was at 4–4.5. Volumetric decoloration rate constants were at least an order of magnitude higher (about 0.23 h−1, Fig. 2) when the RBC was operated in a repeated batch mode with immobilized T. versicolor than in a batch stirred tank reactor with the suspended culture (0.015 h−1, Fig. 1).
Sequential batch decoloration of amaranth by T. versicolor immobilized in a RBC. The first-order reaction rate constant, k, for the last two decolorations is 0.25 and 0.22 h−1 at 50 and 100 mg/l amaranth, respectively (diamond Amaranth; open square percentage of oxygen saturation; filled triangle pH)
Effect of rotational speed and aeration on decoloration in RBC
In wastewater treatment facilities, RBCs are open to air and the rotation brings a thin liquid film on the discs in contact with air then back into the bulk liquid. However, to ensure an axenic culture for experimental purposes, the reactor was operated as a closed system. This also allowed the effect of aeration rate on amaranth decoloration to be determined in sequential batch operation. Biomass growth was minimal during decoloration as nitrogen-free medium was used, and the nitrogen content from the dye was quite low as amaranth concentration ranged from 25 to 100 mg/l with only about 2% of its mass being nitrogen. These dye concentrations were selected because effluent concentrations are typically at the lower end of the range of 10–250 mg/l [14].
Furthermore, no fresh growth medium was added after the colonization stage. In this experiment, the RBC was operated at an airflow rate of either 0.25 or 2.5 l/min at a constant rotational speed. Increased aeration led to higher dissolved oxygen levels in the reactor liquid allowing higher amaranth decoloration rates to be obtained (Table 1). This indicates that, at that biofilm thickness and rate of metabolic activity, oxygen transfer limited the dye decoloration rate. The oxygen transfer rate is known to limit the performance of RBCs in aerobic wastewater treatment [18]. The rotational speed would also impact the transfer rate and, consequently, the decoloration rate as oxygen is necessary for decoloration to occur. The higher the rotational speed, the more contact is achieved between the immobilized biomass, the liquid and the gas phases, so that better treatment efficiencies can be obtained. However, increasing the rotational speed leads to higher power consumption and increased mechanical requirements, which may not be economical for wastewater treatment applications.
An increase in the rotational speed from 18 to 42 rpm (at a fixed aeration rate of 0.25 l/min) resulted in an increase in the dissolved oxygen concentration in the bulk liquid such that oxygen saturation increased from 37 to 99% (Fig. 3). The rate of decoloration tended to be higher at higher rotational speeds when the dissolved oxygen concentration was at saturation. For thicker biofilms, a higher rotational speed may be needed to achieve oxygen saturation. This is the first study which relates the dissolved oxygen concentration and decoloration efficiency and which indicates that decoloration in a RBC might be most efficient when the liquid phase is saturated with oxygen. Although Kapdan and Kargi [10] showed that increasing the rotational speed of a RBC resulted in increased decoloration of Everzol Turquoise Blue G by C. versicolor, no dissolved oxygen measurements were made.
Continuous operation
For the experiment described in Table 2, after 7 days of cultivation of T. versicolor on the jute support, the RBC was operated continuously for 8 days without the addition of fresh growth medium. At a hydraulic retention time of 9.5 h with a feed of nitrogen-free growth medium (i.e., modified Kirk’s medium without ammonium tartrate), the rate and extent of decoloration decreased by about 50% when the glucose concentration was decreased from 10.1 to 1.0 g/l. The drop in decoloration may be due to lowering the glucose concentration but an earlier study had shown that decreasing glucose from 2 to 1 g/l had no effect [19]. Apart from being a potential carbon and energy source for the fungus, glucose may be a substrate for pyranose oxidase which can generate H2O2 [8, 15] required by peroxidases, such as manganese peroxidase, one of the enzymes that T. versicolor secretes during decoloration [21]. It is likely that a trace element in the medium such as copper [7] may have enhanced decoloration.
When the hydraulic retention time was increased with a feed of 1 g/l glucose, the steady state decoloration efficiency increased from 32.7 to 47.6% with an increase in the decoloration rate. Typically dye-decolorizing enzymes are secreted during secondary metabolism when the growth rate is low so that operating a continuous reactor at higher hydraulic retention times would favor decoloration.
Comparison of sequential batch and continuous operation
When sequential batch and continuous operation of the RBC are compared under similar conditions, the first-order reaction rate constant was higher under sequential batch operation. At an initial amaranth concentration of about 50 mg/l and 1 g/l glucose in sequential batch mode, the k value for amaranth decoloration was 0.25 h−1 (Fig. 2) and with a similar medium (56 mg/l amaranth and 1 g/l glucose) in continuous operation, the k value was 0.051 h−1 (Table 2). The batch reaction took about 10 h to achieve greater than 98% decoloration while only about 33% decoloration was achieved at a retention time of 9.5 h in continuous operation. It would appear that repeated batch operation would give a better quality effluent at a faster rate.
In conclusion, T. versicolor immobilized on jute twine in a RBC operated in a sequential batch or continuous mode, successfully decolorized amaranth, an azo dye. Higher decoloration efficiencies were achieved in sequential batch operation (compare Fig. 2; Table 2) and at higher dissolved oxygen concentrations (Table 1 and Fig. 3). Based on all our results, the best decoloration rate would occur in nitrogen-free Kirk’s medium with 1 g/l glucose in sequential batch operation, and at rotational speeds and/or aeration rates which maintain oxygen saturation in the liquid phase.
References
Priority Substances List Assessment Report (2001) Textile mill effluents. Environment Canada and Health Canada (ISBN 066229341X)
Brar SK, Gupta SK (2000) Biodegradation of trichloroethylene in a rotating biological contactor. Water Res 34:4207–4214
Chang H-M, Joyce TW, Kirk TK (1987) Process of treating effluent from a pulp or papermaking operation. US Patent 4,655,926
Cho NS, Hofrishter M, Wesenberg D (2002) Fungal laccase: properties and activity on lignin. J Basic Microbiol 41:185–227
Chung KT, Stevens SE (1993) Decolorization of azo dyes by environmental organisms and helminths. Environ Toxicol Chem 12:2121–2132
Dubrow SF, Boardman GD, Michelsen DL (1996) Chemical pretreatment and aerobic–anaerobic degradation of textile dye wastewater. In: Reife A, Freeman HS (eds) Environmental chemistry of dyes and pigments. Wiley, Toronto, pp 75–94
Galhaup C, Wagner H, Hinterstoisser B, Haltrich D (2002) Increased production of laccase by the wood-degrading basidiomycete Trametes pubescens. Enzyme Microb Technol 30:529–536
Giffron F (2000) Fungal pyranose oxidases: occurrence, properties and biotechnical applications in carbohydrate chemistry. Appl Microbiol Biotechnol 54:727–740
Joyce TW, Chang H-M, Campbell AG Jr, Gerrard ED, Kirk TK (1984) A continuous biological process to decolorize bleach plant effluents. Biotech Advs 2:301–308
Kapdan IK, Kargi F (2002) Biological decolorization of textile dyestuff containing wastewater by Coriolus versicolor in a rotating biological contactor. Enzyme Microb Technol 30:195–199
Kapdan I, Kargi F, McMullan G, Marchant R (2000) Effect of environmental conditions on biological decolorization of textile dyestuff by C. versicolor. Enzyme Microb Technol 26:381–387
Laquidara MJ, Blanc FC, O’Shaughnessy JC (1986) Development of biofilm, operating characteristics and operational control in the anaerobic rotating biological contactor process. J Water Pollut Control Fed 58:107–114
Oguz M, Oguz M (1993) Characterization of Ankara meat packing plant wastewater and treatment with a rotating biological contactor. Int J Environ Stud 44:39
O’Neill C, Hawkes FR, Hawkes DL, Lourenco ND, Pinheiro HM, Delee W (1999) Colour in textile effluents—sources, measurement, discharge consents and simulation: a review. J Chem Technol Biotechnol 74:1009–1018
Machida Y, Nakanishi T (1984) Purification and properties of pyranose oxidase from Coriolus versicolor. Agric Biol Chem 48:2463–2470
Ramsay JA, Goode C (2004) Decoloration of a carpet dye effluent using Trametes versicolor. Biotechnol Lett 26:197–201
Rigden B (1989) Water and wastewater treatment for a small scale island resort. Water Sci Technol 21:189–193
Rittmann BE, Suozzo R, Romero BR (1983) Temperature effects on oxygen transfer to rotating biological contactors. J Water Pollut Control Fed 55:270–217
Shin M, Nguyen T, Ramsay J (2002) Evaluation of support materials for the surface immobilization and decoloration of amaranth by Trametes versicolor. Appl Microbiol Biotechnol 60:218–223
Spadaro JT, Gold MH, Ranganathan V (1992) Degradation of azo dyes by the lignin-degrading fungus Phanerochaete chrysosporium. Appl Environ Microbiol 58:2397–2401
Swamy J, Ramsay J (1999) Effects of glucose and NH +4 concentrations on sequential dye decoloration by Trametes versicolor. Enzyme Microb Technol 25:278–284
Tokuz RY (1989) Biodegradation and removal of phenols in rotating biological contactors. Water Sci Technol 21:1751–1754
van Driessel B, Christov L (2001) Decolorization of bleach plant effluent by mucoralean and white rot fungi in a rotating biological contactor reactor. J Biosci Bioeng 92:271–276
Yang ZY, Fan ZS (1990) Treatment of leather and fur wastewater by a rotating biological contactor. Water Sci Technol 22:119–126
Zollinger H (1991) Color chemistry: syntheses, properties and applications of organic dyes and pigments, 2nd edn. Wiley-VCH, New York
Acknowledgments
The authors acknowledge the financial support of the Natural Science and Engineering Research Council of Canada; Premier’s Research Excellence Award, Government of Ontario; and the Chancellor’s Award of Queen’s University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ramsay, J., Shin, M., Wong, S. et al. Amaranth decoloration by Trametes versicolor in a rotating biological contacting reactor. J IND MICROBIOL BIOTECHNOL 33, 791–795 (2006). https://doi.org/10.1007/s10295-006-0117-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-006-0117-0