Abstract
Using genetic engineering, the Vitreoscilla (bacterial) hemoglobin gene (vgb) was integrated stably into the chromosomes of Pseudomonas aeruginosa and Burkholderia sp. strain DNT. This was done for both wild type vgb and two site-directed mutants of vgb that produce Vitreoscilla hemoglobin (VHb) with lowered oxygen affinities; in all cases functional VHb was expressed. Similar to previous results, the wild type VHb improved growth for both species and degradation of 2,4-dinitrotoluene (Burkholderia sp.) or benzoic acid (P. aeruginosa) under both normal and low aeration conditions. Both mutant vgbs enhanced these parameters compared to wild type vgb, and the improvement was seen in both species. The enhancements were generally greater at low aeration than at normal aeration. The results demonstrate the possibility that the positive effects provided by VHb may be augmented by protein engineering.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
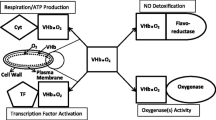
The Vitreoscilla hemoglobin (VHb) of the Gram-negative aerobe Vitreoscilla was the first bacterial hemoglobin discovered [28] and remains one of the best characterized of this growing class of proteins [8]. It was originally proposed that its function is to sequester oxygen, particularly when it is at low concentrations, and deliver it to the terminal respiratory oxidase, thus enhancing oxidative phosphorylation [29]. There is now direct evidence to support this role [2, 18, 20]. VHb may also have other functions. For example, like the related bacterial flavohemoproteins [23], it appears to be involved in detoxification of NO [10]. In addition, VHb may function to supply O2 to oxygenases [12, 14].
The Vitreoscilla (bacterial) hemoglobin gene (vgb) has been used to engineer heterologous bacteria (and other organisms) to improve their growth and other useful properties [22]. One of these applications is transformation of vgb into bioremediating bacteria, in which the presence of vgb/VHb sometimes results in enhancement in growth and metabolism of aromatic compounds; benzoic acid, 2-chlorobenzoic acid, and 2,4-dinitrotoluene have been tested [3, 7, 14, 17, 19, 26, 27]. In most of these studies vgb has been introduced on a plasmid, but we have also been able to stably integrate vgb into the host chromosome using the transposon-conjugation-based system developed by Timmis and coworkers [4, 9], in order to produce more genetically stable strains that may be more useful in practical applications. There is evidence to suggest that, in these recombinant bacteria, VHb has positive effects on both the respiratory chain [18] and the oxygenases that are directly involved in aromatic catabolism [7, 12, 14]. In the latter case, it may be that VHb stimulates oxygenases by direct delivery of oxygen.
In the course of our study of VHb structure and function, we have produced a number of site-directed mutants of the protein; some of these change the oxygen affinity of the protein [6]. It is possible that certain of these alterations could enhance O2 delivery to the terminal respiratory oxidase or the aromatic pathway oxygenases. We thus investigated two of these mutants for such enhancement. In addition, we combined this work with stable integration of the vgbs into host cell chromosomes in order to investigate the potential advantages of using both approaches together to produce even more useful strains for practical applications. The results suggest that engineering of VHb may augment its utility in enhancing bioremediation of aromatic compounds.
Materials and methods
Strains and plasmids
Pseudomonas aeruginosa (strain NRRL B-771) was obtained from L.K. Nakamura at the USDA bacterial culture collection (Peoria, Ill.); Burkholderia sp. strain DNT [21] was a gift from S. Nishino and J. Spain (Tyndall Air Force Base, Fla.). Escherichia coli strain SM10(λ pir) [16] was a gift from K.N. Timmis (GBF-National Research Center for Biotechnology, Braunschweig, Germany). Plasmids pUC18: Not and pUTmini-Tn5 Cm [4, 9] were also gifts from K.N. Timmis. Strain PaJC, which contains wild type vgb integrated into the chromosome of P. aeruginosa strain NRRL B-771, was constructed previously [3]. pBluescript-based plasmids pNKD1 (containing wild type vgb) and plasmids pNKD5 and pNKD6 (encoding VHb mutants Y126H and Y126F, respectively) were gifts from K. Dikshit (Institute of Microbial Technology, Chandigarh, India). The vgb/VHb mutants from plasmids pNKD5 and pNKD6 are denoted here as mutants 5 and 6, respectively.
Cloning of vgb variants and transfer to heterologous species
The vgbs were excised from the appropriate pNKD plasmid, gel purified, and cloned into pUTmini-Tn5 Cm, via a pUC18: Not intermediate step according to the method of Herrero et al. [9]. After transformation of each resulting recombinant plasmid into E. coli SM10(λ pir) [16], the recombinant pUTmini-Tn5 Cm constructs were transferred into Burkholderia sp. strain DNT and P. aeruginosa by conjugation [9]. P. aeruginosa and Burkholderia transformants were selected by growth on 400 μg mL−1 chloramphenicol (kills both untransformed recipient strains) and 50 μg mL−1 kanamycin (both recipient strains have natural resistance, but this kills donor cells). The Burkholderia transformants with wild type vgb, and mutant vgbs 5 and 6 are denoted BsYS, BsYS5, and BsYS6, respectively; the P. aeruginosa strains with wild type vgb, and mutant vgbs 5 and 6 are denoted PaJC, PaYS5, and PaYS6, respectively. The untransformed Burkholderia and P. aeruginosa hosts are denoted BsWT and PaWT, respectively.
Chromosomal DNA was isolated from untransformed Burkholderia sp. strain DNT and P. aeruginosa, as well as the transformants, using Qiagen DNeasy tissue kits (Qiagen, Valencia, Calif.). These DNA samples were used as templates for PCR using primers specific for a 300 bp internal region of vgb [3]. After electrophoresis in agarose gels, these PCR products were confirmed by Southern hybridization using the Amersham ECL system (Amersham Biosciences, Piscataway, N.J.) with vgb as the probe. The same eight strains were grown in tryptic soy broth (TSB) for about 18 h at 200 rpm and 30°C, and cells were harvested for detection of VHb by whole cell CO-difference spectra [5]; the extinction coefficient E419-436 nm = 274 mM−1 cm−1 for wild type VHb was used to quantitate VHb from these spectra.
Growth and biodegradation experiments
Burkholderia strains were grown in 5 mL TSB overnight at 30°C and 200 rpm. Each overnight culture was diluted with fresh TSB to OD600 =0.5 and 100 μL added to a 250 mL Erlenmeyer flask containing TSB plus 200 ppm 2,4-dinitrotoluene (DNT). For normal aeration, the flask contained 50 mL medium and incubation was at 30°C and 200 rpm; for hypoxic conditions the flask contained 200 mL medium and incubation was at 50 rpm and 30°C.
The experiments for the P. aeruginosa strains were set up similarly except that the overnight TSB culture was diluted to OD600 =0.5 with M9 medium, pH 7.4 [15], 100 μL of which was then inoculated into M9 containing 1,000 ppm benzoic acid (BA). The BA was adjusted to pH 7.4 before sterile filtering and addition to the M9.
For all strains, 1–2 mL samples were taken periodically for OD600 measurements (samples diluted as necessary with fresh medium to keep the measured absorbance below 0.5). At the same time samples were taken for viable cell counts (on tryptic soy agar plates after serial dilution) and HPLC analysis of BA and DNT levels. HPLC samples (500 μL) were clarified by centrifugation, and the clarified supernatants stored at −20°C until analyzed. HPLC was performed with a Varian Star Chromatography ISO 9001 system (Varian, Palo Alto, Calif.) using a Spherisorb C18 90A reverse phase column (Waters, Milford, Mass.) eluted with 50% acetonitrile and 50% aqueous trifluoroacetic acid. Both compounds were detected by absorbance at 254 nm. For quantitation of DNT and BA levels, standard curves of peak area versus concentration were constructed for DNT from 0 to 200 ppm and for BA from 0 to 1,000 ppm. These were linear over the entire concentration range in each case.
Results
Confirmation of strains and VHb expression
The presence of the wild type and mutant vgbs in the P. aeruginosa and Burkholderia transformants was confirmed by PCR of chromosomal DNA from the transformants and untransformed host strains. Strain PaJC has previously been proved to bear vgb [3]. For each of the remaining transformants, but for neither untransformed host, the expected 300 bp vgb fragment could be amplified. Southern hybridization using vgb as a probe confirmed that the 300 bp amplified fragments were in fact from vgb and that no vgb-hybridizing sequences could be amplified from either host strain.
Whole cell CO-difference spectra of all strains showed that neither host strain but all transformant strains bearing vgb also expressed VHb (Fig. 1). The levels of VHb expressed (stationary phase; average of three measurements, with standard deviations in parentheses) were 9.6 (0.8), 18.8 (0.9), and 19.7 (1.3) nmol g−1 wet weight of cells for BsYS, BsYS5, and BsYS6, respectively; and 8.8 (0.9), 11.8 (0.5), and 11.8 (0.7) nmol g−1 wet weight of cells for PaJC, PaYS5, and PaYS6, respectively. These levels are similar to those measured previously in P. aeruginosa (8.8 nmol g−1) and Burkholderia (8–10 nmol g−1) transformed with vgb [3, 19]. For Burkholderia, apparent levels of the two mutant VHbs were about twice that of the wild type; for P. aeruginosa, the apparent levels of the mutant VHbs were about one-third higher than that of the wild type.
Whole cell CO-difference spectra (400–500 nm, x-axis) of the eight strains used in this research. a–d Pseudomonas aeruginosa strains PaWT, PaJC, PaYS5, and PaYS6, respectively; e–h Burkholderia strains BsWT, BsYS, BsYS5, and BsYS6, respectively. Vitreoscilla hemoglobin (VHb) gives a characteristic peak at 419 nm and trough at 435 nm in these spectra. y-axis units are absorbance units; horizontal line in each spectrum is the baseline
When Burkholderia strains were compared regarding growth in medium containing DNT, the strain bearing wild type vgb outgrew the untransformed strain regarding both maximum OD600 and maximum viable cells under both normal aeration and hypoxic conditions. The vgb-bearing strain also degraded DNT faster, and its advantages regarding all three parameters were greatest under hypoxic conditions (Table 1). These results parallel those we have seen previously with Burkholderia sp. strain DNT bearing vgb on a plasmid present at ~15 copies/cell [17, 19]. Similar results were observed for untransformed P. aeruginosa versus P. aeruginosa bearing wild type vgb grown in medium containing BA (Table 1), again consistent with what we have observed with the same two strains in a previous study [3].
When the three Burkholderia transformants were compared regarding growth at both normal and low aeration in medium with DNT, there was a slight advantage regarding OD600 of the strains bearing the mutant vgbs, but only at low aeration; viability was increased for the mutants at both aerations, but more so at low aeration. Very similar results were seen for the two mutant vgb-bearing P. aeruginosa strains compared with their wild type vgb counterpart grown in medium with BA (Table 2).
Regarding DNT degradation, the two mutant vgb-bearing Burkholderia strains were essentially identical to their wild type vgb counterpart at normal aeration, but had an advantage over the wild type vgb-bearing strain at low aeration (Fig. 2). In the case of the P. aeruginosa strains, there were generally advantages in BA degradation for both mutant vgb-bearing strains compared with the wild type vgb counterpart, and these advantages were similar at normal and low aeration (Fig. 3).
Discussion
The two mutant VHbs used in this study have Tyr126 changed to His in PaYS5 and BsYS5 or Phe in PaYS6 and BsYS6. Tyr126 is located in the middle of the H-helix and may be involved in positioning the proximal heme iron ligand, His85 (F8) via a hydrogen bond involving the Tyr OH group [24]. The Phe change will result in a side chain of almost identical size and properties except for the inability to form this hydrogen bond; if the His in this position does form a hydrogen bond it would likely have a different orientation than in the native protein. This is the probable mechanism by which both mutants affect oxygen binding, and in both cases it is a decrease in affinity (see below).
The simplest explanation for the increased growth and aromatic compound degradation observed in strains bearing the two mutant VHbs is the increased VHb levels in these strains. These increased levels of VHb could produce the observed increases as a general correlation between VHb levels and positive effects on growth and metabolism has been found in a variety of organisms [22]. Tsai et al. [25] found in recombinant E. coli that an increase in VHb levels from 150 to 360 nmol g−1 wet weight increased the maximum cell mass by about 20% but did not change the maximum growth rate. Positive effects on growth and bioremediation at VHb levels of 8–10 nmol g−1 wet weight were observed for both P. aeruginosa and Burkholderia sp. strain DNT [3, 19]; these levels are the same as those observed for wild type VHb in this study. However, Chung et al. [3] also found that VHb levels of less than 1 nmol g−1 wet weight in recombinant Burkholderia cepacia were insufficient to provide positive effects.
It is possible that the increase in the levels of the mutant VHbs is due to their fortuitous integration into a chromosomal region that supports higher gene expression. However, this is unlikely to have occurred for all four mutant transformants but not for the wild type vgb, as transposition in the pUTminiTn5 system has been shown (in P. putida) to occur at different chromosomal sites in each transformation [9]. It is also possible that the higher mutant VHb levels are due to greater inherent stability compared with wild type VHb, although we have, as yet, no evidence for this, and what the basis for this stability might be is unclear. VHb levels in all strains were estimated using the extinction coefficient for wild type VHb; although the extinction coefficients for the mutant VHbs could differ by a few percent, this will not explain the large differences observed, especially for the Burkholderia strains. These VHb levels were measured for a single time point for cells grown in TSB, and it is not yet known how these levels might differ at different phases of growth, or in TSB plus DNT or M9 plus BA.
Cellular localization studies indicate that VHb is concentrated adjacent to the cytoplasmic side of the plasma membrane in close proximity to the respiratory terminal oxidases [20]. Further, there is evidence that VHb can bind to the cytochrome bo terminal oxidase [20], specifically to the oxidase subunit in which O2 reduction occurs [18]. These data support its proposed role of sequestering O2 and delivering it to the terminal oxidase, thus enhancing oxidative phosphorylation and growth, particularly at low O2. As mentioned above, both mutant VHbs studied here have lower oxygen affinities than wild type VHb. When we measured wild type and the two mutant VHbs together, the Kd values found were 4.5, 5.9, and 12.4 μM for the wild type, mutants 5 and 6 VHbs, respectively (S. Verma, personal communication). The mutants would still be able to saturate with oxygen at low O2 concentrations (O2 solubility at 30°C, for example, is 235 μM), but would likely function better than wild type VHb at unloading O2 to terminal oxidases—the cytochrome bo oxidase of E. coli, for example, has a Km for O2 of 2.9 μM [11]. A similar effect might occur with oxygenases in pathways of aromatic compound catabolism [12, 14], with the mutant VHbs better able than wild type VHb to unload oxygen to them. Such oxygenases are invariant in oxidative pathways for aromatic catabolism in aerobic organisms such as Burkholderia and P. aeruginosa [13] and any enhancement in their function could, in addition to the direct effect of degrading an aromatic compound faster, lead to better growth as the consequent metabolites are eventually used for oxidative phosphorylation.
In agreement with the above is that the two mutant hemoglobins have nearly identical effects on growth (on the basis of both viable cells and OD600) in both species, and in both cases the benefits were better at low aeration. However, the patterns are less consistent regarding enhancement of DNT and BA degradation: there are interspecies differences regarding which mutant is more effective and how the enhancement varies at low versus regular aeration (Figs. 2, 3). One possibility for the interspecies variations are differences in binding affinities for the VHbs and Kd values for O2 of the terminal respiratory oxidases or the aromatic catabolic oxygenases. It is possible that the amino acid changes in VHb mutants 5 and 6 have effects in addition to those on oxygen affinity. As mentioned above, VHb interacts directly with the terminal respiratory oxidases of E. coli, Vitreoscilla, and P. aeruginosa [18, 20] and with the first oxygenase in the DNT degradation pathway in Burkholderia sp. strain DNT [12, 14]. It is possible that mutants 5 and 6 have altered binding to one or more of these proteins in such a way that also contributes to their enhanced effects.
Despite the lack of complete understanding of how the two VHb mutants exert their positive effects on growth and aromatic catabolism, the positive effects occurred in two different species with two different aromatic compounds. This suggests that site-directed mutagenesis of vgb may be of general use in enhancing the positive effects of VHb regarding bioremediation and other useful processes in heterologous hosts. In addition, random error-prone PCR mediated mutagenesis of vgb has been used to select VHb variants with enhanced growth stimulating properties in E. coli, although none of these mutants involved the same amino acids as ours [1]. However generated, the mutants will have to be screened to match the species they will be used with and the metabolic role they will be expected to play in each process or application.
References
Andersson CIJ, Holmberg N, Farres J, Bailey JE, Bulow L, Kallio PT (2000) Error-prone PCR of Vitreoscilla hemoglobin (VHb) to support the growth of microaerobic Escherichia coli. Biotechnol Bioeng 70:446–455
Chen R, Bailey JE (1994) Energetic effect of Vitreoscilla hemoglobin expression in Escherichia coli: an on-line 31 P NMR and saturation transfer study. Biotechnol Prog 10:360–364
Chung JW, Webster DA, Pagilla KR, Stark BC (2001) Chromosomal integration of the Vitreoscilla hemoglobin gene in Burkholderia and Pseudomonas for the purpose of producing stable engineered strains with enhanced bioremediating ability. J Ind Microbiol Biotechnol 27:27–33
De Lorenzo V, Herrero M, Jakubzik U, Timmis KN (1990) Mini-Tn 5 transposon derivatives for insertion mutagenesis, promoter probing, and chromosomal insertion of cloned DNA in Gram-negative eubacteria. J Bacteriol 172:6568–6572
Dikshit KL, Webster DA (1988) Cloning, characterization and expression of the bacterial globin gene from Vitreoscilla in Escherichia coli. Gene 70:377–386
Dikshit KL, Orii Y, Navani N, Patel S, Huang H-Y, Stark BC, Webster DA (1998) Site-directed mutagenesis of bacterial hemoglobin: the role of glutamine (E7) in oxygen-binding in the distal heme pocket. Arch Biochem Biophys 349:161–166
Fish PA, Webster DA, Stark BC (2000) Vitreoscilla hemoglobin enhances the first step in 2,4-dinitrotoluene degradation in vitro and at low aeration in vivo. J Mol Catal B 9:75–82
Hardison RC (1996) A brief history of hemoglobins: plant, animal, protist, and bacteria. Proc Natl Acad Sci USA 93:5675–5679
Herrero M, de Lorenzo V, Timmis KN (1990) Transposon vectors containing non-antibiotic resistance selection markers for cloning and stable chromosomal insertion of foreign genes in Gram-negative bacteria. J Bacteriol 172:6557–6567
Kaur R, Pathania R, Sharma V, Mande SC, Dikshit KL (2002) Chimeric Vitreoscilla hemoglobin (VHb) carrying a flavoreductase domain relieves nitrosative stress in Escherichia coli: new insight into the functional role of VHb. Appl Environ Microbiol 68:152–160
Kita K, Konishi K, Anraku Y (1984) Terminal oxidases of Escherichia coli aerobic respiratory chain II. Purification and properties of cytochrome b558-d complex from cells grown with limited oxygen and evidence of branched electron-carrying systems. J Biol Chem 259:3375–3381
Lee SY, Stark BC, Webster DA (2004) Structure-function studies of the Vitreoscilla hemoglobin D-region. Biochem Biophys Res Commun 316:1101–1106
Lin ECC (1999) Regulation of fermentation and respiration. In: Lengeler JW, Drews G, Schlegel HG (eds) Biology of the prokaryotes. Blackwell, New York, pp 524–537
Lin JM, Stark BC, Webster DA (2003) Effects of Vitreoscilla hemoglobin on the 2,4-dinitrotoluene (DNT) dioxygenase activity of Burkholderia and on DNT degradation in two phase bioreactors. J Ind Microbiol Biotechnol 30:362–368
Miller JP (1972) Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
Miller VL, Mekalanos JJ (1988) A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J Bacteriol 170:2575–2583
Nasr MA, Hwang KW, Akbas M, Webster DA, Stark BC (2001) Effects of culture conditions on enhancement of 2,4-dinitrotoluene degradation by Burkholderia engineered with the Vitreoscilla hemoglobin gene. Biotechnol Prog 17:359–361
Park KW, Kim KJ, Howard AJ, Stark BC, Webster DA (2002) Vitreoscilla hemoglobin binds to subunit I of cytochrome bo ubiquinol oxidases. J Biol Chem 277:33334–33337
Patel SM, Stark BC, Hwang KW, Dikshit KL, Webster DA (2000) Cloning and expression of the Vitreoscilla hemoglobin gene in Burkholderia sp. strain DNT for enhancement of 2,4-dinitrotoluene degradation. Biotechnol Prog 16:26–30
Ramandeep, Hwang KW, Raje M, Kim KJ, Stark BC, Dikshit KL, Webster DA (2001) Vitreoscilla hemoglobin: intracellular localization and binding to membranes. J Biol Chem 276:24781–24789
Spanggord RJ, Spain JC, Nishino SF, Mortelmans KE (1991) Biodegradation of 2,4-dinitrotoluene by a Pseudomonas sp. Appl Environ Microbiol 57:3200–3205
Stark BC, Webster DA, Dikshit KL (1999) Vitreoscilla hemoglobin: molecular biology, biochemistry, and practical applications. Recent Res Dev Biotechnol Bioeng 2:155–174
Stevanin TM, Ioannidis N, Mills CE, Kim SO, Hughes MN, Poole RK (2000) Flavohemoglobin Hmp affords inducible protection for Escherichia coli respiration, catalyzed by cytochromes bo or bd, from nitric oxide. J Biol Chem 275:35868–35875
Tarricone C, Galizzi A, Coda P, Ascenzi P, Bolognesi M (1997) Unusual structure of the oxygen-binding site in the dimeric bacterial hemoglobin from Vitreoscilla sp. Structure 5:497–507
Tsai PS, Hatzimanikatis V, Bailey JE (1996) Effect of Vitreoscilla hemoglobin dosage on microaerobic Escherichia coli carbon and energy metabolism. Biotechnol Bioeng 49:139–150
Urgun-Demirtas M, Pagilla KR, Stark BC, Webster DA (2003) Biodegradation of 2-chlorobenzoate by recombinant Burkholderia cepacia expressing Vitreoscilla hemoglobin under variable levels of oxygen availability. Biodegradation 14:357–365
Urgun-Demirtas M, Pagilla KR, Stark BC (2005) 2-Chlorobenzoate biodegradation by recombinant Burkholderia cepacia under hypoxic conditions in a membrane bioreactor. Water Environ Res (in press)
Wakabayashi S, Matsubara H, Webster DA (1986) Primary sequence of a dimeric bacterial haemoglobin from Vitreoscilla. Nature 322:481–483
Webster DA (1987) Structure and function of bacterial hemoglobin and related proteins. In: Eichhorn GL, Marzilli LG (eds) Advances in inorganic biochemistry Heme proteins, vol 7. Elsevier, New York, pp 245–265
Acknowledgement
This work was supported by NSF Grant No. MCB-9910356.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, Y., Webster, D.A. & Stark, B.C. Improvement of bioremediation by Pseudomonas and Burkholderia by mutants of the Vitreoscilla hemoglobin gene (vgb) integrated into their chromosomes. J IND MICROBIOL BIOTECHNOL 32, 148–154 (2005). https://doi.org/10.1007/s10295-005-0215-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-005-0215-4