Abstract
Objective
Familial dysautonomia (FD) is a rare genetic disease that involves extreme blood pressure fluctuations secondary to afferent baroreflex failure. The diurnal blood pressure profile, including the average, variability, and day–night difference, may have implications for long-term end organ damage. The purpose of this study was to describe the circadian pattern of blood pressure in the FD population and relationships with renal and pulmonary function, use of medications, and overall disability.
Methods
We analyzed 24-h ambulatory blood pressure monitoring recordings in 22 patients with FD. Information about medications, disease severity, renal function (estimated glomerular filtration, eGFR), pulmonary function (forced expiratory volume in 1 s, FEV1) and an index of blood pressure variability (standard deviation of systolic pressure) were analyzed.
Results
The mean (± SEM) 24-h blood pressure was 115 ± 5.6/72 ± 2.0 mmHg. The diurnal blood pressure variability was high (daytime systolic pressure standard deviation 22.4 ± 1.5 mmHg, nighttime 17.2 ± 1.6), with a high frequency of a non-dipping pattern (16 patients, 73%). eGFR, use of medications, FEV1, and disability scores were unrelated to the degree of blood pressure variability or to dipping status.
Interpretation
This FD cohort had normal average 24-h blood pressure, fluctuating blood pressure, and a high frequency of non-dippers. Although there was evidence of renal dysfunction based on eGFR and proteinuria, the ABPM profile was unrelated to the measures of end organ dysfunction or to reported disability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Familial dysautonomia (FD) is a rare autosomal recessive disorder with high carrier frequency in the Ashkenazi population. FD is caused by deficiency of the elongator complex protein 1 [1]. Patients with FD have afferent baroreflex lesion [2], with orthostatic hypotension, supine hypertension, and extreme fluctuations in blood pressure. Moreover, FD patients are susceptible to crises related to high circulating levels of catecholamines [3]. The crises are characterized by high blood pressure, diaphoresis, and vomiting that may last for days [4].
Chronic kidney disease (CKD) is a clinically important late sequelae of FD, and the incidence of CKD is increasing along with the increased life expectancy of FD patients [5]. Blood pressure dysregulation in FD might be a factor contributing to the target organ damage. It has been proposed that renal hypoperfusion related to hemodynamic instability may be the cause of renal failure in FD [6]. On the other hand, Norcliffe-Kaufmann et al. reported that FD patients with more severe hypertension and excessive blood pressure variability identified by 24-h blood pressure monitoring have worse renal function [5].
A particular pattern of nocturnal blood pressure called non-dipping involves a failure of pressure to decrease at night when the patient is asleep recumbent. Other forms of autonomic failure involving renal dysfunction are associated with the non-dipping pattern [7]. The prevalence of non-dipping in FD and the relationship with renal function are unknown. It is also not known whether pulmonary function and overall disability score, clinical factors that affect adults with FD, correlate with the blood pressure profile. Based on the scarcity of relevant literature on this topic, we thought it worthwhile to assess whether ABPM patterns in FD are related to target organ damage. Such correlations have been described in general in hypertension, but one cannot infer that the same associations obtain in FD. This study was designed to fill these gaps in knowledge. We characterized blood pressure variation in patients with FD and examined correlations between indices of circadian blood pressure and variability and renal function as well as other relevant clinical parameters such as pulmonary function and overall disease severity.
Methods
Study population
The study was conducted according to a clinical research protocol approved by the Institutional Review Board of the Chaim Sheba Medical Center and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments; specific national laws have also been observed. The study is retrospective and all data were extracted from the patients’ files.
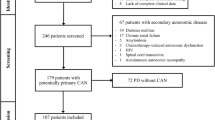
Data were analyzed from 22 patients followed in the Familial Dysautonomia Treatment and Evaluation Center at the Safra Children’s Hospital of the Sheba Medical Center, for whom records from ambulatory blood pressure monitoring were available. FD was diagnosed according to standard clinical criteria and molecular genetic testing [1]. Renal function, pulmonary function test and FuSS were assessed within 6 months from the ABPM.
Ambulatory blood pressure monitoring
Twenty-four-hour ambulatory blood pressure monitoring (ABPM) was conducted in 22 outpatients while performing their usual daily activities. Blood pressure and heart rate were measured at 20-min intervals throughout the day and night. Fewer than 10% of the BP readings were rejected as artifacts on the basis of these criteria. Overall 24-h averages of systolic blood pressure (SBP), diastolic blood pressure (DBP), and heart rate (HR) were recorded.
ABPM recordings were divided into a baseline “daytime” portion from 1000 to 2000 hours and a sleeping “nighttime” portion from 0000 to 0600 hours [7, 8]. This stratification was carried out to exclude data from the transition periods in the morning and evening when blood pressure is known to fluctuate widely [9, 10].
ABPM parameters
The nocturnal percent fall in SBP (SBP dipping) was calculated as 100 × [1 − (nighttime SBP/daytime SBP)]. We sub-classified the patients by the amount of SBP dipping as follows: “extreme dippers” for SBP dipping ≥ 20%, “dippers” for SBP dipping ≥ 10% but < 20%, “non-dippers” for SBP dipping ≥ 0% but < 10%, and “reverse dippers” for SBP increasing. Normal dipping is SBP 10–20% [7, 11].
Renal function
Renal function was assessed from the serum creatinine concentration at a regular outpatient visit. eGFR was calculated using the Cockcroft–Gault equation [12]. Midstream urine samples were assayed by dipstick for proteinuria.
Pulmonary function tests
Spirometry measurements were performed according to American Thoracic Society/European Respiratory Society (ATS/ERS) guidelines [13]. Airflow limitation was assessed using the Global Initiative for Chronic Obstructive Lung Disease (GOLD) classification of the ATS/ERS [14], and airflow limitation severity was measured as below or above 80% of predicted FEV1%.
Functional assessment
Overall clinical function was assessed using a functional severity scale (FuSS) for FD [15].
Statistics
Continuous variables were evaluated for normal distribution. All mean values are reported as ± SEM. Comparisons between dippers and non-dippers were analyzed by t tests for independent means. Fisher’s exact test was used for categorical variables. Linear regression analysis with Pearson correlation coefficients was used to assess the relationship between continuous variables. All tests were two-tailed, and a p value of < 0.05 defined statistical significance. Analyses were performed with SPSS statistical software (v.24.0).
Results
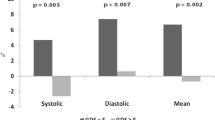
The mean age of the cohort was 26 ± 2.2 years, with 10/22 being females and the average BMI 18 ± 0.7 kg/m2. The average 24-h blood pressure was 115 ± 5.6/72 ± 2.0 mmHg, with daytime blood pressure averaging 120 ± 3.1/73 ± 2.4 mmHg and nighttime 120 ± 3.9/72 ± 2.8 mmHg. The indices of blood pressure variability, daytime and nighttime standard deviations of systolic pressure were 22.4 ± 1.5 and 17.2 ± 1.6 mmHg (normal < 15 and < 12 mmHg [16]). Table 1 outlines additional information regarding the study population.
A non-dipper pattern was observed in 16 patients (73%). The distribution of dipping patterns was as follows: extreme dippers 2 patients (9%); dippers 4 patients (18%); non-dippers 7 patients (32%); and reverse dippers 9 patients (41%). There were no differences in age, gender, weight, height, body mass index, or FuSS between the dipping and non-dipping groups (Table 2). Most patients in both the dipping and non-dipping groups had CKD stage 1 or 2 and trace proteinuria (Table 3; Fig. 1). Both groups had reduced predicted FEV1% (46.8 ± 4.9% in dippers, 54.8 ± 6.0% in non-dippers). Dippers and non-dippers did not differ in airflow limitation severity (Fig. 2). Dipping status was independent of treatment with clonidine, midodrine, or fludrocortisone (Fig. 3); supplementary Table 1 summarizes the medication treatment in both groups.
Prevalence of chronic kidney disease (CKD) staging in dippers and non-dippers. The proportion of patients is shown for categories of CKD. CKD was defined as estimated GFR of ≥ 90 ml/min/1.73 m2 (stage 1), 60–89 ml/min/1.73 m2 (stage 2), 30–59 ml/min/1.73 m2 (stage 3) and 15–29 ml/min/1.73 m2 (stage 4)
There were no relationships between the percent fall in SBP (%, SBP dipping) and FEV1% (p = 0.920, r = 0.023) or eGFR (p = 0.591, r = 0.121).
There were no relationships between 24-h SBP coefficient of variation and eGFR, FEV1%, or FuSS (p = 0.177, r = − 0.299, p = 0.904, r = 0.027, p = 0.718, r = − 0.082, respectively).
Discussion
ABPM provides a means to quantify several important blood pressure indices and characterizes the diurnal pressure profile. In our small cohort of FD patients in whom ABPM was carried out on an ordinary day, blood pressure was optimized, but there was a high degree of blood pressure variability and a high prevalence (73%) of non-dipping status. Among these patients, 56% actually had “reverse dipping,” with an increase in nighttime blood pressure compared to daytime. This undesirable pattern, however, was not related to renal or pulmonary function nor to general disability.
The mechanism of non-dipping is not well understood. Non-dipping may be associated with failure of sympathetic noradrenergic outflows to decrease and of cardiovagal outflow to increase during the nighttime [17,18,19]. It is not known whether this explanation applies to FD.
Among patients with primary, or essential, hypertension, non-dippers have an increased prevalence of target organ damage [11]; however, the applicability of data from hypertensives to our cohort, who were normotensive, is not known. We found no association between the dipping status and laboratory or clinical findings suggesting renal or pulmonary dysfunction. Among our FD patients, the non-dippers also did not differ from the dippers in terms of functional severity scores nor other indices of their general clinical status.
Previous studies showed an association between supine hypertension and low baroreflex cardiovagal gain in chronic autonomic failure [20, 21]. In our FD cohort, high blood pressure variability could have reflected afferent baroreflex dysfunction. If so, however, this was unrelated to values for indices of end organ damage. There were no differences between dippers and non-dippers in daytime, nighttime, or 24-h indices of blood pressure variability.
Our FD cohort had normal or even low mean blood pressure, and end organ damage in FD might reflect hypertension rather than non-dipping or blood pressure variability. Norcliffe-Kaufmann et al. reported that the coefficient of variation of blood pressure was inversely correlated with eGFR in FD [5]. Failure to replicate this finding in the present study may be due to the different blood pressure status, since most of the FD patients in the previous study were hypertensive.
Our study had several limitations. First, the study involved only a small number of patients. FD is a rare disease with fewer than 400 patients worldwide, and, because of the many burdens posed by the disease, participation in clinical studies is limited. Second, drawing inferences from some of the test results was difficult. The FuSS score could not separate neurological impairment from disability related to blood pressure variability or other medical complications; and estimated eGFR from creatinine clearance data in patients with reduced muscle mass may underestimate the severity of renal disease. Third, medical treatments were not controlled, and goals of treatments in terms of target BP likely changed over time, so that there was no way to assess validly the effects of particular medications. Most importantly, this was a retrospective study without a scientifically rigorous design, and we could not draw conclusions from the obtained associations about cause and effect.
In summary, FD patients exhibit high blood pressure variability and a high prevalence of the non-dipping pattern. Neither of these parameters is related to indices of renal or pulmonary damage. In our cohort, which was normotensive, renal function was approximately normal. Whether careful regulation of the blood pressure is useful in preventing end organ target damage in this patient cohort cannot be determined from our data.
Change history
04 April 2020
Unfortunately, the original version of this article contained
References
Slaugenhaupt SA et al (2001) Tissue-specific expression of a splicing mutation in the IKBKAP gene causes familial dysautonomia. Am J Hum Genet 68(3):598–605
Norcliffe-Kaufmann L, Axelrod F, Kaufmann H (2010) Afferent baroreflex failure in familial dysautonomia. Neurology 75(21):1904–1911
Norcliffe-Kaufmann L et al (2013) Hyperdopaminergic crises in familial dysautonomia: a randomized trial of carbidopa. Neurology 80(17):1611–1617
Palma JA et al (2014) Current treatments in familial dysautonomia. Expert Opin Pharmacother 15(18):2653–2671
Norcliffe-Kaufmann L, Axelrod FB, Kaufmann H (2013) Developmental abnormalities, blood pressure variability and renal disease in Riley Day syndrome. J Hum Hypertens 27(1):51–55
Pearson J et al (1980) Renal disease in familial dysautonomia. Kidney Int 17(1):102–112
Okamoto LE et al (2009) Nocturnal blood pressure dipping in the hypertension of autonomic failure. Hypertension 53(2):363–369
van Ittersum FJ et al (1995) Analysis of twenty-four-hour ambulatory blood pressure monitoring: what time period to assess blood pressures during waking and sleeping? J Hypertens 13(9):1053–1058
Fagard R et al (1996) Prediction of the actual awake and asleep blood pressures by various methods of 24 h pressure analysis. J Hypertens 14(5):557–563
Verdecchia P (2000) Prognostic value of ambulatory blood pressure : current evidence and clinical implications. Hypertension 35(3):844–851
Kario K et al (2001) Stroke prognosis and abnormal nocturnal blood pressure falls in older hypertensives. Hypertension 38(4):852–857
Cockcroft DW, Gault MH (1976) Prediction of creatinine clearance from serum creatinine. Nephron 16(1):31–41
Miller MR et al (2005) Standardisation of spirometry. Eur Respir J 26(2):319–338
Celli BR, MacNee W, Force AET (2004) Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J 23(6):932–946
Axelrod FB et al (2012) A rating scale for the functional assessment of patients with familial dysautonomia (Riley Day syndrome). J Pediatr 161(6):1160–1165
Sega R et al (2002) Blood pressure variability and organ damage in a general population: results from the PAMELA study (Pressioni Arteriose Monitorate E Loro Associazioni). Hypertension 39(2 Pt 2):710–714
Kohara K et al (1995) Autonomic nervous function in non-dipper essential hypertensive subjects. Evaluation by power spectral analysis of heart rate variability. Hypertension 26(5):808–814
Nakano Y et al (2001) Non-dipper phenomenon in essential hypertension is related to blunted nocturnal rise and fall of sympatho-vagal nervous activity and progress in retinopathy. Auton Neurosci 88(3):181–186
Sherwood A et al (2002) Nighttime blood pressure dipping: the role of the sympathetic nervous system. Am J Hypertens 15(2 Pt 1):111–118
Goldstein DS et al (2003) Association between supine hypertension and orthostatic hypotension in autonomic failure. Hypertension 42(2):136–142
Sharabi Y, Goldstein DS (2011) Mechanisms of orthostatic hypotension and supine hypertension in Parkinson disease. J Neurol Sci 310(1–2):123–128
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Goldberg, L., Bar-Aluma, BE., Krauthammer, A. et al. Ambulatory blood pressure profiles in familial dysautonomia. Clin Auton Res 28, 385–390 (2018). https://doi.org/10.1007/s10286-018-0507-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10286-018-0507-1