Abstract
Expression of vgb, encoding Vitreoscilla hemoglobin (VHb), in Burkholderia strain YV1 was previously shown to improve cell growth and enhance 2,4-dinitrotoluene (2,4-DNT) degradation compared with control strain DNT, especially under hypoxic conditions. In the work reported here, the ratio of 2,4-DNT degraded to oxygen uptake was approximately 5-fold larger for strain YV1 than for strain DNT. The addition of purified VHb to cytosolic fractions of strain DNT increased 2,4-DNT degradation 1.5-fold, compared with 1.1-fold for control bovine Hb, but increased the 2,4-DNT degradation 2.7-fold when added to partially purified 2,4-DNT dioxygenase, compared with 1.3-fold for bovine Hb. This suggests a direct transfer of oxygen from VHb to the oxygenase. In a bioreactor at high 2,4-DNT concentration (using 100 ml oleyl alcohol containing 2 g 2,4-DNT as the second phase) with 1.5 l culture, both strains could remove 0.8 g 2,4-DNT by 120 h; and, under the same conditions in a fed-batch reactor, the degradation increased to 1 g for strain YV1 but not for strain DNT.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hemoglobin (VHb) from the gram-negative, gliding, filamentous, and strictly aerobic bacterium Vitreoscilla [33] is the best characterized prokaryotic Hb [40, 41]. Its function may be to enable the organism to survive in oxygen-limited environments by facilitating oxygen diffusion to terminal oxidases under oxygen-limiting conditions [9, 20, 30, 34, 42]. Positive effects on growth and productivity due to engineering with VHb have been shown in bacteria [1, 3, 4, 10, 11, 21, 22, 25, 26, 39, 43, 44], fungi [7, 27], plants [15], and mammalian cells [32].
2,4-Dinitrotoluene (2,4-DNT) is an aromatic compound and the major impurity resulting from the manufacture of 2,4,6-trinitrotoluene (TNT). Waste from this process has contaminated waterways and soils and, because of its toxicity, 2,4-DNT is listed as a priority pollutant by the United States Environmental Protection Agency [19]. Burkholderia sp. strain DNT, formerly known as Pseudomonas sp. strain DNT, is an aerobic, gram-negative bacterium that can degrade 2,4-DNT as a source of carbon and energy under aerobic conditions. A biodegradation pathway for 2,4-DNT by Burkholderia sp. strain DNT was first proposed by Spanggord et al. [35]. 2,4-DNT dioxygenase is the first enzyme in this pathway and the genes encoding this enzyme were identified by Suen et al. [37]. Recent studies showed that VHb enhances the first step in 2,4-DNT degradation [12], that Burkholderia sp. strain DNT engineered with the gene (vgb) encoding VHb (strain YV1) enhances 2,4-DNT degradation [29, 31], and vgb is stably maintained and expressed in a functional form in strain YV1 [31]. Reports on the beneficial effects of VHb prompted this study to explore further the enhancement by VHb of 2,4-DNT biodegradation by Burkholderia sp. strain DNT in vivo and in vitro.
Regarding our in vivo experiments, bioremediation is an increasingly popular alternative to conventional physical and chemical methods for treating aromatic compounds. The use of a two-phase organic/aqueous bioreactor configuration has been shown to permit biological treatment of xenobiotic wastes, such as phenol [5] and a mixture of benzene, toluene, and p-xylene [6]. To test whether strain YV1 has advantages for 2,4-DNT degradation over strain DNT in bioremediation bioreactors, we examined their performance in one- and two-phase bioreactors.
Materials and methods
Bacterial strains and cell growth
Burkholderia sp. strain DNT [35] was kindly supplied by Drs. J. Spain and S. Nishino of Tyndall Air Force Base (Fla.). Burkholderia sp. strain YV1 was constructed in our laboratory by transforming strain DNT with plasmid pSC160 [31]. Plasmid pSC160 was constructed by inserting vgb-bearing plasmid pUC8:16 into the broad-host-range vector pKT230 [25]; and vgb expression was under control of the native promoter in pSC160.
Cultures of Burkholderia strain DNT were maintained on agar plates containing 2,4-DNT minimal medium [35]. The cells were transferred from agar plates to sterilized tryptic soy broth (TSB) containing 30 g TSB l−1 and grown in 500-ml flasks containing 250 ml medium for 18 h with shaking at 150 rpm at 37 °C. The cells were harvested by centrifugation at 6,000 g for 10 min and washed twice with 20 mM sodium phosphate buffer (pH 7.2). The washed cells were either stored at –80 °C until used for the purification of 2,4-DNT dioxygenase or resuspended in the same buffer to an appropriate cell concentration and kept on ice for the measurement of oxygen uptake and 2,4-DNT degradation rate.
Burkholderia strain YV1 was maintained and grown in the same way as Burkholderia strain DNT, except that 40 mg kanamycin (Kan) l−1 and 100 mg ampicillin (Amp) l−1 were added to agar plates and liquid media.
Vitreoscilla strain C1 was grown in PYA medium (1% peptone, 1% yeast extract, 0.02% sodium acetate, pH 7.8). The cells were harvested (as described above for Burkholderia) from 2,800-ml flasks containing 1,500 ml PYA after growth for 22 h with shaking at 200 rpm at 30 °C. The cells were washed twice with 0.01 M Tris buffer, pH 7.5, and kept on ice until used for the purification of VHb.
Cell lysis
Cells of Burkholderia strains were lysed by sonication. Freshly harvested cells were suspended in an equal volume (w/v) of deionized, sterilized water or 20 mM sodium phosphate buffer (pH 7.2) in a 15-ml plastic bottle and sonicated at 0 °C, using repeats of a 30 s burst with a 30 s interval and a 50% duty cycle at power level 7, using a Sonifier 350 (Branson, Danbury, Conn.), for a total of 1 min for each gram of cells. The lysis of Vitreoscilla was performed using the procedure of Georgiou and Webster [13].
Partial purification of 2,4-DNT dioxygenase from Burkholderia strain DNT and purification of VHb from Vitreoscilla
All purification procedures were carried out at 4 °C, unless stated otherwise. Cell extracts for enzyme purification were prepared from 15 g (wet weight) frozen cells suspended in an equal volume (w/v) of 20 mM sodium phosphate buffer (pH 7.2). The cell suspension was lysed as described above. Cytosolic fractions were prepared from the cell extracts by centrifugation at 45,000 g for 1 h and applied to a DEAE-cellulose column (2.5×40 cm) equilibrated with 20 mM sodium phosphate buffer (pH 7. 2). The column was washed with about 1,000 ml of the same buffer. Bound proteins were eluted with a linear gradient of sodium chloride (0.0–0.5 M in the same buffer) at a flow rate of approximately 26 ml h−1. Fractions with 2,4-DNT degradation activity were pooled, concentrated using a concentrator (Amicon, Danvers, Mass.) or aquacide (Calbiochem, San Diego, Calif.), dialyzed in 20 mM sodium phosphate (pH 7.2), and chromatographed on a gel filtration column (Sephadex G150, 2.0×80 cm, flow rate 9.3 ml h−1) equilibrated with the same buffer. All fractions with 2,4-DNT degradation activity were collected and stored frozen at –20 °C. The purification of VHb from Vitreoscilla was performed as described previously [8].
Measurement of oxygen uptake and 2,4-DNT dioxygenase activity assay
Whole-cell oxygen uptake was measured with an oxygen monitor (model 53; Yellow Springs Instruments, Yellow Springs, Ohio) and a recorder (model SRG-2; Sargent–Welch, Skokie, Ill.). The temperature was controlled at 37 °C by a circulator (model 1267-62; Cole Parmer, Chicago, Ill.). The cells were grown and harvested as described above. The 2,4-DNT solution was adjusted to an appropriate concentration with water. The cells and 3 ml air-saturated 2,4-DNT solution were loaded into the respirometer chamber. When the oxygen concentration decreased to 50%, the cell suspension was removed from the chamber and centrifuged immediately. The supernatant was saved and the 2,4-DNT disappearance was calculated by measuring the absorbance at 254 nm (A 254).
For cell extracts and purified fractions, 0.02 ml were loaded into the respirometer chamber with air-saturated 2,4-DNT substrate solution (2 ml of 5.5×10−4 M 2,4-DNT, 0.1 ml of 10 mM NADH in 20 mM sodium phosphate buffer, pH 7.2) at 37 °C. After 3 min, aliquots were removed from the chamber to measure 2,4-DNT disappearance by high-pressure liquid chromatography (HPLC). Alternatively, previously frozen fractions were assayed in 1.5-ml microtubes containing 0.5 ml air-saturated 5.5×10−4 M 2,4-DNT and 1.0 mM NADH in 20 mM sodium phosphate buffer, pH 7.2. The microtubes were equilibrated in a 37 °C water bath for 5 min and then 20 μl of the thawed samples were added and the tubes were incubated for another 5 min. The 2,4-DNT disappearance was measured by HPLC.
Measurement of 2,4-DNT concentration using HPLC and A 254
The HPLC system used was from Varian (Walnut Creek, Calif.), using solvent delivery system 9012 Q and variable wavelength UV-VIS detector 9050. After a minimum 5 min warm-up, 50 ml of combined solvent [1:1 v/v 0.1% trifluoroacetic acid (v/v in water) with acetonitrile (HPLC grade)] were used to purge the solvent line. Then, the combined solvent was pumped (1.4 ml min−1 with constant pressure) through a C18 column (150×4.6 mm; Varian) and 5 μl of sample were loaded with a 10-μl syringe into the injector (Rheodyne, Cotati, Calif.). All data were collected by a computer with the Star Chromatography Workstation software (Varian). For the measurement of 2,4-DNT in oleyl alcohol, the HPLC conditions were the same as those above (2,4-DNT in aqueous solvents), except the 2,4-DNT samples were diluted 1:100 (1:10 serial dilution twice) with absolute ethanol, the combined solvent was 1:1 ethanol (HPLC grade) and acetonitrile, and the C18 column was 250×4.6 mm (Resolution Systems, Holland, Mich.). The 2,4-DNT extinction coefficient (15.2 mM−1 cm−1) was determined at 254 nm.
Kinetic measurements for 2,4-DNT dioxygenase
In a microtube, 10 μl of 20 mM NADH in 20 mM sodium phosphate buffer (pH 7.2) were added to a 10 μl enzyme sample and incubated in a 37 °C water bath for 1 min. Then, 20 μl (37 °C) of varying concentrations of 2,4-DNT in 20 mM sodium phosphate buffer (pH 7.2) were added to the tube. This tube was flicked, centrifuged to sediment any droplets, and returned to the 37 °C water bath for 10 s. The 2,4-DNT disappearance from the sample was immediately measured by HPLC. Kinetic parameters (K M2,4-DNT, V max2,4-DNT) were determined from Lineweaver–Burk plots.
Measurements of protein concentrations and SDS-PAGE
Protein concentrations in cell extracts and column fractions were determined by the Folin–Ciocalteau method, as described by Switzer and Garrity [38], using the A 280 values with lysozyme as the standard. Protein concentrations in the cytosolic fractions (centrifuged cell extracts) of Burkholderia sp. strain DNT were determined by the dye-binding assay [2], using bovine serum albumin as the standard. For SDS-PAGE, 15% polyacrylamide gels were prepared as described by Laemmli [24] and used to analyze the 2,4-DNT dioxygenase fractions from the G150 Sephadex chromatography. The gels were stained by the silver-staining method [28].
Determination of the native molecular weight of 2,4-DNT dioxygenase by gel filtration on Bio-Gel P-300
Standard proteins [cytochrome c (13 kDa), myoglobin (16.9 kDa), egg albumin (45 kDa), Hb (64 kDa), β-galactosidase (116 kDa), myosin (200 kDa)] were individually chromatographed to determine their elution times. Their elution volume (V) was calculated by multiplying the flow rate (10 ml h−1) by the peak elution time. Blue dextran (2,000 kDa) was used to measure Vo, the void volume (22 ml). The standard curve was constructed by plotting V/Vo versus log molecular mass.
Effects of VHb and bovine Hb on 2,4-DNT degradation by cell extracts of Burkholderia sp. strain DNT and purified 2,4-DNT dioxygenase
Cytosolic fractions of Burkholderia were obtained by centrifuging lysed cells at 10,000 g for 10 min at 4 °C. In microtubes, 50 μl of 200 mM NADH in 20 mM sodium phosphate buffer, pH 7.2, were added to 50 μl of 40 μM Hb. The tubes were incubated at room temperature for at least 10 min. Then, in order, 50 μl of 0.1 mM 2,4-DNT in the same phosphate buffer and 50 μl of lysed cells, cytosolic fractions, or purified 2,4-DNT dioxygenase were added to the tubes. The tubes were incubated in a 37 °C water bath for 10 min (20 min for the purified enzymes). The 2,4-DNT disappearance from these samples was measured by HPLC.
One- and two-phase bioreactors for 2,4-DNT bioremediation
A 2-l fermentor (model F-2000, New Brunswick Scientific Co., Edison, N.J.) was used in all experiments. The conditions common to the five types of experiment performed were as follows. The basic medium (1.5 l) was either 2,4-DNT minimal medium [35] or TSB, which was saturated with oxygen by using forced air before inoculation. The inoculum was 4 ml of a TSB overnight culture. During growth, temperature was controlled at 37 °C, the culture was always agitated, and the pH was monitored continuously. According to the parameter(s) being investigated, individual experiments varied in the following ways: (1) additions of oleyl alcohol, glucose, or 2,4-DNT were made (or not) to the basic medium, (2) the strain used did (or did not) contain vgb, (3) pH was (or was not) controlled, (4) forced air was (or was not) added during the run, (5) the number of impellers and the impeller speed was varied, and (6) the mode used was batch or fed-batch.
For the initial investigation of the abilities of strains DNT and YV1 to degrade 2,4-DNT in bioreactors, 2,4-DNT minimal medium plus 5 g glucose l−1 was used, there was one impeller at 400 rpm, and there was no pH control. For high aeration, forced air was added at a rate of 0.04 m3 h−1and, for hypoxic conditions, no forced air was added. For all other runs, forced air was added at a rate of 0.04 m3 h−1. For measuring the effects of oleyl alcohol on growth, TSB was used with or without the addition of 100 ml of oleyl alcohol, one impeller at 400 rpm was used, and there was no pH control. Two sets of conditions (A, B; Table 2) were used to investigate 2,4-DNT degradation in batch mode with the two-phase system. The fed-batch conditions were the same as condition B in Table 2, except for the addition of 10 ml of 10× TSB at 24-h intervals.
Results
Partial purification of 2,4-DNT dioxygenase
Table 1 shows that the total purification of 2,4-DNT dioxygenase from the cytosol of Burkholderia sp. strain DNT was 20.3-fold. The purest fraction from Sephadex G150 chromatography was analyzed on a SDS-PAGE gel. It was estimated to be about 5–10% pure by visual examination and four bands at the molecular sizes expected for the subunits [37] were identified. The native molecular mass of 2,4-DNT dioxygenase determined by gel filtration was 187 kDa; and the molecular size calculated from the deduced amino acid sequences of its subunits, including one ORF of unknown function, was 171 kDa [37]. The K M for 2,4-DNT dioxygenase, determined using the partially purified enzyme, was 40 μM, in good agreement with the 27 μM obtained using cell extracts [12].
2,4-DNT biodegradation relative to oxygen consumption for whole cells of Burkholderia sp. strains DNT and YV1
The increased efficiency of 2,4-DNT degradation by cells of strain YV1 (that contain VHb) is shown in Fig. 1, which relates the ratio of 2,4-DNT degraded to oxygen uptake at varying 2,4-DNT concentrations. This ratio was roughly 5-fold higher for strain YV1 than for strain DNT at all concentrations of 2,4-DNT.
Ratio of 2,4-dinitrotoluene (2,4-DNT) degradation to oxygen uptake for whole cells at different 2,4-DNT concentrations. This ratio was roughly 5-fold higher for strain YV1 than for strain DNT at all concentrations of 2,4-DNT. 2,4-DNT concentrations were calculated from measuring the absorbance at 254 nm (A 254). Circles Strain YV1, diamonds strain DNT
Effects of Hb on 2,4-DNT degradation by lysed cells and cytosolic fractions of Burkholderia sp. strain DNT and by purified 2,4-DNT dioxygenase
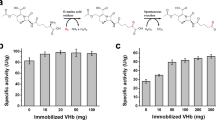
VHb increased 2,4-DNT degradation 46% by the cytosol, compared with a 9% stimulation by bovine Hb (BHb; Fig. 2a), but increased it 174% for purified 2,4-DNT dioxygenase, compared with only 32% for BHb (Fig. 2b).
Effect of Vitreoscilla (VHb) and bovine (BHb) hemoglobins. a Effect of hemoglobins on 2,4-DNT degradation by cytosolic fractions of Burkholderia sp. strain DNT. b Effects of hemoglobins on partially purified 2,4-DNT dioxygenase activity. The G150 Sephadex fraction containing the highest enzyme activity was used for these experiments. For both a and b, 2,4-DNT dioxygenase activity was determined in 1.5-ml microtubes, as described in Materials and methods; and 2,4-DNT concentration was measured by HPLC. Values are averages of five individual measurements (standard deviations are indicated)
2,4-DNT degradation in bioreactors by Burkholderia sp. strains DNT and YV1
Glucose (5 g l−1) was selected as a carbon source for both strains to enhance 2,4-DNT degradation by co-metabolism. Under high aeration, strain YV1 showed no advantage in growth or in 2,4-DNT degradation over the control strain DNT; and this was observed previously [12, 29]. Under hypoxic conditions, however, strain YV1 did show advantages over strain DNT (Fig. 3). Although strain DNT initially consumed 2,4-DNT at a faster rate in this one-phase bioreactor experiment, both its growth and 2,4-DNT degradation leveled off, whereas strain YV1 consumed 100% of the 2,4-DNT and grew to a cell density about 3-fold larger.
2,4-DNT degradation by and growth of Burkholderia sp. strains DNT and YV1 in bioreactors with 2,4-DNT minimal medium and glucose (no aeration). 2,4-DNT disappearance was measured by HPLC. Each sample taken from the medium was 1 ml. The first six samples were taken at 8-h intervals and the following samples were taken at 12-h intervals
One hindrance to the bioremediation of 2,4-DNT is its low solubility in water, 187 mg l−1 [35]. The solubility of 2,4-DNT in oleyl alcohol was tested and found to be about 20 g l−1, more than 100-fold greater than its solubility in water. But 2,4-DNT also showed high diffusibility from oleyl alcohol to water, the concentration in the aqueous phase reaching 230 mg l−1 after 2 h. This is a value 23% higher than the solubility, 187 mg l−1, reported by Spanggord et al. [35]. This increased solubility is likely due to the different conditions we used, i.e., temperature and agitation. The growth of Burkholderia in a two-phase system compared with a one-phase system using TSB as the medium is shown in Fig. 4 (top panel) for strains DNT and YV1. Surprisingly, both strains grew better in the two-phase bioreactor, i.e., in the presence of oleyl alcohol; and, because of this result, oleyl alcohol was selected to be the 2,4-DNT carrier to enhance 2,4-DNT degradation in two-phase bioreactors.
Growth of Burkholderia sp. strains DNT and YV1. Top panel Growth of strains DNT and YV1 in tryptic soy broth (TSB) medium with and without oleyl alcohol in the bioreactor. Parameters were the same as condition A in Table 2, except there was no 2,4-DNT in the oleyl alcohol. Solid lines represent the one-phase bioreactors with 1.5 l of TSB medium and dashed lines represent the two-phase bioreactors with 1.5 l of TSB medium plus 100 ml oleyl alcohol. Middle panel 2,4-DNT degradation by and growth of Burkholderia sp. strains DNT and YV1 in the two-phase bioreactor under condition A of Table 2. The total remaining 2,4-DNT was measured by HPLC and calculated by combining the remaining 2,4-DNT in the aqueous phase with the remaining 2,4-DNT in the oleyl alcohol phase. Bottom panel 2,4-DNT degradation by and growth of Burkholderia sp. strains DNT and YV1 in the two-phase bioreactor under condition B of Table 2. The total remaining 2,4-DNT was measured and calculated as in the middle panel
Table 2 shows the two set-up conditions (A, B) for the two-phase bioreactors. In condition A, after an initial lag phase, strain YV1 had a faster growth rate and had the higher 2,4-DNT degradation rate until after 100 h (Fig. 4, central panel). In condition B, there was more 2,4-DNT and one more impeller was added for better mixing of the two phases. This resulted in a steep initial 2,4-DNT degradation rate for about the first 24 h for both strains, which then leveled off for the rest of the experiment (Fig. 4, bottom panel). There was little difference in growth rate and 2,4-DNT degradation for the two strains under these conditions. In a fed-batch experiment, aliquots of 10 ml of 10× TSB medium were added to the two-phase bioreactor under condition B every 24 h after the first 24 h. Strain YV1 but not strain DNT showed a better 2,4-DNT degradation rate after 48 h under these conditions, compared with the non-fed conditions, degrading half (about 1 g) of the total 2,4-DNT in the reactor (Fig. 5).
Discussion
In the one-phase bioreactor (Fig. 3), the beneficial effects of VHb occurred only under hypoxic conditions; and similar results were previously observed in 2,4-DNT degradation [12, 29] and in other systems [3, 17, 20]. Two major systems involved in oxygen consumption by strain DNT are the respiratory chain and the oxygenases in the 2,4-DNT degradation pathway. Thus, stimulation of 2,4-DNT degradation by VHb observed in vivo under hypoxic conditions could be a direct effect, resulting from increased oxygen delivery to the oxygenases, or an indirect effect on cell respiration. The latter could be an increased respiratory efficiency at low oxygen levels leading to higher ATP synthesis and increased protein (i.e., oxygenase) synthesis; and there is evidence for this in other systems [16, 17, 22]. Previous work showed that cells of strains expressing vgb did have a higher level of 2,4-DNT dioxygenase activity [12] and the increased efficiency of 2,4-DNT degraded per oxygen consumed observed here (Fig. 1) supports this. However, this result and the direct stimulation of 2,4-DNT degradation by exogenous VHb for both cytosolic fractions and partially purified 2,4-DNT dioxygenase (Fig. 2) are evidence that VHb directly stimulates this enzyme, presumably by delivering oxygen to it. These results also show the superiority of VHb relative to BHb for this purpose; and this has been observed for other systems [18]. The purest fractions containing 2,4-DNT dioxygenase were not likely to contain DntB and DntC, because the three oxygenases have molecular sizes of 171 kDa, 60 kDa, and 44 kDa, respectively [14, 36], which would result in their separation in the gel filtration chromatography step. Thus, the effects of Hbs on 2,4-DNT degradation by the purified 2,4-DNT dioxygenases are likely due to interactions solely with that enzyme. It appears, then, that 2,4-DNT dioxygenase activity is elevated both by increased production and by a direct effect of VHb on the enzyme.
Neither strain could completely degrade the relatively large amount of 2,4-DNT in the two-phase bioreactors under the conditions tested, but under condition B, 1.5 l of culture was capable of degrading gram quantities of 2,4-DNT (Figs. 4, 5). Leveling-off of the degradation at the end of the experiment was not due to nutrient deficiency, since adding additional nutrient at these times did not result in further degradation. The amount of 2,4-DNT degraded was approximately 0.8 g for both strains in the two-phase bioreactor under condition B. To degrade this amount would require about 5 l of a TSB culture in a one-phase bioreactor, a saving of 3.5 l of TSB medium for the two-phase partitioning bioreactor. The two-phase bioreactor has another advantage. The 2,4-DNT in a contaminated site could be extracted with an organic solvent, the 2,4-DNT transferred from this primary solvent to oleyl alcohol, and degraded in a two-phase bioreactor. Both the extraction solvent and the oleyl alcohol would then be available for further cycles of extraction and biodegradation.
Surprisingly, strains YV1 and DNT grew better in the presence of oleyl alcohol in the two-phase bioreactor than in its absence (Fig. 4, top panel). There is evidence that Pseudomonas putida S12 has an efflux pump involved in solvent tolerance [23], but whether Burkholderia has any such efflux pump is not known. The possibility that Burkholderia may be able to use oleyl alcohol as a carbon source also needs to be examined.
References:
Aydin S, Webster DA, Stark BC (2000) Nitrite inhibition of Vitreoscilla hemoglobin (VHb) in recombinant E. coli: direct evidence that VHb enhances recombinant protein production. Biotechnol Prog 16:917–921
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Buddenhagen RE, Webster DA, Stark BC (1996) Enhancement by bacterial hemoglobin of amylase production in recombinant E. coli occurs under conditions of low O2. Biotechnol Lett 18:695–700
Chung JW, Webster DA, Pagilla KR, Stark BC (2001) Chromosomal integration of the Vitreoscilla hemoglobin gene in Burkholderia and Pseudomonas for the purpose of producing stable engineered strains with enhanced bioremediating ability. J Ind Microbiol Biotechnol 27:27–33
Collins LD, Daugulis AJ (1996) Biodegradation of phenol at high initial concentrations in two-phase partitioning batch and fed-batch bioreactors. Biotechnol Bioeng 55:155–162
Collins LD, Daugulis AJ (1999) Simultaneous biodegradation of benzene, toluene, and p-xylene in a two-phase partitioning bioreactor: concept demonstration and practical application. Biotechnol Prog 15:74–80
DeModena JA, Gutierrez S, Velasco J, Fernandez FJ, Fachini RA, Galazzo JL, Hughes DE, Martin JF (1993) The production of cephalosporin C by Acremonium chrysogenum is improved by the intracellular expression of a bacterial hemoglobin. Bio/Technology 11:926–929
Dikshit KL, Webster DA (1988) Cloning, characterization and expression of the bacterial globin gene from Vitreoscilla in Escherichia coli. Gene 70:377–386
Dikshit KL, Spaulding D, Braun A, Webster DA (1989) Oxygen inhibition of globin gene transcription and bacterial haemoglobin synthesis in Vitreoscilla. J Gen Microbiol 135:2601–2609
Dikshit RP, Dikshit KL, Liu Y, Webster DA (1992) The bacterial hemoglobin from Vitreoscilla can support the aerobic growth of Escherichia coli lacking terminal oxidases. Arch Biochem Biophys 293:241–245
Enayati N, Tari C, Parulekar SJ, Stark BC, Webster DA (1999) Production of α-amylase in fed-batch cultures of vgb + and vgb − recombinant Escherichia coli: some observations. Biotechnol Prog 15:640–645
Fish PA, Webster DA, Stark BC (2000) Vitreoscilla hemoglobin enhances the first step in 2,4-dinitrotoluene degradation in vitro and at low aeration in vivo. J Mol Catal B Enzym 9:75–82
Georgiou CD, Webster DA (1987) Identification of b, c, and d cytochromes in the membrane of Vitreoscilla. Arch Microbiol 148:328–333
Haigler BE, Suen WC, Spain JC (1996) Purification and sequence analysis of 4-methyl-5-nitrocatechol oxygenase from Burkholderia sp. strain DNT. J Bacteriol 178:6019–6024
Holmberg N, Lilius G, Bailey JE, Bülow L (1997) Transgenic tobacco expressing Vitreoscilla hemoglobin exhibits enhanced growth and altered metabolic production. Nat Botechnol 15:244–247
Kallio PT, Bailey JE (1996) Intracellular expression of Vitreoscilla hemoglobin (VHb) enhances total protein secretion and improves the production of alpha-amylase and neutral protease in Bacillus subtilis. Biotechnol Prog 12:31–39
Kallio PT, Kim DJ, Tsai PS, Bailey JE (1994) Intracellular expression of Vitreoscilla hemoglobin alters Escherichia coli energy metabolism under oxygen-limited conditions. Eur J Biochem 219:201–208
Kallio PT, Tsai PS, Bailey JE (1996) Expression of Vitreoscilla hemoglobin is superior to horse heart myoglobin or yeast flavohemoglobin expression for enhancing Escherichia coli growth in a microaerobic bioreactor. Biotechnol Prog 6:751–757
Keith LH, Telliard WA (1979) Priority pollutants. I. A perspective view. Environ Sci Technol 13:416–423
Khosla C, Bailey JE (1988a) Heterologous expression of a bacterial haemoglobin improves the growth properties of recombinant Escherichia coli. Nature 331:633–635
Khosla C, Bailey JE (1988b) The Vitreoscilla hemoglobin gene: molecular cloning, nucleotide sequence, and genetic expression in Escherichia coli. Mol Gen Genet 214:158–161
Khosravi M, Webster DA, Stark BC (1990) Presence of the bacterial hemoglobin gene improves α-amylase production of a recombinant Escherichia coli strain. Plasmid 24:190–194
Kieboom J, Dennis JJ, Bont JAM de, Zylstra GJ (1998) Identification and molecular characterization of an efflux pump involved in Pseudomonas putida S12 solvent tolerance. J Biol Chem 273:85–91
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Liu SC, Webster DA, Stark BC (1995) Cloning and expression of the Vitreoscilla hemoglobin gene in Pseudomonas: effects on cell growth. Appl Microbiol Biotechnol 44:419–424
Liu SC, Webster DA, Wei ML, Stark BC (1996) Genetic engineering to contain the Vitreoscilla hemoglobin gene enhances degradation of benzoic acid by Xanthomonas maltophilia. Biotechnol Bioeng 49:101–105
Magnolo SK, Leenutaphong DL, DeModena JA, Curtis JE, Bailey JE, Galazzo JL, Hughes DE (1991) Actinorhodin production by Streptomyces coelicolor and growth of Streptomyces lividans are improved by the expression of a bacterial hemoglobin. Bio/Technology 9:473–476
Morrissey JH (1981) Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem 117:307–310
Nasr MA, Hwang KW, Akbas M, Webster DA, Stark BC (2000) Effects of culture conditions on enhancement of 2,4-dinitrotoluene degradation by Burkholderia engineered with the Vitreoscilla hemoglobin gene. Biotechnol Prog 17:359–361
Park KW, Kim KJ, Howard AJ, Stark BC, Webster DA (2002) Vitreoscilla hemoglobin binds to subunit I of cytochrome bo ubiquinol oxidases. J Biol Chem 277:33334–33337
Patel SM, Stark BC, Hwang KW, Dikshit KL, Webster DA (2000) Cloning and Vitreoscilla hemoglobin gene in Burkholderia sp. strain DNT for enhancement of 2,4-dinitrotoluene degradation. Biotechnol Prog 16:26–30
Pendse GJ, Bailey JE (1994) Effects of Vitreoscilla hemoglobin expression on growth and specific tissue plasminogen activator productivity in recombinant Chinese hamster ovary cells. Biotechnol Bioeng 44:1367–1370
Pringsheim EG (1951) The Vitreoscillaceae: a family of colourless, gliding, filamentous organisms. J Gen Microbiol 5:124–149
Ramandeep, Hwang KW, Raje M, Kim KJ, Stark BC, Dikshit KL, Webster DA (2001) Vitreoscilla hemoglobin. Interacellullar localization and binding to membranes. J Biol Chem 27:24781–24789
Spanggord RJ, Spain JC, Nishino SF, Mortelmans KE (1991) Biodegradation of 2,4-dinitrotoluene by a Pseudomonas sp. Appl Environ Microbiol 57:3200–3205
Suen WC, Spain JC (1993) Cloning and characterization of Pseudomonas sp. DNT genes for 2,4-dinitrotoluene degradation. J Bacteriol 175:1831–1837
Suen WC, Haigler BE, Spain JC (1996) 2,4-dinitrotoluene dioxygenase from Burkholderia sp. strain DNT: similarity to naphthalene dioxygenase. J Bacteriol 178:4926–4934
Switzer RL, Garrity LF (1999) Experimental biochemistry. Julet, New York
Tari C, Parulekar SJ, Stark BC, Webster DA (1998) Synthesis and excretion of α-amylase in vgb + and vgb − recombinant Escherichia coli: a comparative study. Biotechnol Bioeng 59:673–678
Tarricone C, Galizzi A, Coda A, Ascenzi P, Bolognesi M (1997) Unusual structure of the oxygen-binding site in the dimeric bacterial hemoglobin from Vitreoscilla sp. Structure 5:497–507
Wakabayashi S, Matsubara H, Webster DA (1986) Primary sequence of a dimeric bacterial haemoglobin from Vitreoscilla. Nature 322:481–483
Webster DA (1987) Structure and function of bacterial hemoglobin and related proteins. In: Eichhorn GC, Marzilli LG (eds) Advances in inorganic biochemistry. Elsevier, New York, pp 245–265
Wei ML, Webster DA, Stark BC (1998) Genetic engineering of Serratia marcescens with bacterial hemoglobin gene: effects on growth, oxygen utilization, and cell size. Biotechnol Bioeng 57:477–483
Wei ML, Webster DA, Stark BC (1998) Metabolic engineering of Serratia marcescens with the bacterial hemoglobin gene: alterations in fermentation pathways. Biotechnol Bioeng 59:640–646
Acknowledgements
This work was supported by grant number MCB-9910356 from the National Science Foundation and grant number F49620-95-1-0325 from the Air Force Office of Scientific Research. Purified VHb was a gift from Dr. K.W. Hwang.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lin, JM., Stark, B.C. & Webster, D.A. Effects of Vitreoscilla hemoglobin on the 2,4-dinitrotoluene (2,4-DNT) dioxygenase activity of Burkholderia and on 2,4-DNT degradation in two-phase bioreactors. J IND MICROBIOL BIOTECHNOL 30, 362–368 (2003). https://doi.org/10.1007/s10295-003-0054-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-003-0054-0