Abstract
Patients with autonomic failure are characterized by orthostatic hypotension, supine hypertension, high blood pressure variability, blunted heart rate variability, and often have a “non-dipping” or “reverse dipping” pattern on 24-h ambulatory blood pressure monitoring. These alterations may lead to cardiovascular and cerebrovascular changes, similar to the target organ damage found in hypertension. Often patients with autonomic failure are on treatment with anti-hypotensive drugs, which may worsen supine hypertension. The aim of this review is to summarize the evidence for cardiac, vascular, renal, and cerebrovascular damage in patients with autonomic failure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Autonomic failure (AF) is defined as the loss of function of the autonomic nervous system and includes, both primary and secondary forms. Primary neurodegenerative disorders, such as pure AF (PAF), multiple system atrophy (MSA), and Parkinson disease (PD), are characterized by the accumulation of the protein alpha-synuclein in the cytoplasm of central nervous system neurons, glia, and pre- and post-ganglionic peripheral autonomic neurons. Secondary forms of autonomic failure are primarily due to small-fiber neuropathies. The main secondary forms of autonomic neuropathy are those related to diabetes mellitus (DM), amyloidosis, immune-mediated neuropathies, and other systemic diseases [1].
Patients with AF often have high blood pressure (BP) variability [2]; on 24-h ambulatory BP monitoring (24-h ABPM), over 60 % of these patients show a “non-dipping” or “reverse dipping” pattern [2–4]. Supine hypertension is due to baroreflex dysfunction, nocturnal fluid retention, adrenergic hypersensitivity, and drug treatment for orthostatic hypotension [3, 5]. Orthostatic hypotension, supine hypertension, increased BP variability, and “non dipping” BP pattern may expose these patients to an increased risk of cardiovascular events, according to evidence in the literature on the general population and hypertensive patients [6–11].
We reviewed the evidence for cardiac, vascular, renal, and cerebrovascular damage in patients with AF.
Studies were selected from the PubMed database using the following keywords: autonomic failure, autonomic neuropathy, pure autonomic failure, multiple system atrophy, Parkinson disease AND left ventricular hypertrophy, arterial stiffness, peripheral artery disease, intima-media thickness, white matter hyperintensities, stroke, renal impairment, and renal failure. Retrospective, prospective, and cross-sectional studies were included. We selected articles in English language from 1984 to 2014. We also included conceptually related articles and expanded the bibliography by hand-searching references from selected articles. Main results are shown in Table 1. Similarities and differences in cardiovascular organ damage between essential hypertension and autonomic failure are summarized in Table 2.
Heart
Several studies described cardiac damage in small groups of patients with primary forms of AF [12, 13]. Cardiac morphology and function were evaluated by transthoracic echocardiography and compared to patients with essential hypertension. Left ventricular mass was similar in subjects with AF and essential hypertension, but higher than in normotensive subjects. No differences were found between patients with MSA and those with PAF; patients affected by PD were not included in these studies. Vagaonescu et al. [12] found a weak relationship between left ventricular mass and mean 24-h BP values in a group of 14 patients with AF. Maule et al. [13] found higher BP standard deviations (marker of BP variability) in patients with left ventricular hypertrophy compared with those with normal left ventricular mass. A history of hypertension anteceding AF and treatment for OH does not seem to be related to the development of left ventricular hypertrophy [13].
In secondary forms of AF, such as diabetes mellitus (DM), heart damage has been evaluated after exclusion of patients with overt cardiovascular disease. In these patients, AF is related to an increased left ventricular mass evaluated by echocardiography [14] and cardiac magnetic resonance [15], independently of age, sex, 24-h ABPM values, and other clinical characteristics. Moreover, in these patients, diastolic function is also impaired [16]. These changes are related to increased sympathetic tone, increased BP variability, decreased heart rate variability, and impaired myocardial blood flow regulation [17–19]. Parasympathetic impairment seems to represent one of the main determinants of diastolic dysfunction [20].
Arterial stiffness and blood vessels
To our knowledge, only one study evaluated arterial stiffness and central hemodynamics in patients with primary AF [21]. The study aimed to evaluate determinants of Pulse Wave Velocity (PWV), an index of arterial stiffness, and Augmentation Index (AIx), an index of central hemodynamics, using AF as a model of peripheral denervation. Ten patients affected by severe primary AF and a group of healthy controls were evaluated in supine and head-up tilt positions. Compared to controls, in AF patients, it was observed that carotid-femoral PWV, AIx, brachial and central BP values, and aortic and brachial pulse pressures were higher in the supine position. During head-up tilt, both carotid–femoral PWV and AIx decreased in AF, and the change was correlated to the drop in BP.
Many studies evaluated arterial stiffness in secondary forms of AF. In DM, arterial stiffness is inversely related to autonomic function [22–26]. Parasympathetic dysfunction (expressed as blunted heart rate variability) is strongly related to increased arterial stiffness and alterations of central hemodynamics [25, 27].
The relationship between cardiovascular autonomic function, arterial stiffness, and central hemodynamics was also studied in healthy volunteers. Heart rate response to deep breathing, Valsalva ratio, and the overall AF score correlated with PWV and AIx, while cardiovascular sympathetic tests did not correlate with aortic stiffness parameters [28].
In DM, the presence of autonomic neuropathy has been related to an increased intima-media thickness and to a higher burden of atherosclerotic lesions in the carotid arteries, but the actual causality relationship between these two factors is unknown [29–31]. Some authors argued that a reduction in heart rate variability, which may express an early autonomic dysfunction, could be related to a faster progression of atherosclerotic damage in patients with DM [32]. However, other studies did not find a relationship between atherosclerotic disease, defined by intima-media thickness, and the existence of AF [22].
Evidence is lacking regarding the exact relationship between AF and peripheral artery disease—but a lower heart rate variability has been described in patients with Type 2 DM and peripheral artery disease [33].
Kidney
Supine hypertension seems to have a preponderant role in causing renal functional impairment in primary AF. Garland et al. [34] retrospectively evaluated hemodynamic and laboratory data of 64 patients affected by PAF. Serum creatinine and urinary albumin were higher in PAF, while estimated glomerular filtration rate (eGFR) was lower in PAF than controls. Patients with PAF and supine hypertension had a higher serum creatinine level and a lower eGFR than those without supine hypertension.
In Type 1 DM, autonomic neuropathy is related to diabetic nephropathy [35, 36]. In Type 2 DM, patients with moderate to severe AF had a higher decline in eGFR during a mean follow-up of 9 years compared to those with normal autonomic function or early autonomic dysfunction at the time of diagnosis [37], as well as an increased prevalence of diabetic neuropathy [38]. In autonomic function tests, heart-rate response to deep breathing was independently associated with reduction in eGFR [37].
Brain
Cerebrovascular damage, represented by lacunar infarct, territorial infarct, and white matter hyperintensities (WMH), has been extensively studied in primary AF.
Patients with MSA have a higher degree of WMH compared to controls with similar age and cardiovascular risk factors [39–41]. BP in the supine position and the presence of orthostatic BP drop seem to be the most important determinants of cerebrovascular damage in AF. These factors, along with age, are independently related to the amount of WMH in MSA [39–41]. In PD, the presence and the extent of supine and nocturnal hypertension are strongly related to cerebrovascular damage [42]. In a recent retrospective study in PAF [43], 70 % of the patients had pathologic cerebral findings (WMH, lacunar strokes, hemispheric strokes, and microbleeds), but the prevalence of WMH seems to be lower in PAF than in the general population. Age and supine systolic BP were significantly higher in patients with pathologic findings. However, mean BP (as measured during the daytime, nighttime, and over a 24 h period), “non-dipper” status, and plasma catecholamine levels did not differ in patients with cerebrovascular lesions when compared to those without cerebrovascular lesions.
In secondary AF, diabetic autonomic dysfunction is an independent predictor of stroke in Type 2 DM [44–46]. The relationship between WMH and autonomic dysfunction has not been studied in secondary AF.
Other forms of organ damage
Hypertensive retinopathy is a relevant expression of hypertensive organ damage [47]. It has not been evaluated in primary forms of AF so far. In secondary forms of AF, namely diabetes mellitus, retinopathy is secondary to microangiopathy and the contribution of autonomic damage to the development of retinopathy is difficult to discern.
Pathogenesis of cardiovascular damage in AF
Cardiovascular complications in AF might share some pathophysiological aspects with target organ damage in essential hypertension.
AF patients are characterized by a very marked BP variability. In essential hypertension, target organ damage is correlated with BP variability, independent of baseline BP values [11, 48]. In AF, long-term BP variability indexes were found to be significantly higher in patients with left ventricular hypertrophy compared with those with normal left ventricular mass [13].
In PAF and in other forms of primary AF, the relationship between BP variability and impaired renal function has yet to be studied [34]. Long-term BP variability does not seem to play a role in cerebrovascular damage in AF [42].
In essential hypertension, the non-dipping BP pattern is related to a higher degree of cardiac alterations than a dipping pattern [49]. In AF, supine hypertension and reverse BP pattern may also play a role in cardiac [12], renal [34], and cerebrovascular damage [40, 43].
Orthostatic hypotension, the hallmark of AF, is associated with left ventricular hypertrophy in hypertensive patients [50], and is a risk factor for incident chronic kidney disease, heart failure, stroke, atrial fibrillation, coronary events, and overall mortality in the general population [6–9, 50, 51]. However, the relationship between orthostatic BP drop and the presence of cardiovascular damage is not well defined in AF, except for the white matter lesions [40, 41].
A blunted heart rate variability, typical of AF, is related to increased renal and vascular damage in hypertensive patients [52] and may contribute to the pathogenesis of vascular stiffness in AF [25, 27].
Except for rare, congenital forms, AF is more common in middle-aged and elderly patients; nonetheless, ageing does not seem to be significantly related to an increased prevalence of cardiovascular damage in AF in most studies.
Increased arterial stiffness is related to higher BP variability [53], impaired heart rate variability [27], and orthostatic hypotension [54, 55] in hypertensive patients. The interactions between arterial stiffness, hemodynamics, and subtypes of AF have yet to be studied.
Other mechanisms, unique to AF, might contribute to cardiovascular alterations in these patients.
In AF, anti-hypotensive drugs are widely employed. Fludrocortisone is a synthetic mineral corticoid analogue that increases sodium reabsorption and extracellular volume [56]. It may also induce fibrosis, with a mechanism of action similar to that of aldosterone. Few studies have evaluated the influence of this therapy on cardiovascular alterations in AF. In patients with and without left ventricular hypertrophy, the number of AF patients treated with fludrocortisone was similar in both groups [13]. The influence of anti-hypotensive therapy on cardiovascular alterations in AF has yet to be addressed in specifically designed studies.
The cardiovascular alterations in MSA may in part be explained by residual adrenergic sympathetic tone on super-sensitized receptors, in coordination with baroreflex failure [5]. Patients with PAF have very low levels of plasma norepinephrine and plasma renin activity, however, plasma aldosterone levels are normal [57], and angiotensin II is significantly higher than in controls [58]. Angiotensin II might also play a role in cardiovascular damage in AF.
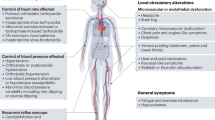
Pathophysiological aspects and potential mechanisms determining cardiovascular alterations in AF are summarized in Fig. 1.
Pathophysiological aspects and potential mechanisms determining cardiovascular alterations in AF. Black boxes: target organ damage occurring in essential hypertension and AF. Red boxes: potential mechanisms of target organ damage in AF that are similar to essential hypertension. Blue boxes: potential mechanisms of target organ damage unique to AF. Black arrows: evidence discussed in the text
Secondary forms of AF are characterized by additional and different mechanisms of organ damage. In diabetes mellitus, microvascular and macrovascular alterations elicit cardiovascular complications; in amyloidosis, the deposit of amyloid in the heart determines the typical restrictive cardiomyopathy. In primary forms of AF, pathophysiological mechanisms are primarily related to alterations of blood pressure and cardiovascular regulation, typical of autonomic dysregulation.
Prognostic implications of cardiovascular damage in primary AF
The presence of AF is related to an increased mortality in diabetes [59], and orthostatic hypotension is related to an increased risk of all-cause mortality in the general population [10]. Hypertensive organ damage is related to an increased cardiovascular risk and therefore to a worse prognosis in essential hypertension [47]. The prognostic role of cardiovascular damage in primary AF is not known.
MSA patients have a poor prognosis overall [60]. Major causes of death in MSA include infectious diseases and sudden death [61–63]. Due to the rapid course of the disease, early detection of cardiovascular damage may be less relevant in these patients.
Causes of death in PAF patients are less well-known, although the prognosis seems good [63, 64]. These patients may be exposed to an increased risk of cardiovascular complications and mortality due to orthostatic hypotension and high BP variability [6–11].
PD has a better prognosis than MSA. Infectious diseases such as pneumonia, are a common cause of death in patients with PD. Cardiovascular mortality and sudden death have also been reported [65, 66]. Early detection of AF in PD is important so that patients may begin treatment for orthostatic hypotension, which is a common side-effect of anti-parkinsonian drugs. Specific treatment of orthostatic hypotension will address symptoms associated with low blood pressure, reduce the risk of falls, and improve quality of life for these patients.
In conclusion, for clinical practice, the detection of supine hypertension is crucial and allows clinicians to choose the most appropriate drug therapies—indeed, 24 h ABPM is a valuable diagnostic tool that can be used to identify this in patients with AF. Orthostatic hypotension is usually treated with short-acting drugs such as midodrine, and careful adjustments need to be made to the time of administration in patients who have severe cases of nocturnal hypertension.
Prospective studies evaluating the impact of cardiovascular alterations on the prognosis of AF are still lacking, as are studies evaluating the impact of the treatment of supine hypertension on the prognosis. It is not known whether the early treatment of supine hypertension may prevent the onset of cardiovascular damage in AF.
The study of cardiovascular alterations in AF patients allows for better understanding of the natural history of AF and the pathophysiological mechanisms of hypertension-induced cardiovascular damage.
References
Freeman R (2008) Clinical practice. Neurogenic orthostatic hypotension. N Engl J Med 358(6):615–624
Stuebner E, Vichayanrat E, Low DA, Mathias CJ, Isenmann S, Haensch CA (2013) Twenty-four hour non-invasive ambulatory blood pressure and heart rate monitoring in Parkinson’s disease. Front Neurol 4:49
Okamoto LE, Gamboa A, Shibao C, Black BK, Diedrich A, Raj SR et al (2009) Nocturnal blood pressure dipping in the hypertension of autonomic failure. Hypertension 53(2):363–369
Plaschke M, Trenkwalder P, Dahlheim H, Lechner C, Trenkwalder C (1998) Twenty-four-hour blood pressure profile and blood pressure responses to head-up tilt tests in Parkinson’s disease and multiple system atrophy. J Hypertens 16(10):1433–1441
Goldstein DS, Pechnik S, Holmes C, Eldadah B, Sharabi Y (2003) Association between supine hypertension and orthostatic hypotension in autonomic failure. Hypertension 42(2):136–142
Rose KM, Tyroler HA, Nardo CJ, Arnett DK, Light KC, Rosamond W et al (2000) Orthostatic hypotension and the incidence of coronary heart disease: the Atherosclerosis Risk in Communities study. Am J Hypertens 13(6 Pt 1):571–578
Eigenbrodt ML, Rose KM, Couper DJ, Arnett DK, Smith R, Jones D (2000) Orthostatic hypotension as a risk factor for stroke: the atherosclerosis risk in communities (ARIC) study, 1987–1996. Stroke 31(10):2307–2313
Fedorowski A, Engstrom G, Hedblad B, Melander O (2010) Orthostatic hypotension predicts incidence of heart failure: the Malmo preventive project. Am J Hypertens 23(11):1209–1215
Fedorowski A, Hedblad B, Engstrom G, Gustav Smith J, Melander O (2010) Orthostatic hypotension and long-term incidence of atrial fibrillation: the malmo preventive project. J Intern Med 268(4):383–389
Fedorowski A, Stavenow L, Hedblad B, Berglund G, Nilsson PM, Melander O (2010) Orthostatic hypotension predicts all-cause mortality and coronary events in middle-aged individuals: the malmo preventive project. Eur Heart J 31(1):85–91
Parati G, Ochoa JE, Lombardi C, Bilo G (2013) Assessment and management of blood-pressure variability. Nat Rev Cardiol 10(3):143–155
Vagaonescu TD, Saadia D, Tuhrim S, Phillips RA, Kaufmann H (2000) Hypertensive cardiovascular damage in patients with primary autonomic failure. Lancet 355(9205):725–726
Maule S, Milan A, Grosso T, Veglio F (2006) Left ventricular hypertrophy in patients with autonomic failure. Am J Hypertens 19(10):1049–1054
Gambardella S, Frontoni S, Spallone V, Maiello MR, Civetta E, Lanza G et al (1993) Increased left ventricular mass in normotensive diabetic patients with autonomic neuropathy. Am J Hypertens 6(2):97–102
Pop-Busui R, Cleary PA, Braffett BH, Martin CL, Herman WH, Low PA et al (2013) Association between cardiovascular autonomic neuropathy and left ventricular dysfunction: DCCT/EDIC study (diabetes control and complications trial/epidemiology of diabetes interventions and complications). J Am Coll Cardiol 61(4):447–454
Irace L, Iarussi D, Guadagno I, Tedesco MA, Perna B, Ratti G et al (1996) Left ventricular performance and autonomic dysfunction in patients with long-term insulin-dependent diabetes mellitus. Acta Diabetol 33(4):269–273
Taskiran M, Rasmussen V, Rasmussen B, Fritz-Hansen T, Larsson HB, Jensen GB et al (2004) Left ventricular dysfunction in normotensive Type 1 diabetic patients: the impact of autonomic neuropathy. Diabet Med 21(6):524–530
Karamitsos TD, Karvounis HI, Didangelos T, Parcharidis GE, Karamitsos DT (2008) Impact of autonomic neuropathy on left ventricular function in normotensive type 1 diabetic patients: a tissue Doppler echocardiographic study. Diabetes Care 31(2):325–327
Mogensen UM, Jensen T, Kober L, Kelbaek H, Mathiesen AS, Dixen U et al (2012) Cardiovascular autonomic neuropathy and subclinical cardiovascular disease in normoalbuminuric type 1 diabetic patients. Diabetes 61(7):1822–1830
Willenheimer RB, Erhardt LR, Nilsson H, Lilja B, Juul-Moller S, Sundkvist G (1998) Parasympathetic neuropathy associated with left ventricular diastolic dysfunction in patients with insulin-dependent diabetes mellitus. Scand Cardiovasc J 32(1):17–22
Huijben AM, Mattace-Raso FU, Deinum J, Lenders J, van den Meiracker AH (2012) Aortic augmentation index and pulse wave velocity in response to head-up tilting: effect of autonomic failure. J Hypertens 30(2):307–314
Meyer C, Milat F, McGrath BP, Cameron J, Kotsopoulos D, Teede HJ (2004) Vascular dysfunction and autonomic neuropathy in Type 2 diabetes. Diabet Med 21(7):746–751
van Ittersum FJ, Schram MT, van der Heijden-Spek JJ, Van Bortel LM, Elte JW, Biemond P et al (2004) Autonomic nervous function, arterial stiffness and blood pressure in patients with Type I diabetes mellitus and normal urinary albumin excretion. J Hum Hypertens 18(11):761–768
Prince CT, Secrest AM, Mackey RH, Arena VC, Kingsley LA, Orchard TJ (2010) Cardiovascular autonomic neuropathy, HDL cholesterol, and smoking correlate with arterial stiffness markers determined 18 years later in type 1 diabetes. Diabetes Care 33(3):652–657
Liatis S, Alexiadou K, Tsiakou A, Makrilakis K, Katsilambros N, Tentolouris N (2011) Cardiac autonomic function correlates with arterial stiffness in the early stage of type 1 diabetes. Exp Diabetes Res 2011:957901
Secrest AM, Marshall SL, Miller RG, Prince CT, Orchard TJ (2011) Pulse wave analysis and cardiac autonomic neuropathy in type 1 diabetes: a report from the Pittsburgh epidemiology of diabetes complications study. Diabetes Technol Ther 13(12):1264–1268
Theilade S, Lajer M, Persson F, Joergensen C, Rossing P (2013) Arterial stiffness is associated with cardiovascular, renal, retinal, and autonomic disease in type 1 diabetes. Diabetes Care 36(3):715–721
Nemes A, Takacs R, Gavaller H, Varkonyi TT, Wittmann T, Forster T et al (2010) Correlations between aortic stiffness and parasympathetic autonomic function in healthy volunteers. Can J Physiol Pharmacol 88(12):1166–1171
Gottsäter A, Ryden-Ahlgren A, Szelag B, Hedblad B, Persson J, Berglund G et al (2003) Cardiovascular autonomic neuropathy associated with carotid atherosclerosis in Type 2 diabetic patients. Diabet Med 20(6):495–499
Sinha PK, Santr G, De D, Sah A, Biswas K, Bhattachary P et al (2012) Carotid intima-media thickness in type 2 diabetes mellitus patients with cardiac autonomic neuropathy. J Assoc Physicians India 60:14–18
Jung CH, Baek AR, Kim KJ, Kim BY, Kim CH, Kang SK et al (2013) Association between Cardiac Autonomic Neuropathy, Diabetic Retinopathy and Carotid Atherosclerosis in Patients with Type 2 Diabetes. Endocrinol Metab (Seoul) 28(4):309–319
Gottsäter A, Ahlgren AR, Taimour S, Sundkvist G (2006) Decreased heart rate variability may predict the progression of carotid atherosclerosis in type 2 diabetes. Clin Auton Res 16(3):228–234
Canani LH, Copstein E, Pecis M, Friedman R, Leitao CB, Azevedo MJ et al (2013) Cardiovascular autonomic neuropathy in type 2 diabetes mellitus patients with peripheral artery disease. Diabetol Metab Syndr 5(1):54
Garland EM, Gamboa A, Okamoto L, Raj SR, Black BK, Davis TL et al (2009) Renal impairment of pure autonomic failure. Hypertension 54(5):1057–1061
Torffvit O, Lindqvist A, Agardh CD, Pahlm O (1997) The association between diabetic nephropathy and autonomic nerve function in type 1 diabetic patients. Scand J Clin Lab Invest 57(2):183–191
Pavy-Le Traon A, Fontaine S, Tap G, Guidolin B, Senard JM, Hanaire H (2010) Cardiovascular autonomic neuropathy and other complications in type 1 diabetes. Clin Auton Res 20:153–160
Kim YK, Lee JE, Kim YG, Kim DJ, Oh HY, Yang CW et al (2009) Cardiac autonomic neuropathy as a predictor of deterioration of the renal function in normoalbuminuric, normotensive patients with type 2 diabetes mellitus. J Korean Med Sci 24(Suppl):S69–S74
Bilal N, Erdogan M, Ozbek M, Cetinkalp S, Karadeniz M, Ozgen AG et al (2008) Increasing severity of cardiac autonomic neuropathy is associated with increasing prevalence of nephropathy, retinopathy, and peripheral neuropathy in Turkish type 2 diabetics. J Diabetes Complicat 22:181–185
Lim TS, Lee PH, Kim HS, Yong SW (2009) White matter hyperintensities in patients with multiple system atrophy. J Neurol 256(10):1663–1670
Umoto M, Miwa H, Ando R, Kajimoto Y, Kondo T (2012) White matter hyperintensities in patients with multiple system atrophy. Parkinsonism Relat Disord 18(1):17–20
Tha KK, Terae S, Yabe I, Miyamoto T, Soma H, Zaitsu Y et al (2010) Microstructural white matter abnormalities of multiple system atrophy: in vivo topographic illustration by using diffusion-tensor MR imaging. Radiology 255(2):563–569
Oh YS, Kim JS, Yang DW, Koo JS, Kim YI, Jung HO et al (2013) Night time blood pressure and white matter hyperintensities in patients with Parkinson disease. Chronobiol Int 30(6):811–817
Struhal W, Lahrmann H, Mathias CJ (2013) Incidence of cerebrovascular lesions in pure autonomic failure. Auton Neurosci 179(1–2):159–162
Toyry JP, Niskanen LK, Lansimies EA, Partanen KP, Uusitupa MI (1996) Autonomic neuropathy predicts the development of stroke in patients with non-insulin-dependent diabetes mellitus. Stroke 27(8):1316–1318
Cohen JA, Estacio RO, Lundgren RA, Esler AL, Schrier RW (2003) Diabetic autonomic neuropathy is associated with an increased incidence of strokes. Auton Neurosci 108(1–2):73–78
Ko SH, Song KH, Park SA, Kim SR, Cha BY, Son HY et al (2008) Cardiovascular autonomic dysfunction predicts acute ischaemic stroke in patients with Type 2 diabetes mellitus: a 7-year follow-up study. Diabet Med 25(10):1171–1177
Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Bohm M et al (2013) 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens 31(7):1281–1357
Leoncini G, Viazzi F, Storace G, Deferrari G, Pontremoli R (2013) Blood pressure variability and multiple organ damage in primary hypertension. J Hum Hypertens 27(11):663–670
Cuspidi C, Meani S, Salerno M, Valerio C, Fusi V, Severgnini B et al (2004) Cardiovascular target organ damage in essential hypertensives with or without reproducible nocturnal fall in blood pressure. J Hypertens 22(2):273–280
Fan XH, Wang Y, Sun K, Zhang W, Wang H, Wu H et al (2010) Disorders of orthostatic blood pressure response are associated with cardiovascular disease and target organ damage in hypertensive patients. Am J Hypertens 23(8):829–837
Franceschini N, Rose KM, Astor BC, Couper D, Vupputuri S (2010) Orthostatic hypotension and incident chronic kidney disease: the atherosclerosis risk in communities study. Hypertension 56(6):1054–1059
Melillo P, Izzo R, De Luca N, Pecchia L (2012) Heart rate variability and target organ damage in hypertensive patients. BMC Cardiovasc Disord 12:105
Schillaci G, Bilo G, Pucci G, Laurent S, Macquin-Mavier I, Boutouyrie P et al (2012) Relationship between short-term blood pressure variability and large-artery stiffness in human hypertension: findings from 2 large databases. Hypertension 60(2):369–377
Protogerou AD, Stergiou GS, Lourida P, Achimastos A (2008) Arterial stiffness and orthostatic blood pressure changes in untreated and treated hypertensive subjects. J Am Soc Hypertens 2:372–377
Valbusa F, Labat C, Salvi P, Vivian ME, Hanon O, Benetos A (2012) Orthostatic hypotension in very old individuals living in nursing homes: the PARTAGE study. J Hypertens 30(1):53–60
Chobanian AV, Volicer L, Tifft CP, Gavras H, Liang CS, Faxon D (1979) Mineralocorticoid-induced hypertension in patients with orthostatic hypotension. N Engl J Med 301(2):68–73
Biaggioni I, Garcia F, Inagami T, Haile V (1993) Hyporeninemic normoaldosteronism in severe autonomic failure. J Clin Endocrinol Metab 76(3):580–586
Arnold AC, Okamoto LE, Gamboa A, Shibao C, Raj SR, Robertson D et al (2013) Angiotensin II, independent of plasma renin activity, contributes to the hypertension of autonomic failure. Hypertension 61(3):701–706
Pop-Busui R, Evans GW, Gerstein HC, Fonseca V, Fleg JL, Hoogwerf BJ et al (2010) Effects of cardiac autonomic dysfunction on mortality risk in the action to control cardiovascular risk in diabetes (ACCORD) trial. Diabetes Care 33(7):1578–1584
Wenning GK, Geser F, Krismer F, Seppi K, Duerr S, Boesch S et al (2013) The natural history of multiple system atrophy: a prospective European cohort study. Lancet Neurol 12(3):264–274
Papapetropoulos S, Tuchman A, Laufer D, Papatsoris AG, Papapetropoulos N, Mash DC (2007) Causes of death in multiple system atrophy. J Neurol Neurosurg Psychiatry 78(3):327–329
Shimohata T, Ozawa T, Nakayama H, Tomita M, Shinoda H, Nishizawa M (2008) Frequency of nocturnal sudden death in patients with multiple system atrophy. J Neurol 255(10):1483–1485
Maule S, Milazzo V, Maule MM, Di Stefano C, Milan A, Veglio F (2012) Mortality and prognosis in patients with neurogenic orthostatic hypotension. Funct Neurol 27:101–106
Mabuchi N, Hirayama M, Koike Y, Watanabe H, Ito H, Kobayashi R et al (2005) Progression and prognosis in pure autonomic failure (PAF): comparison with multiple system atrophy. J Neurol Neurosurg Psychiatry 76(7):947–952
Gorell JM, Johnson CC, Rybicki BA (1994) Parkinson’s disease and its comorbid disorders: an analysis of Michigan mortality data, from 1970 to 1990. Neurology 44(10):1865–1868
Driver JA, Kurth T, Buring JE, Gaziano JM, Logroscino G (2008) Parkinson disease and risk of mortality: a prospective comorbidity-matched cohort study. Neurology 70(16 Pt 2):1423–1430
Oh YS, Kim JS, Lee KS (2013) Orthostatic and supine blood pressures are associated with white matter hyperintensities in Parkinson disease. J Mov Disord 6(2):23–27
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Milazzo, V., Di Stefano, C., Milan, A. et al. Cardiovascular complications in patients with autonomic failure. Clin Auton Res 25, 133–140 (2015). https://doi.org/10.1007/s10286-015-0275-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10286-015-0275-0