Abstract
The production and purification of a calcium-dependent protease by Bacillus cereus BG1 were studied. The production of the protease was found to depend specifically on the calcium concentration in the culture medium. This suggests that this metal ion is essential for the induction of protease production and/or stabilisation of the enzyme after synthesis. The calcium requirement is highly specific since other metal ions (such as Mg2+ and Ba2+, which both activate the enzyme) are not able to induce protease production. The most appropriate medium for growth and protease production comprises (g L−1) starch 5, CaCl2 2, yeast extract 2, K2HPO4 0.2 and KH2PO4 0.2. The protease of BG1 strain was purified to homogeneity by ultrafiltration, heat treatment, gel filtration on Sephacryl S-200, ion exchange chromatography on DEAE-cellulose and, finally, a second gel filtration on Sephacryl S-200, with a 39-fold increase in specific activity and 23% recovery. The molecular weight was estimated to be 34 kDa on SDS-PAGE. The optimum temperature and pH of the purified enzyme were determined to be 60°C and 8.0, respectively, in 100 mM Tris-HCl buffer + 2 mM CaCl2.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Proteases constitute one of the most important groups of industrial enzymes. They have diverse applications in a wide variety of industries such as the detergent, food, agrochemical and pharmaceutical industries [12, 15, 26].
Bacteria, moulds and yeasts are some of the microorganisms that produce proteases. Among bacteria, Bacillus strains are the most important producers of commercial proteases [11, 22].
Protease production depends on many factors. For instance, the growth rate of the culture and the composition of the medium play important roles. Indeed, carbon and nitrogen sources were considered determinant factors [6, 10]. Several studies have reported that proteins and peptides are necessary for effective protease production, while glucose repressed protease formation [4, 5, 7]. However, some works reported better protease synthesis in the presence of glucose as carbon source [8, 20]. Gessesse and Gashe [8] showed that the alkaliphilic strain of microbacterium isolated from an alkaline soda lake is insensitive to repression by glucose. Other medium compounds, such as metal ions and phosphorus source may also affect the amount of enzyme formation. Secades et al. [23] reported the production of a calcium-induced metalloprotease from the fish pathogen Flavobacterium psychrophilum. The enzyme was detected only when the strain was cultivated in the presence of calcium.
Several alkaline proteases from the genus Bacillus have been purified and characterised [5, 24]. However, few reports are available on proteolytic enzymes from Bacillus cereus. Hayano et al. [13] reported a neutral metalloprotease from B. cereus. The DNA sequence of the B. cereus thermolysin-like enzyme has a high degree of homology with the sequences of other metalloproteases, especially with the gene for neutral protease from Bacillus thuringiensis (97% homology over 945 bp [3]).
In a previous search for alkaline-protease-producing strains, which have potential industrial applications, B. cereus BG1 strain, producing an organic solvent-stable protease, was isolated from an activated sludge reactor treating fishing industry wastewater [9]. The protease activity and thermal stability were dependent on calcium ions. At 60°C, Ca2+ (2 mM) stimulated the protease activity by 500%. In the presence of 10 mM Ca2+, the enzyme retained 100, 93 and 26% of its initial activity after being heated for 15 min at 55, 60 and 70°C, respectively. However, the enzyme was completely inactivated when incubated at 55°C for 15 min in the absence of calcium.
In this paper, we describe the production and purification of a calcium-dependant protease from B. cereus BG1.
Materials and methods
Bacterial strain
The strain used in this study was isolated from an activated sludge reactor treating fishing industry wastewater. It was identified as B. cereus BG1.
Cultivation and media
The medium used for isolation of protease-producing strains was composed of (g L−1): peptone, 5; yeast extract, 3; skimmed milk, 250 ml and bacteriological agar, 12. Inocula were routinely grown in Luria-Bertani (LB) broth medium (g L−1): peptone, 10; yeast extract, 5 and NaCl, 5 [21]. The initial medium for protease production (M1) was composed of (g L−1): carbon source, 10; CaCl2, 1; yeast extract, 2; K2HPO4, 0.1; and KH2PO4 0.1, pH 8.0. Media were autoclaved at 120°C for 20 min.
Cultivations were performed on a rotatory shaker (200 rev min−1) for 48 h at 37°C, in 250 mL conical flasks with a working volume of 25 mL. The cultures were centrifuged and the supernatants were used for estimation of proteolytic activity.
Cell growth determination
The growth of the microorganism was determined by measuring optical density at 600 nm. All experiments were carried out in duplicate and repeated at least twice.
Protease purification
The culture broth was centrifuged and the cell-free supernatant (41 mL at 4,000 U mL−1) was concentrated to 2 ml by ultrafiltration using a 10-kDa membrane.
The concentrated enzyme was then heat-treated in a water bath at 60°C for 30 min in the presence of 10 mM CaCl2. Insoluble material was separated by centrifugation at 13,000 g for 10 min. The supernatant was then subjected to gel filtration on a Sephacryl S-200 column (3×100 cm) pre-equilibrated with 25 mM Tris-HCl (pH 8.0), 2 mM CaCl2 and 0.025% Triton X-100 (buffer A). Fractions of 4 ml each were collected at a rate flow of 30 mL h−1 and analysed for protease activity and protein concentration.
Fractions showing protease activities were pooled (56 mL) from the Sephacryl S-200 column and applied to a DEAE-cellulose column (3×25 cm) pre-equilibrated with 25 mM Tris-HCl (pH 8.5), 2 mM CaCl2 (buffer B). The unadsorbed protein fractions were eluted with buffer B (150 mL). The chromatography was carried out at a flow rate of 45 mL h−1. Fractions containing protease activity were pooled, concentrated by ultrafiltration to 2.3 mL as described previously and applied to a second Sephacryl S-200 column (2×160 cm) equilibrated with buffer A, and eluted at a flow rate of 0.46 mL min−1. Fractions of 4.5 mL were collected.
All the purification steps were conducted at temperatures not exceeding 4°C.
Polyacrylamide gel electrophoresis
Sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) was carried out for the determination of purity and molecular weight of the enzyme as described by Laemmli [17]. The molecular weight of the enzyme was calculated using a low molecular weight calibration kit (Lot 100K9314: Sigma, St Louis, Mo.) as markers. The molecular mass markers used were: bovine serum albumin (BSA; 66,000), egg white ovalbumin (45,000), glyceraldehyde-3-P dehydrogenase (36,000), bovine carbonic anhydrase (29,000), bovine trypsinogen (24,000), soybean trypsin inhibitor (20,100) and bovine α-lactalbumin (14,200). Protein bands were visualised after staining with Coomassie Brilliant blue R250.
Influence of pH and temperature on protease activity
The pH and temperature optima of the purified enzyme were determined with casein as substrate. The optimum pH for activity was studied in the range of 6–12 at 60°C. The effect of temperature on protease activity was studied from 50°C to 75°C at pH 8.0 in the presence of 2 mM CaCl2.
Assay of proteolytic activity
Protease activity was measured by the method of Kembhavi et al. [16] using casein as substrate. A 0.5 mL aliquot of the culture supernatant, suitably diluted, was mixed with 0.5 mL 100 mM Tris-HCl (pH 8.0) + 2 mM Ca2+ containing 1% casein, and incubated for 15 min at 60°C. The reaction was stopped by addition of 0.5 mL 20% trichloroacetic acid. The mixture was allowed to stand at room temperature for 15 min and then centrifuged at 10,000×g for 15 min to remove the precipitate. The absorbance was measured at 280 nm. A standard curve was generated using solutions of 0–50 mg L−1 tyrosine. One unit of protease activity was defined as the amount of enzyme required to liberate 1 μg tyrosine per millilitre in 1 min under the experimental conditions used.
Protein concentration
Protein concentration was determined by the method of Bradford [1] with BSA as a standard.
Results and discussion
Effect of different carbon sources on protease synthesis
Protease production was first tested in medium M1 containing different carbon sources at a concentration of 10 g L−1. B. cereus BG1 strain grew well in media containing maltose, glucose and starch, and the proteolytic activity varied with each carbon source. As shown in Table 1, the best carbon source for protease production was starch (3,393 U mL−1), followed by maltose (3,062 U mL−1). In a similar study, Johnvesly and Naik [14] showed that soluble starch was the best carbon source for protease production by Bacillus sp. JB-99.
Enzyme production was significantly low (1,094 and 1,283 U mL−1) when B. cereus strain was grown on lactose and saccharose, respectively, and was the same as that of the control without carbon source.
In the present study, glucose was found to be a good carbon source for the growth of the BG1 strain but this substrate repressed the synthesis of the enzyme. Johnvesly and Naik [14] also reported that 1% glucose completely repressed the synthesis of alkaline protease by Bacillus sp. JB-99. However, other studies showed better protease synthesis in the presence of glucose as carbon source [20].
Since starch was the best carbon source, the effect of various concentrations of starch on protease production was studied. The optimum concentration of starch for protease production was of 5 g L−1 ; further additions of starch decreased the level of protease while biomass increased (Table 2).
Effect of various nitrogen sources on protease synthesis
In general, both organic and inorganic nitrogen were used efficiently by Bacillus sp. for protease production. In the present study, different organic (casein peptone, yeast extract, casein, soy peptone and urea) and inorganic (ammonium sulphate) nitrogen sources, at a concentration of 2 g L−1, were tested in a medium containing starch at 5 g L−1 as carbon source. As shown in Table 3, the best nitrogen source for protease production by this strain was yeast extract (3,720 U mL−1) followed by casein peptone (3,542 U mL−1). These results also showed that the strain could produce protease efficiently from casein as nitrogen source (3,076 U mL−1), indicating that the strain can obtain its nitrogen requirement directly from undigested proteins. Enzyme production was significantly lower with ammonium sulphate and urea as nitrogen sources. This result differs from the findings of Do Nascimento and Martins [2], who reported maximum enzyme activity by thermophilic Bacillus sp. SMIA-2 with ammonium nitrate as inorganic nitrogen source, and found that protease production was repressed by organic nitrogen sources.
Based on these observations, yeast extract was selected and its various concentrations were tested for the production of protease. As shown in Table 4, the enrichment of growth medium with yeast extract enhanced significantly both bacterial growth and the protease production. Maximum activity was achieved at a concentration of 2 g L−1 (3,755 U mL−1).
Effect of metal ions on protease synthesis
Several studies have shown that divalent cations can stimulate or inhibit enzyme formation in microorganisms. Mabrouk et al. [19], reported that Ca2+ at 0.07% markedly affected protease production by Bacillus licheniformis ATCC 21415 and caused 26.6% increase in activity over the control.
The effects of addition of some inorganic salts to the culture at a concentration of 10 mM on growth and protease production by B. cereus BG1 are shown in Table 5. When the strain was grown in the absence of metal ions, no protease activity was detected in the culture supernatant, although such a medium yielded a higher concentration of biomass. Among all the inorganic salts tested, only CaCl2 led to a strong increase in protease synthesis. The addition of CaCl2 (10 mM) enhanced protease production to a level of 3,800 U mL−1. Very low levels of activity—about 28 U mL−1—were detected in medium containing MgSO4, although the strain grew well in the presence of this divalent cation. However, with ZnSO4, MnSO4 and CuSO4, growth was inhibited. Therefore, biosynthesis of protease by B. cereus BG1 can be considered calcium-dependent since other metals such as Ba2+ and Mg2+, which stimulated protease activity, were unable to induce protease production.
In a second experiment, the strain was grown in the presence of different concentrations of CaCl2 ranging from 0.05 g L to 10 g L−1. As shown in Table 6, protease production was significantly lower with CaCl2 concentrations up to 0.5 g L−1. Maximum protease induction was observed with 2 g L−1, about 4,036 U mL−1, which was about 16-fold over the medium containing 0.2 g L−1 CaCl2. Beyond 2 g L−1 CaCl2, protease activity decreased while biomass increased. These results clearly indicated that production of the BG1 protease is dependent on the CaCl2 concentration.
Effect of the concentration of K2HPO4 and KH2PO4 on protease production
The amount of phosphorus source was also varied (Table 7). A high level of activity, about 3,710 U mL−1, was detected in medium lacking a phosphorus source. Maximum protease was observed with 0.2 g L−1 K2HPO4 and 0.2 g L−1 KH2PO4. As the phosphorus level increased, the level of protease activity and bacterial growth decreased. Since Ca2+ has a significant stabilising effect on protease, the decrease in protease synthesis could be explained by the lowering of the available Ca2+ in the culture with increasing phosphorus concentration.
Role of calcium in protease production
Minor studies have been conducted to investigate the biological role of calcium in enzyme production. Secades et al. [23] showed that the level of Fpp1 protease produced by F. psychrophilum is specifically dependent on the CaCl2 concentration. We therefore examined three hypotheses that could explain the enhancement of protease synthesis by CaCl2:
-
First hypothesis.
Ca2+ might be required for enzyme secretion.
-
Second hypothesis.
Enzyme production might be induced by calcium.
-
Third hypothesis.
The active conformation of the enzyme might be stabilised by ionic calcium after synthesis.
The first hypothesis seems improbable since no activity was detected within the cells when the strain was grown in a medium lacking CaCl2. Furthermore, no activity was detected in the culture supernatant and within the cells when they were grown in media containing MgCl2. When cultivated in a medium containing CaCl2, more than 88% of the enzyme activity was detected in the culture supernatant. In contrast to our results, Liao and McCallus [18] reported that more than 60% of the enzyme activity was retained within the cells when Pseudomonas aeruginosa CY091 was grown in a medium containing ZnCl2, MgCl2, or MnCl2.
To study hypotheses concerning the induction or stabilisation of the enzyme by CaCl2, additional experiments were conducted to elucidate the role of Ca2+. In the first experiment the time-courses of protease production and growth of B. cereus BG1 were studied. As shown in Fig. 1, B. cereus BG1 grew well in medium supplemented with CaCl2 and reached stationary phase after 15 h. Biosynthesis of protease by the strain appeared to be growth-related, since the activity was detected from early stages in the growth of the microorganism, with values increasing exponentially at the end of the exponential phase, then continuing to increase even during stationary phase. These results differ from those of previous works, which showed that induction of metalloprotease from the fish pathogen F. psychrophilum was only growth related; addition of calcium during the stationary phase did not induce production of the enzyme [23].
Time-course of alkaline protease production and growth of Bacillus cereus BG1 in 100 mL medium consisting of (g L−1): starch 5, CaCl2 2, yeast extract 2, K2HPO4 0.2 and KH2PO4 0.2. Cell growth was measured by absorbance at 600 nm (A600). Protease activity was determined in culture filtrates obtained after removal of cells by centrifugation, as described in Materials and methods. Values are means of three independent experiments. Standard deviations were less than 5%. Arrows Time-points of addition of 2 g L−1 CaCl2 in a subsequent experiment
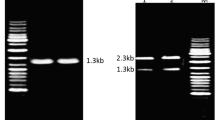
Ca-induction experiments were also performed by incubating the microorganism in a medium composed of (g L−1): starch 5, CaCl2 2, yeast extract 2, K2HPO4 0.1 and KH2PO4 0.1. During the exponential or stationary phases, 2 g L−1 CaCl2 was added to the cultures after 4, 8, 16 and 24 h incubation. Protease production throughout this study was determined after 48 h of growth, which corresponds to the optimum period for enzyme production (Table 8). Furthermore, proteins in the culture supernatants were also analysed by SDS-PAGE (Fig. 2). As shown in Table 8 and Fig. 2, addition of CaCl2 during the exponential and stationary phases resulted in the presence of the BG1 protease in the medium (the molecular weight of the purified protease was estimated to be approximately 34,000 by SDS-PAGE, see Fig. 6). Protease production was improved by 30% when CaCl2 was applied during exponential growth phase (4 h), compared to that obtained with the medium initially supplemented with CaCl2. However, addition of CaCl2 during stationary phase led to a significant reduction in enzyme formation.
Electrophoretic analyses of supernatants from various cultures after acetone treatment. B. cereus was initially grown in medium consisting of (g L−1): starch 5, CaCl2 2, yeast extract 2, K2HPO4 0.1, KH2PO4 0.1. Lanes:1 Molecular weight markers, 2 culture conducted with 2 mM CaCl2 initially; 3–6 cultures to which CaCl2 was added after 4 h (lane 3), 8 h (lane 4), 16 h (lane 5) and 24 h (lane 6); 7 culture conducted in medium lacking CaCl2
No activity was detected when the strain was grown in medium lacking CaCl2. However, a minor band, which migrated in SDS-PAGE to a position corresponding to a molecular weight of 34 kDa, was discernable. Furthermore, protein bands having molecular weight greater than 34 kDa, and other protein bands lower than 34 kDa, which may correspond to degradation products of the BG1 protease, were also observed. Therefore, the hypothesis that protease production is stimulated by calcium seems to be invalidated. From these results, and those showing that the thermoactivity and the thermostability of the enzyme were considerably increased in the presence of CaCl2 [9], it seems likely that CaCl2 is required for maintaining the molecular conformation needed for enzyme activity. On the other hand, enzyme activity inhibited by treatment with metal chelators such as EDTA, was not restored at all by the addition of calcium ions (2–50 mM). This suggests that the tertiary structure of the enzyme was irreversibly destroyed by chelating metal ion(s) in the protein molecule. These findings are in line with several earlier reports showing that the three-dimensional structure of the protease from B. cereus contains four Ca2+ binding sites [25].
Protease purification
In the first step, the culture supernatant obtained by centrifugation (13,000 g for 10 min at 4°C) was concentrated by ultrafiltration using a 10-kDa membrane then heat treated. These two steps resulted in 1.6-fold purification, with a recovery of 68% and a specific activity of 70×103 U mg−1. The heated enzyme was subjected to gel filtration on a Sephacryl S-200 column with buffer A. The elution profiles of protease and proteins from Sephacryl S-200 are shown in Fig. 3. This procedure yielded a single peak of protease activity.
Chromatography on Sephacryl S-200 of the concentrated and treated enzyme preparation. The enzyme preparation was applied to a 3×100 cm column, equilibrated and eluted with 25 mM Tris-HCl (pH 8.0), 2 mM CaCl2 and 0.025% Triton X-100. Fractions (4 mL) collected from the column were assayed for protease activity. Flow rate =0.5 mL min−1
Fractions containing protease activity were pooled, then loaded on a DEAE-cellulose column pre-equilibrated with buffer B. Protease activity appeared in a single peak together with unadsorbed fractions. The elution profile is shown in Fig. 4. Active fractions were pooled then concentrated by ultrafiltration before being applied to a second Sephacryl S-200 gel. The elution profile is shown in Fig. 5.
Elution profile of B. cereus BG1 protease from a DEAE-cellulose column. The column (3×25 cm) was equilibrated with 20 mM Tris-HCl (pH 8.0) and 2 mM CaCl2. The unadsorbed protein fraction was eluted with the same buffer at a flow rate of 0.66 mL min−1. The protease activity of each fraction was determined as described in Materials and methods
Application of active fractions from DEAE-Cellulose to a second Sephacryl S-200 column. The enzyme preparation was applied to a 2×160 cm column, equilibrated and eluted with 25 mM Tris-HCl (pH 8.0), 2 mM CaCl2 and 0.025% Triton X-100. Fractions (4 mL), collected from the column at a flow rate 0.46 mL min−1, were assayed for protease activity
Active fractions eluted from the second Sephacryl S-200 gel filtration were assayed for activity, and fraction 124 was analysed on a 15% SDS-polyacrylamide gel. As shown in Fig. 6, a unique protein band was obtained. The molecular weight of the BG1 protease was estimated to be 34 kDa by SDS-PAGE using molecular weight markers.
The results of the purification procedure are summarised in Table 9. After the final purification step, the protease was purified 39-fold with a recovery of 23% and a specific activity of 1,682×103 U mg−1.
Influence of pH and temperature on enzyme activity
The enzyme was active between pH 6.0 and 9.0, with an optimum at pH 8.0. At pH values 8.5 and 7.0, 75 and 32% activity remained, respectively (Fig. 7). The optimal temperature for the activity of the purified protease was 60°C, when incubated for 15 min at pH 8.0 (Fig. 8).
The thermostability of the purified protease was examined by incubating the enzyme at 60°C for 1 h in the presence of various concentrations of CaCl2. As shown in Fig. 9, the stability of the enzyme was considerably enhanced by Ca2+. The half-life of the purified enzyme at 60°C was determined to be of the order of 23 and 51 min, in the presence of 1 and 2 mM CaCl2, respectively.
Effect of calcium on protease thermostability. Purified enzyme was preincubated at 60°C in the presence of various concentration of CaCl2. Residual activity was determined from 0 to 60 min as described in Materials and methods. The non-heated enzyme was considered as control (100%). Values are means of three independent experiments. Standard deviations were ±2.5% (based on three replicates)
Conclusion
This study described the production and purification of a calcium-dependent protease from B. cereus BG1. Protease activity was detected only when the cells were grown in medium containing CaCl2. The biosynthesis of protease can be said to be absolutely calcium-dependent since no other divalent cation was able to induce enzyme production. The data presented here show that calcium might be required for stabilising the enzyme after synthesis. Further work is needed to confirm this hypothesis.
An optimised induction medium, which supported good growth as well as enzyme production, was formulated and consisted of (g L−1): starch 5, CaCl2 2, yeast extract 2, K2HPO4 0.2 and KH2PO4 0.2.
The enzyme was purified to homogeneity by ultrafiltration, heat treatment, and gel filtration on Sephacryl S-200, DEAE-cellulose ion exchange chromatography, and by a second Sephacryl S-200 gel filtration chromatography. After the final purification step the enzyme was purified 39-fold with a specific activity of 1,682×103 U mg−1 and 23 % recovery. The purified protease preparation was homogenous on SDS-PAGE and its molecular weight was estimated to be 34 kDa. The optimum pH and temperature for the proteolytic activity were pH 8.0 and 60°C, respectively.
References
Bradford M (1976) A rapid and sensitive method for the quantification of microorganism quantities of protein utilizing the principle of dye binding. Anal Biochem 72:248–254
Do Nascimento WCA, Martins MLL (2004) Production and properties of an extracellular protease from thermophilic Bacillus sp. Braz J Microbiol 35:91–96
Donovan WP, Tan Y, Slaney AC (1997) Cloning of the nprA gene for neutral protease A of Bacillus thuringiensis and effect of in vivo deletion of nprA on insecticidal crystal protein. Appl Environ Microbiol 63:2311–2317
Drucker H (1972) Regulation of exocellular protease in Neurospora crassa: induction and repression of enzyme synthesis. J Bacteriol 110:1041–1049
Ferrero MA, Castro GR, Abate CM, Baigori MD, Sineriz F (1996) Thermostable alkaline proteases of Bacillus licheniformis MIR 29: isolation, production and characterization. Appl Microbiol Biotechnol 45:327–332
Frankena J, Koningstein GM, van Verseveld HW, Stouthamer AH (1986) Effect of different limitations in chemostat cultures on growth and production of exocellular protease by Bacillus licheniformis. Appl Microbiol Biotechnol 24:106–112
Fukushima Y, Itoh H, Fukase T, Motai H (1989) Continuous protease production in a carbon-limited chemostat culture by salt tolerant Aspergillus oryzae. Appl Microbiol Biotechnol 30:604–608
Gessesse A, Gashe BA (1997) Production of alkaline protease by an alkaliphilic bacteria isolated from an alkaline soda lake. Biotechnol Lett 19:479–481
Ghorbel B, Sellami-Kamoun A, Nasri M (2003) Stability studies of protease from Bacillus cereus BG1. Enzyme Microb Technol 32:513–518
Giesecke UE, Bierbaum G, Rudde H, Spohn U, Wandrey C (1991) Production of alkaline protease with Bacillus licheniformis in a controlled fed-batch process. Appl Microbiol Biotechnol 35:720–724
Godfrey TA, Reichelt P (1985) Industrial enzymology: the application of enzymes in industry. The Nature Press, London
Gupta R, Beg QK, Lorenz P (2002) Bacterial alkaline proteases: molecular approaches and industrial applications. Appl Microbiol Biotechnol 59:13–32
Hayano K, Takeuchi M, Ichishima E (1987) Characterization of a metalloproteinase component extracted from soil. Biol Fertil Soils 4:179–183
Johnvesly B, Naik GR (2001) Studies on production of thermostable alkaline protease from thermophilic and alkaliphilic Bacillus sp. JB-99 in a chemically defined medium. Process Biochem 37:139–144
Kelly CT, Fogarty WM (1976) Microbial alkaline enzymes. Process Biochem 11:3–9
Kembhavi AA, Kulkarni A, Pant AA (1993) Salt-tolerant and thermostable alkaline protease from Bacillus subtilis NCIM No.64. Appl Biochem Biotechnol 38:83–92
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Liao CHS, McCallus DE (1998) Biochemical and genetic characterization of an extracellular protease from Pseudomonas fluorescens CY091. Appl Environ Microbiol 64:914–921
Mabrouk SS, Hashem AM, El-Shayeb NMA, Ismail AMS, Abdel-Fattah AF (1999) Optimisation of alkaline protease productivity by Bacillus licheniformis ATCC 21415. Bioresour Technol 69:155–159
Mehrotra S, Pandey PK, Gaur R, Darmwal NS (1999) The production of alkaline protease by a Bacillus species isolate. Bioresour Technol 67:201–203
Miller JH (1972) Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y., pp 431–435
Priest FG (1977) Extracellular enzyme synthesis in the genus Bacillus. Bacteriol Rev 41:711–753
Secades P, Alvarez B, Guijarro JA (2001) Purification and characterization of a Psychrophilic, calcium-induced, growth-phase-dependent metalloprotease from the fish pathogen Flavobacterium psychrophilum. Appl Environ Microbiol 67:2436–2444
Singh J, Batra N, Sobti RC (2001) Serine alkaline protease from a newly isolated Bacillus sp. SSR1. Process Biochem 36:781–785
Stark W, Pauptit RA, Wilson KS, Jansonius JN (1992) The structure of neutral protease of Bacillus cereus at 0.2 nm resolution. Eur J Biochem 207:781–791
Zukowski MM (1992) Production of commercially valuable products. In: Doi RH, McGloughlin M (eds) Biology of bacilli: application to industry. Butterworth-Heinemann, London, pp 311–337
Acknowledgements
We are grateful to Mr. A. Hajji from the Engineering School of Sfax for his help with English. This work was funded by the “Ministère de la Recherche Scientifique, de la Technologie et du Développement des Compétences, Tunisie”.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ghorbel-Frikha, B., Sellami-Kamoun, A., Fakhfakh, N. et al. Production and purification of a calcium-dependent protease from Bacillus cereus BG1. J IND MICROBIOL BIOTECHNOL 32, 186–194 (2005). https://doi.org/10.1007/s10295-005-0228-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-005-0228-z