Abstract
Undersea deposition of unexploded ordnance (UXO) constitutes a potential source of contamination of marine environments by hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) and octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine (HMX). The goal of the present study was to determine microbial degradation of RDX and HMX in a tropical marine sediment sampled from a coastal UXO field in the region of Oahu Island in Hawaii. Sediment mixed cultures growing in marine broth 2216 (21°C) anaerobically mineralized 69% or 57% (CO2, 25 days) of the total carbon of [UL-14 C]-RDX (100 μM) or [UL-14 C]-HMX (10 μM), respectively. As detected by PCR-DGGE, members of γ-proteobacteria (Halomonas), sulfate-reducing δ-proteobacteria (Desulfovibrio), firmicutes (Clostridium), and fusobacterium appeared to be dominant in RDX-enrichment and/or HMX-enrichment cultures. Among 22 sediment bacterial isolates screened for RDX and HMX biodegradation activity under anaerobic conditions, 5 were positive for RDX and identified as Halomonas (HAW-OC4), Marinobacter (HAW-OC1), Pseudoalteromonas (HAW-OC2 and OC5) and Bacillus (HAW-OC6) by their 16S rRNA genes. Sediment bacteria degraded RDX to N2O and HCHO via the intermediary formation of hexahydro-1-nitroso-3,5-dinitro-1,3,5-triazine (MNX) and methylenedinitramine. The present findings demonstrate that cyclic nitramine contaminants are likely to be degraded upon release from UXO into tropical marine sediment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) and octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine (HMX) (Fig. 1) are two powerful cyclic nitramine explosives, the wide use of which has resulted in contamination of both terrestrial and aquatic environments [8, 20, 25]. Unexploded ordnances (UXO) from sunken warships, improper disposal of military waste, and navy training, are potential sources of contamination of marine environments by explosives [8, 26, 27]. RDX and HMX are toxic to both terrestrial and aquatic organisms [16, 22], thus necessitating their removal from contaminated marine environments. Although biodegradation of cyclic nitramines by microorganisms, especially anaerobic bacteria, has been widely studied in soil [17, 24], anaerobic sludge [1, 11] and fresh water [4, 13], their degradation by microorganisms from marine environments, especially in sediments from tropical areas, has rarely been reported [26, 27].
Structures of nitroaromatic cyclic nitramine explosives and their derivatives. TNT 2,4,6-Trinitrotoluene; 2,6-DNT 2,6-dinitrotoluene; 2,4-DNT 2,4-dinitrotoluene; RDX hexahydro-1,3,5-trinitro-1,3,5-triazine; MNX hexahydro-1-nitroso-3,5-dinitro-1,3,5-triazine; DNX hexahydro-5-nitro-1,3-dinitroso-1,3,5-triazine; TNX hexahydro-1,3,5-trinitroso-1,3,5-triazine; HMX octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine; MEDINA methylenedinitramine; NDAB 4-nitro-2,4-diazabutanal
In the present study, tropical marine sediment obtained from a coastal region of Oahu Island of Hawaii was found to contain traces of nitro aromatic compounds such as 2,4,6-trinitrotoluene (TNT) or 2,4-dinitrotoluene or 2,6-dinitrotoluene (DNT) (Fig. 1). Neither of the two cyclic nitramines, RDX and HMX, were detected despite their suspected presence, suggesting the possible occurrence of biodegradation of nitramines at this site. The objective of the present study was thus first to determine if the microbial community indigenous to the sediment was capable of degrading RDX and HMX, and second to isolate and characterize specific bacteria involved in biodegradation. We hope that data generated from this study can be used to provide insight into the fate (in situ natural attenuation) of cyclic nitramines in marine sediment.
Materials and methods
Chemicals and microbiological media
RDX (99% pure), HMX (98% pure), uniformly labeled [UL-14 C]-RDX (chemical purity, >98%; radiochemical purity, 96%; specific radioactivity, 28.7 µCi mmol−1) and [UL-14 C]-HMX (chemical purity, >94%; radiochemical purity, 91%; specific radioactivity, 93.4 µCi mmol−1), and hexahydro-1,3,5-trinitroso-1,3,5-triazine (TNX, 99% pure) were provided by Defense Research and Development Canada (DRDC), QC, Canada. Hexahydro-1-nitroso-3,5-dinitro-1,3,5-triazine (MNX, 98% pure) and 4-nitro-2,4-diazabutanal (NDAB) (99% pure) were provided by R.J. Spanggord (SRI International; Menlo Park, Calif.), and methylenedinitramine (MEDINA) was purchased from Aldrich, Canada (rare chemical department). The marine salts medium was prepared as described previously [26, 27]. The liquid and solid marine media used were marine broth or marine agars 2216 (Becton Dickinson, Sparks, Md.) [6]. The pH of marine broth 2216 was readjusted to 6.5 in all experiments. All media used were sterilized by autoclaving. All other chemicals were of reagent grade.
Sampling and characterization of Hawaii marine sediment
Marine sediment was sampled from a coastal region (Station ORD2, UXO field) of Oahu Island in Hawaii (15–21 m deep) and characterized. Briefly, the sediment comprised sand mixed with coral reef, was alkaline (pH 8.4), and the temperature at the sampling site was relatively warm (26°C). Lots of heavily degraded large ordnance with brass caps, shell casings, and rings were found at the site; identification of specific ordnance type was not possible due to the extent of degradation. The analysis of explosive in the sediment showed the presence of trace amounts of TNT (<0.01 mg kg−1), 2,4-DNT (0–2.5 mg kg−1) and 2,6-DNT (0–0.1 mg kg−1). Neither RDX nor HMX was detected. The sealed sediment samples were kept in a cold room at 4°C until use.
Biodegradation and mineralization of cyclic nitramines in sediment slurry and by enriched mixed cultures
For biodegradation experiments, serum bottles (120 mL) containing 1 g wet sediment (dry weight, 0.85 g) and 75 mL marine broth 2216 were charged separately with either RDX (final concentration, 100 μM) or HMX (final concentration, 10 μM) from stock solutions (in acetone). The pH of the sediment-slurry was adjusted (using 0.1 N HCl) to 6.5. The bottles were sealed with Teflon-coated sterile butyl rubber caps and aluminum seals, and were then briefly degassed and flushed with argon three times. The sealed bottles were incubated statically at room temperature (21°C). Controls contained sterile (γ-irradiated) sediment, sterile marine broth 2216, and the cyclic nitramines. To analyze remaining RDX or HMX and products formed in the aqueous phase of sediment-slurry, 2 mL slurry medium was withdrawn and filtered through a 0.45-μm filter. The filtrate was analyzed by HPLC as described below. The final redox potential of the RDX and HMX enrichment cultures reached −292 mV as measured using an Accumet platium/Ag/AgCl combination electrode (Fisher Scientific, Nepean, ON, Canada). The experiment was conducted in triplicate.
For the mineralization experiment, a nitramine-adapted mixed culture was obtained by three consecutive subculturings of the original sediment microbial population in RDX-containing or HMX-containing marine broth 2216. The third subculture (0.5 mL, 14 days old) was inoculated into anoxic marine broth 2216 (9.5 mL) containing either [UL-14 C]-RDX (101,387 dpm) or [UL-14 C]-HMX (100,273 dpm) and incubated under conditions similar to those used for the biodegradation experiment. Mineralization (liberated 14 CO2) was measured as described previously [11]. Abiotic controls contained sterile marine broth 2216 and [UL-14 C]-RDX (101,387 dpm) or [UL-14 C]-HMX (100,273 dpm).
PCR/denaturing gradient gel electrophoresis-based analysis of 16S rRNA genes of mixed cultures
Denaturing gradient gel electrophoresis (DGGE) was used to analyze the microbial community in untreated sediment and in three enriched sediment cultures prepared as follows: (1) incubation in marine broth 2216 for 5 weeks in the absence of the cyclic nitramine; (2) in the presence of RDX (103 μM); (3) in the presence of HMX (11.4 μM).
DGGE was conducted by Microbial Insight (Knoxville, Tenn.). Briefly, extractions of genomic DNA from sediment and enrichment cultures were performed using UltraClean DNA extraction kits (MoBio Laboratories, Solana Beach, Calif.) following the manufacturer’s instructions. Eubacterial PCR primer 341f (5′-CCTACGGGAGGCAGCAG-3′) with a 5′-end GC-clamp (CGCCCGCCGCGCGCGGCGGGCGGGGCGGGGGCACGGGGGG), and 519r (5′-ATTACCGCGGCTGCTGG-3′) [18] were used to amplify 16S rRNA genes under the following conditions: 60 ng genomic DNA; 10 pmol primer; 12 pmol each deoxyribonucleoside triphosphate; 1.25 units High Fidelity polymerase (Taq enzyme) (Boehringer, Indianapolis, Ind.); 34 thermocycles (each cycle: 94°C for 45 s, 55°C for 30 s, and 68°C for 45 s) and 2.5 μL PCR buffer as described by Muyzer et al. [18]. Thermocycling was performed with a Robocycler PCR Block (Stratagene, La Jolla, Calif.). PCR products (150 ng DNA) were applied to an 8% (w/v) polyacrylamide gel with a denaturant gradient of 30–65% (100% denaturant: 7 M urea/40% v/v formamide) using the D-Code DGGE gel system (Bio-Rad, Hercules, Calif.), and run at 60°C, 55 V for 16 h. The dominant (visually dark) DGGE bands (C–F, H–N) were excised (central portion, 1 mm) using a razor blade and soaked in purified water (Milli-Q, 50 μL) overnight. Aliquots of the latter samples (2 μL) were used as templates for subsequent PCR amplification. The resulting amplicon was further purified using a MoBio Laboratories PCR clean up kit, followed by sequencing (about 127–150 bases) on an ABI-Prism automatic sequencer model 377 with dye terminators (Global Medical Instrumentation, Ramsey, Minn.).
Isolation of cyclic nitramine-degrading bacteria and biodegradation by isolates
Anaerobic sediment enrichment cultures incubated in marine broth 2216 at 21°C in the presence of RDX or HMX for 5 weeks were used to isolate bacteria. Colonies differing in morphology were selected and streaked on fresh agar plates for further purification. Isolates grown on marine agars for 3–5 days were suspended in sterile marine broth 2216 to OD540 nm =0.8–1.0. The resultant cellular suspensions (0.1 mL) were added to sterile marine broth 2216 (2 mL) containing RDX (100 μM) or HMX (4 μM), followed by static and anaerobic incubation at room temperature (21°C) for 2 weeks. The potential of the bacterial isolates to degrade RDX and HMX was measured by analyzing the two cyclic nitramines remaining in the medium by HPLC (see below).
RDX degradation by resting cells of selected isolates (HAW-OC2, HAW-OC4, HAW-OC5, HAW-OC6) was conducted using the following protocol. Cells were first grown in marine broth 2216 (600 mL) in the presence of RDX (100 μM) for 5 days followed by harvesting by centrifugation at 10,000 g for 20 min. Centrifuged cells were washed (with anaerobic marine salts medium) and re-suspended in marine salts solution [60 mL; final biomass (isolate/gram wet cells per liter): HAW-OC2/59, HAW-OC4/52, HAW-OC5/31, HAW-OC6/65] containing RDX (113 μM), followed by static incubation under anaerobic conditions at room temperature (21°C). Removal of nitrite (NO −2 , 100 μM) by resting cells of isolates was conducted under the conditions used for RDX.
Phylogenetic analyses of 16S gene sequences of DGGE bands and bacterial isolates
The 16S rRNA gene sequences of the DGGE bands and of microbial isolates were compared to known sequences in GenBank using BLAST. The gene sequences of the DGGE bands and those of closely related bacteria in the databank were aligned using ClustalX (ver. 1.81). The neighbor-joining method in the MEGA2 package [15], based on the pairwise nucleotide distance of Kimura 2-parameter, was used to build a phylogenetic tree (the number of bootstrap repetitions was 4,000). GenBank accession numbers of 16S rRNA gene sequences are: DGGE bands C, AY669152; D, AY669153, E, AY669155; F, AY669154; H, AY669156; I, AY669157; J, AY669158; K, AY669159; L, AY669160; M, AY669161; N, AY669162. GenBank accession numbers of 16S rRNA gene sequences of isolates are: HAW-OC1, AY669163; HAW-OC2, AY669164; HAW-OC4, AY669165; HAW-OC5, AY669166; HAW-OC6, AY669167.
Analyses of cyclic nitramines and their products
The concentrations of RDX, MNX, DNX, TNX, and HMX were determined by HPLC as described previously [11]. MEDINA and NDAB were determined on an AnionSep Ice-Ion-310 Fast organic acids HPLC lane (6.5×150 mm, Cobert, St Louis, Mo.) at 225 nm and 35°C. HCHO, N2O and NO −2 were analyzed as described previously [10, 11].
Results and discussion
Biodegradation of RDX and HMX by mixed culture in marine sediment
Anaerobic incubation of RDX and HMX in Hawaii marine sediment (1 g wet sediment) in the presence of marine medium (marine broth 2216, 75 mL, pH 6.5) at 21°C led to their removal (101 μM of initial 103 μM RDX; 2.3 μM of initial 11.4 μM HMX) in 18 days [final E (versus Ag/AgCl) −292 mV]. Biodegradation of HMX was slower relative to that of RDX, consistent with our previous observations with Halifax sediment [26] and other anaerobic systems [11]. No significant loss of RDX (0.7 μM of 101 μM) or HMX (0.15 μM of 11 μM) was found in abiotic controls containing sterile sediment under similar conditions [pH 6.5, final E (versus Ag/AgCl) −80 mV]. However, when the latter experiment was conducted at the original pH (8.4) of the Hawaii sediment, loss of RDX and HMX occurred (data not shown) possibly due to alkaline hydrolysis [2, 7]. Using HPLC-UV and LC-MS methods [11], we observed the transient formation of MEDINA (2.5±0.3 μM), MNX (5.2±0.7 μM), traces of DNX (unquantified), and TNX (0.26±0.06 μM) after 18 days of incubation with RDX.
The RDX-enrichment culture mineralized 69% of [UL-14 C] RDX in 25 days (Fig. 2). Likewise, HMX-adapted mixed cultures mineralized 57% of [UL-14 C]-HMX after 25 days (Fig. 2).
Identification of bacteria in RDX- or HMX-enrichment cultures
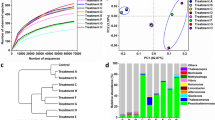
PCR-DGGE analysis of the 16S rRNA genes of the RDX-enrichment revealed 15 bands (lane III, Fig. 3), among which 4 (E, J, K, and L) were dominant. Two of the latter (E and L) were also found in a control obtained by incubating sediment in the absence of nitramines (lane II, Fig. 3). Twelve bands were detected in the HMX-enrichment with four (D, M, N, and F) being dominant (lane IV, Fig. 3). Phylogenetic analyses indicated that the dominant DGGE bands (E, J, K, and L in Fig. 3) in RDX-enrichment belonged to δ-proteobacteria (Desulfovibrio, band E) and γ-proteobacteria (Vibrio, bands J and K; Halomonas, band L). The four dominant DGGE bands in HMX-enrichment (D, M, N, and F in Fig. 3) were similar to that of δ-proteobacteria (Desulfovibrio, bands D and M), firmicutes (Clostridium, band F) and fusobacteria (Leptotrichia, band N). Detection of sulfate-reducing bacteria, fusobacteria and clostridia as dominant bacteria in RDX- and HMX-enrichments is suggestive of their involvement in biodegradation or tolerance of the two chemicals. Previously, bacteria of similar groups (sulfate-reducing bacteria, fusobacteria and clostridia) were found capable of degrading both RDX and HMX [5, 27]. The sequences of the sulfate-reducing bacteria bands (bands D, E, and M in Fig. 3) in the present Hawaii sediment are mostly similar (>94%) to that of an RDX-degrading and HMX-degrading sediment-isolate, HAW-EB18, previously found in Halifax sediment [27].
Denaturing gradient gel electrophoresis (DGGE) profiles of PCR-amplified 16S rRNA gene fragments. Lanes: I Original untreated sediment, II sediment slurry not spiked with cyclic nitramines, III RDX-spiked sediment slurry, IV HMX-spiked sediment slurry, V 16S rRNA fragment standards. Banding patterns and relative intensities of the recovered bands provide a means of comparing bacterial communities. Bacteria must constitute at least 1–2% of the total bacterial community to form a visible band. Labeled bands C–F and H–N were excised and sequenced
To further characterize nitramine-degrading bacteria in the tropical marine sediment, oxygen-tolerant anaerobic bacteria were isolated from RDX- and HMX-enrichment cultures and screened for their potential to biodegrade these two chemicals. Of 22 bacterial isolates, 5 were found capable of anaerobically degrading RDX. The isolates did not significantly remove RDX under aerobic conditions. None of the isolates removed HMX under either aerobic or anaerobic conditions. All five of the above strains are rod-shaped, catalase- and oxidase-positive bacteria identified as HAW-OC1 (light yellow, rough-wrinkled and elevated/1–1.5×3–4 μm), HAW-OC2 (pale white and transparent/1.5–2×4–6 μm), HAW-OC4 (grayish yellow, irregular and sticky/0.5–1×1.5–2 μm), HAW-OC5 (light yellow and slimy/1–1.5×2–4 μm), and HAW-OC6 (pale white, opaque and smooth/1.5–2×2–3 μm). The five isolates grew well in marine medium containing 2% NaCl, but did not grow in NaCl-free nutrient broth, confirming their marine origin.
Analysis of 16S rRNA genes showed that isolate HAW-OC4 belongs to Halomonas (similarities: H. venusta, 99.5%; H. aquamarine, 97.0%), HAW-OC1 to Marinobacter (99.7% similar to M. aquaeolei and M. hydrocarbonoclasticus), HAW-OC6 to Bacillus (similarities: B. cereus, 99.9%; B. anthracis, 99.8%), and HAW-OC2 and HAW-OC5 to Pseudoalteromonas (similarities: P. luteoviolacea, 96.3%) (Fig. 4). Halomonas, Marinobacter, and Pseudoalteromonas are typical halotolerant or halophilic bacteria found in marine and salty environments [9, 19], but have never been reported as RDX degraders. Bacteria belonging to Pseudomonas were both isolated and detected by PCR-DGGE (band I in Fig. 3) but the isolates showed only poor ability to degrade RDX and HMX (data not shown).
Phylogenetic tree of 16S rRNA genes of selected DGGE bands and bacterial isolates from Hawaii sediment based on pairwise nucleotide distance of Kimura 2-parameter using the neighbor-joining method included in the MEGA2 software package. Bar Difference of 10 nucleotides per 100 bases. GenBank accession numbers of the16S rRNA genes appear in parentheses
Differences were found between the bacterial profile identified by DGGE and that identified by the isolation method. For example, Marinobacter, Pseudoalteromonas, and Bacillus, isolated as RDX degraders, were not detected by PCR-DGGE. This is possibly due to the reported low sensitivity of DGGE [18], and/or PCR bias in gene amplification [12, 23]. It is also known that some environmental bacteria (>90%) are hard to cultivate, or are not culturable [3, 14, 21]; therefore, the two methods can complement each other in identifying bacteria involved in biodegradation of cyclic nitramines.
Biodegradation of RDX by bacterial isolates
Biotransformation of RDX by resting cells of the three isolates HAW-OC2 (Pseudoalteromonas), HAW-OC4 (Halomonas), and HAW-OC6 (Bacillus) was accompanied by the intermediary formation of MNX, the ring cleavage intermediate MEDINA, and HCHO (Fig. 5). The latter was not included in the time course study because it mineralized to CO2 rapidly as reported previously [11]. A trace amount of DNX was also found in all isolates, but no TNX was detected. The three isolates HAW-OC2, HAW-OC4, and HAW-OC6 degraded RDX at rates of 0.45, 0.30, and 0.44 μmol h−1 g−1 dry cellular weight, respectively. After 10 h of incubation, approximately 70–85% of the total N of degraded RDX was recovered as MNX, MEDINA, and N2O. We did not find nitrite; however, resting cells of HAW-OC2, HAW-OC4, and HAW-OC6 removed NO −2 at rates (0.8–1.1 μmol h−1 g−1 dry cellular weight) faster than those observed for RDX removal (0.3–0.45 μmol h−1 g−1 dry cellular weight).
Anaerobic biotransformation of RDX by resting cells of bacterial isolates HAW-OC2, HAW-OC4, and HAW-OC6 in sterile marine salts solution (biomass, 31–65 g wet cells per liter, static incubation at 21°C). RDX [cntl] RDX in the control containing sterile anoxic marine salts solution and heat-killed cells
Product distributions in the three isolates tested were similar to those found in mixed cultures of the present sediment, and to that obtained in Halifax marine sediment [27] and anaerobic sludge [11], suggesting the involvement of similar RDX degradation pathways. As in previous studies [11, 27], we suggest that, in the present study, RDX was also degraded through initial denitration or initial reduction to MNX, followed by ring cleavage and autodecomposition in water.
As shown by DGGE (Fig. 3), bacteria belonging to δ-proteobacteria and firmicutes that were detected in Hawaii sediment (20 m depth) were also found in Halifax sediment (215 m depth). However, the mesophilic isolates γ-proteobacteria (Marinobacter and Halomonas) from Hawaii sediment differed from the psychrophilic Shewanella isolates from Halifax sediment, which is consistent with the warm nature of Hawaii sediment (26°C) as opposed to the cold environment in Halifax.
Conclusion
The present study shows that marine sediment in Hawaii contains a microbial community capable of biodegrading RDX and HMX. The sediment, which was also found to be alkaline (pH 8.4), could also lead to the hydrolysis (abiotic decomposition) [2, 7] of RDX. Therefore, we suggest that if RDX or HMX leak from UXO to the sediment, then they would undergo natural attenuation in situ.
References
Adrian NR, Arnett CM (2004) Anaerobic biodegradation of hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) by Acetobacterium malicum strain HAAP-1 isolated from a methnaogenic mixed culture. Curr Microbiol 48:332–340
Balakrishnan VK, Halasz A, Hawari J (2003) Alkaline hydrolysis of the cyclic nitramine explosives RDX, HMX, and CL-20: new insights into degradation pathways obtained by the observation of novel intermediates. Environ Sci Technol 37:1838–1843
Barer MR, Harwood CR (1999) Bacterial viability and culturability. Adv Microbial Physiol 41:93–137
Beller HR (2002) Anaerobic biotransformation of RDX (hexahydro-1,3,5-trinitro-1,3,5-triazine) by aquifer bacteria using hydrogen as the sole electron donor. Water Res 36:2533–2540
Boopathy R, Gurgas M, Ullian J, Manning JF (1998) Metabolism of explosive compounds by sulfate-reducing bacteria. Curr Microbiol 37:127–131
Bowman JP (2001) Methods for psychrophilic bacteria. In: Paul JH (ed) Methods in microbiology. Academic, Cambridge, pp 13–43
Crose M, Okamoto Y (1979) Cationic micellar catalysis of the aqueous alkaline hydrolysis of 1,3,5-triaza-1,3,5-trinitrocyclohexane and 1,3,5,7-tetraaza-1,3,5,7-tetranitrocyclooctane. J Org Chem 44:2100–2103
Darrach MR, Chutjian A, Plett GA (1998) Trace explosives signatures from World War II unexploded undersea ordnance. Environ Sci Technol 32:1354–1358
Dobson SJ, McMeekin TA, Franzmann PD (1993) Phylogenetic relationships between some members of the genera Deleya, Halomonas, and Halovibrio. Int J Syst Bacteriol 43:665–673
Environmental Protection Agency Environmental Monitoring and Support Laboratory Office of Research and Development (1979) USA EPA method 345.1. Methods for chemical analysis of water and wastes. Environmental Protection Agency, Cincinnati, Ohio
Hawari J, Halasz A, Sheremata TW, Beaudet S, Groom C, Paquet L, Rhofir C, Ampleman G, Thiboutot S (2000) Characterization of metabolites during biodegradation of hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) with municipal anaerobic sludge. Appl Environ Microbiol 66:2652–2657
Ishii K, Fukui M (2001) Optimization of annealing temperature to reduce bias caused by a primer mismatch in multitemplate PCR. Appl Environ Microbiol 67:3753–3755
Jeffrey L, Davis JL, Wani AH, O’Neal BR, Hansen LD (2004) RDX biodegradation column study: comparison of electron donors for biologically induced reductive transformation in groundwater. J Hazard Mater 112:45–54
Kaeberlein T, Lewis K, Epstein SS (2002) Isolating “Uncultivable” microorganisms in pure culture in a simulated natural environment. Science 296:1127–1129
Kumar S, Tamura K, Jakobsen IB, Nei M (2001) MEGA2: molecular evolutionary genetics analysis software. Bioinformatics 17:1244–1245
Lotufo GR, Farrar JD, Inouye LS, Bridges TS, Ringelberg DB (2001) Toxicity of sediment-associated nitroaromatic and cyclonitramine compounds to benthic invertebrates. Environ Toxicol Chem 20:1762–1771
McCormick NG, Cornell JH, Kaplan AM (1981) Biodegradation of hexahydro-1,3,5-trinitro-1,3,5-triazine. Appl Environ Microbiol 42:817–823
Muyzer G, De Waal EC, Uitterlinden AG (1993) Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl Environ Microbiol 59:695–700
Patel P, Callow ME, Joint I, Callow JA (2003) Specificity in the settlement—modifying response of bacterial biofilms towards zoospores of the marine alga Enteromorpha. Environ Microbiol 5:338–349
Pennington JC, Jenkins TF, Ampleman G, Thiboutot S, Brannon JM, Lynch J, Ranney TA, Stark JA, Walsh ME, Lewis J, Hayes CA, Mirecki JE, Hewitt AD, Perron N, Lambert D, Clausen J, Delfino JJ (2002) Distribution and fate of energetics on DoD test and training ranges: interim report 1 ERDC TR-02-8. US Army Corps of Engineers, Washington, D.C.
Rappé MS, Giovannoni SJ (2003) The uncultured microbial majority. Annu Rev Microbiol 57:369–394
Robidoux PY, Svendsen C, Caumartin J, Hawari J, Ampleman G, Thiboutot S, Weeks JM, Sunahara GI (2000) Chronic toxicity of energetic compounds in soil determined using the earthworm (Eisenia andrei) reproduction test. Environ Toxicol Chem 19:1764–1773
Suzuki MT, Giovannoni SJ (1996) Bias caused by template annealing in the amplification of mixtures of 16S rRNA genes by PCR. Appl Environ Microbiol 62:625–630
Waisner S, Hansen L, Fredrickson H, Nestler C, Zappi M, Banerji S, Bajpai R (2002) Biodegradation of RDX within soil-water slurries using a combination of differing redox incubation conditions. J Hazard Mater 95:91–106
Yinon J (1990) Toxicity and metabolism of explosives. CRC, Ann Arbor
Zhao J-S, Greer CW, Thiboutot S, Ampleman G, Hawari J (2004) Biodegradation of cyclic nitramine explosives hexahydro-1,3,5-trinitro-1,3,5-triazine and octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine in cold marine sediment under anaerobic and oligotrophic conditions. Can J Microbiol 50:91–96
Zhao J-S, Spain J, Thiboutot S, Ampleman G, Greer C, Hawari J (2004) Phylogeny of cyclic nitramine-degrading psychrophilic bacteria in marine sediment and their potential role in the natural attenuation of explosives. FEMS Microbiol Ecol 49:349–357
Acknowledgements
The sampling of surface sediments of offshore UXO fields was conducted by Drs. Michael T. Montgomery and Christopher L. Osburn (Marine Biogeochemistry Section, United States Naval Research Laboratory), Washington, D.C. We thank Louise Paquet, Annamaria Halasz, and Alain Corriveau for technical assistance. Manish Bhatt thanks the Natural Sciences and Engineering Research Council (NSERC) for a fellowship. Funding for this research was provided by the Office of Naval Research (ONR, United States Navy) (Grant N00014-03-1-0269) and the United States DoD/DoE/EPA Strategic Environmental Research and Development Program (SERDP CP-1431).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bhatt, M., Zhao, JS., Monteil-Rivera, F. et al. Biodegradation of cyclic nitramines by tropical marine sediment bacteria. J IND MICROBIOL BIOTECHNOL 32, 261–267 (2005). https://doi.org/10.1007/s10295-005-0239-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-005-0239-9