Abstract
Objective
To determine normal values for pupillometry indices in healthy control subjects and to examine these indices in patients with autonomic dysfunction and healthy controls.
Methods
Infrared video pupillometry was used to investigate the pupil response to a brief light flash in 79 healthy controls, 28 patients with normal autonomic function (composite autonomic severity score, CASS < 2), and 26 patients with moderate to severe autonomic failure (CASS > 4) seen in our autonomic laboratory from January 2008 to June 2011. In six subjects, we examined the effects of varying light stimulus intensity and light stimulus duration. Descriptive analysis, correlation, and ANCOVA adjusted for age were performed.
Results
We determined eight indices corresponding to parasympathetic and sympathetic pupil function. Baseline pupil diameter (BPD), maximum constriction velocity (MCV), absolute constriction amplitude (ACA), and maximum dilation velocity (MDV) negatively correlated with age (p < 0.01) among controls. MCV and ACA increased with increasing intensity of light stimulus from 3.5 to 112 μW. Indices of parasympathetic pupil innervation (MCV and ACA) were lower in the high CASS group compared to others (p < 0.0001). Indices of sympathetic pupil function, time to reach 75 % of initial resting diameter during pupillary dilation (T¾), and dilation velocity at T¾ (DV¾) did not differ significantly in the three study groups. However, T¾ corrected for the magnitude of pupillary constriction (T¾:ACA) was higher in the high CASS group suggesting sympathetic dysfunction in that group (p = 0.0003).
Conclusions
Indices of pupillomotor function significantly differ between patients with moderate to severe autonomic failure and healthy controls.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Disorders of autonomic function are common and can affect multiple organ systems including the eye. While impaired pupil function does not lead to the same degree of disability as autonomic impairments in cardiovascular control, gastrointestinal motility or thermoregulation, the examination of pupillomotor function may improve diagnostic accuracy in the autonomic laboratory. It is likely that disorders affecting brainstem autonomic centers or peripheral autonomic ganglia will be associated with pupillary impairment, while those affecting only the peripheral autonomic nerves might not have significant pupillary dysfunction. Various non-invasive tests are available to assess global or regional autonomic dysfunction, but most assessments of the parasympathetic cholinergic function rely on examination of baroreflex-mediated changes in heart rate [1]. Current methods for assessment of parasympathetic secretomotor function (lacrimation and salivation) in the clinical laboratory are challenging and have poor reproducibility. On the other hand, assessment of pupil constriction and dilation in response to light provides information about the parasympathetic and sympathetic innervation of the pupil and the responses can be quantified.
Infrared video pupillometry has been available for many years; however, recent technical improvements have allowed for the development of quantitative pupillometry as an autonomic testing tool [2]. The assessment of pupil diameter changes in response to topical drugs is important in certain disorders, but the results are usually qualitative and static and therefore are not easily amenable to quantification of response. The effect of topical agents can also persist for hours. Light pupillometry, on the other hand, is easy, reproducible, safe, and provides dynamic information about pupil function without any residual effects.
Previous investigators have compared various pupillometry indices in patients with autonomic neuropathy and healthy controls [3] and in patients with focal autonomic dysfunction [4]. In a pilot study, pupillary function indices derived from infrared pupillometry were significantly different in patients with diabetes, with or without cardiac autonomic neuropathy when compared with controls [5]. In autoimmune autonomic ganglionopathy (AAG), a rare disorder resulting in global autonomic failure, pupillometry revealed characteristic parasympathetic deficits [6, 7]. Recently, pupillometry has been used in patients with Parkinson’s disease to identify the extent of pupillary abnormalities and pupillary reflex measures were decreased compared to healthy controls [8], but this was not related to dopaminergic loss [9]. These studies indicate that pupillometry could be used to detect early autonomic dysfunction; but to develop pupillometry further as a non-invasive routine test for autonomic dysfunction, standardization and consensus of testing protocols are needed, including the establishment of optimal duration and intensity of light stimuli using commercially available equipment. It is also important to determine the most sensitive indices of the pupil light reflex that will consistently differentiate patients with autonomic failure from controls. In this study, we attempted to address these issues and improve our understanding about the role of pupillometry as an autonomic testing tool.
Methods
Healthy controls
We recruited 79 healthy controls with no known autonomic dysfunction at UTSW Medical Center. Informed consent was obtained from controls as per the guidelines of the institutional research board at our center. In six of the healthy individuals, we assessed the effects of different durations and intensity of light stimulus.
Infrared pupillometry
Pupillometry studies were performed using a binocular pupillometer (A-1000, Neuroptics Inc, San Clemente, CA). This device uses two infrared cameras with a digital image capture rate of 30 Hz. Pupil diameter was detected by threshold detection of the dark pupil and corrected for distance from the camera. A light stimulus (calibrated for intensity and duration) was presented to one eye using a circumferential array of white light-emitting diodes. All data were obtained while subjects were in a comfortable seated position in a darkened room after they were dark adapted for more than 1 min. The only ambient light in the examination room (from the computer screen of the pupillometry device) was directed away from the subject. Dynamic recordings of pupil diameter were saved for off-line analysis.
In six healthy subjects, the effects of variation in intensity and duration of light stimulus were examined. In these six subjects, we also examined the effects of background lighting and frequency of presentation of light stimuli. Based on these results, a standard brief light stimulus (40 ms duration light flash at 14 μW light intensity) was used in all further studies. Analysis was performed on the average of at least three pupillary responses to light to correct for any noise in the signal, especially during the slow redilation part of pupillary light reflex.
Indices of pupillary light reflex
Based on reports from other investigators [2], we calculated several indices from the constriction and dilation phases of the pupillary light reflex in an effort to quantitatively represent parasympathetic and sympathetic pupillary function. The eight indices are described below and are represented graphically in Fig. 1. For analysis, the slope (derivative) of the pupil diameter data at each time point was determined using a linear regression of seven consecutive data points centered on the time point of interest.
Normal pupillograph with description of various indices. Responses from both eyes are superimposed. BPD baseline pupil diameter, MCV maximum constriction velocity, ACA absolute constriction amplitude, RCA relative constriction amplitude, MDV maximum dilation velocity, DV1 dilation velocity during the first second of dilation, DV¾ dilation velocity at time of 3/4th of pupil diameter, Time ¾ time to reach 3/4th of BPD
Baseline pupil diameter (BPD) is the pupil diameter at rest in a darkened room. Maximum constriction velocity (MCV) is the maximum negative slope (pupil size decreasing) during pupillary constriction. Absolute constriction amplitude (ACA) is the magnitude of pupil constriction: the difference between the BPD and the minimum pupil diameter during the pupil constriction. Relative constriction amplitude (RCA) is the ratio of ACA divided by BPD, expressed as a percentage. Maximum dilation velocity (MDV) is the maximum slope during the dilation phase (pupil size increasing). Pupil redilation after light stimulus has two phases. The early rapid phase is influenced both by parasympathetic withdrawal and sympathetic activation, while the later slower phase of redilation predominantly results from sympathetic innervation [10]. Early and late phases of pupillary dilation were assessed by different indices. Dilation velocity at 1 s (DV1) is the slope of pupil diameter change determined 1 s after the minimum pupil size. Time to reach ¾ of the BPD during dilation (T¾) is the time required for pupil diameter to return to 75 % of the initial BPD from the point of maximal pupillary constriction. This has been previously reported as an indicator of pupillary sympathetic function [11]. We also calculated the dilation velocity at T¾ (DV¾) by determining the slope of pupil diameter change at the time of T¾.
Patients with normal and high composite autonomic severity score
Since January 2008, standardized quantitative pupillometry assessment has been incorporated into the clinical practice of the autonomic testing laboratory at UT Southwestern. As part of an autonomic reflex screen [12], we routinely perform Quantitative Sudomotor Axon Reflex Test (QSART), HRDB range (heart rate change during deep breathing), blood pressure and heart rate monitoring in response to Valsalva maneuver, and head-up tilt table testing. Composite autonomic severity score (CASS) is a composite score (range from 0 to 10) derived from the autonomic reflex screen and has been used extensively to quantify autonomic dysfunction [13]. A CASS of 0–1 is considered normal, 2–3 suggests mild autonomic dysfunction, 4–7 moderate autonomic dysfunction, and greater than 7 is considered severe autonomic dysfunction [12]. We identified 28 consecutive patients with a CASS of 0–1. We also identified 26 patients with moderate to high CASS (greater than 4). The high CASS included seven patients with diabetes, eight with Parkinson’s disease, three with multiple systems atrophy, and eight with autonomic impairment secondary to unknown or presumed immune-mediated process. Patients (in both low and high CASS groups) with known ocular disease (defined for the study as requiring current use of topical ocular medication or known to have clearly defined ocular disease which affects visual acuity) or history of prior ophthalmic surgery (other than for refractive error) were excluded. Controls were not on any medication that could affect pupillary function. Patients with high and normal CASS were asked to stop medications (anticholinergic or antiadrenergic) that could affect autonomic testing at least for 24 h prior to autonomic reflex testing. Control subjects were not on any medication. Pupillometry was performed in all patients referred to our autonomic laboratory as part of clinical autonomic reflex testing.
Statistical analysis
There was no side-to-side difference in pupil responses, so all pupillary data reported here were obtained from the left eye of each individual. To analyze the changes in the eight pupillometric indices with variation in duration and intensity of the light stimulus, we used repeated measures or random regression analysis. Descriptive analysis of pupillometry data from the 79 controls and Pearson correlation of pupillometric indices with each other and with age was performed. We used analysis of covariance adjusted for age (ANCOVA) to compare the pupillary indices in controls, and normal and high CASS patients. A p value of <0.05 was considered significant. Statistical analyses were performed with SAS software (version 9.2, SAS Institute, Cary, NC).
Results
Use of different intensity and duration of light stimulus
In six healthy subjects, ages 23–44 years (M:F, 4:2), we analyzed the changes in the eight indices with different durations or intensities of light stimulus. On increasing the duration of stimulus from 20 to 240 ms with a fixed light intensity (14 μW), there was no statistically significant change in any of the eight indices. On increasing the light intensity stimulus from 3.5 to 112 μW with the same duration (40 ms), MCV, ACA (Fig. 2), and RCA exhibited a statistically increasing linear trend. There were no significant changes in other indices with increasing duration or light intensity. Since the purpose of this testing is to identify the ideal duration and light stimulus that provides reliable pupillary indices and is tolerable to subjects, we did not conduct further experiments on the effects of simultaneously increasing both duration and intensity of light stimulus.
Based on the above experiments, we used a fixed light stimulus (14 μW and 40 ms) for the remainder of the studies in controls and patients, since the subjects did not tolerate more intense light stimuli without blinking.
Pupillometry data in normal controls
Descriptive data for the eight pupil light reflex indices is summarized in Table 1. We were able to obtain all eight indices in 73 controls. The late measures of redilation (T¾ and D¾) could not be obtained in six controls because they were unable to suppress blinking. We assessed the correlation of the eight indices with age. BPD, MCV, ACA, and MDV correlated negatively with age (all p values <0.02). RCA positively correlated with age (p < 0.0001). The rest of the indices, T¾, DV1, and D¾ did not change significantly with age. Figure 3 summarizes the correlations for BPD and ACA with age.
Association of pupillary indices with age. Linear regression (solid line) of BPD and ACA with age in healthy controls. Both had negative association (p < 0.001). MCV, RCA, and MDV were all negatively correlated with age. Age in X-axis and BPD and ACA indices in Y-axis. Dashed lines indicate the 95 % prediction intervals
Comparison of high and normal CASS groups with controls
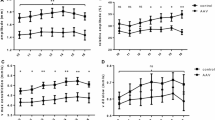
We, then compared the above eight indices of pupillometry in patients with normal autonomic function (CASS < 2) and in those with moderate to severe autonomic failure (CASS > 4). ANCOVA analysis was used instead of ANOVA, as the mean age of the high CASS group (65.1 years) was higher than that of other groups (see Table 1). BPD was not different in the three groups when corrected for age. MCV, ACA, RCA, and MDV were significantly lower in the high CASS group compared to the other two groups (p < 0.0001) indicative of impairment in parasympathetic innervation of the pupil. DV1 was also significantly different between the groups. While DV¾ and T¾ did not differ between groups, when the T¾ was adjusted for the magnitude of pupillary constriction (ACA), the resulting ratio (T¾:ACA) was higher in the high CASS group compared to others (p < 0.0003). Table 1 provides a summary of various indices in different groups and Fig. 4 shows a composite of six of the indices in all three groups.
A composite figure of BPD, MCV, ACA, MDV, Time ¾, and Time ¾:ACA correlation with age. ANCOVA analysis of the three groups. Open circles represent 79 controls, blue dots represent 28 normal CASS patients, and red dots represent 26 high CASS patients. By ANCOVA analysis, BPD was not significantly different in the three groups. MCV, ACA, and MDV were statistically significant in the three groups (p < 0.0001). While Time ¾ was not different between the groups, Time ¾ when corrected for the absolute constriction amplitude (Time ¾:ACA) was different in the three groups (p < 0.0001). Solid and dashed lines represent the regression line and 95 % prediction intervals, respectively, for the control subjects
Discussion
Our study provides additional guidance on the use of infrared pupillometry as an autonomic testing tool. This study was performed using readily available commercial equipment. We were able to (1) obtain normative data for various pupillary indices using a standardized light stimulus (14 μW intensity and 40 ms duration), (2) identify markers of pupillary light reflex from the constriction and dilation phases, corresponding to the parasympathetic and sympathetic pupillary function, and (3) demonstrate that these indices of pupil autonomic function differ in patients with moderate to severe autonomic dysfunction compared to healthy controls.
Quantitative pupillometry can supplement routine autonomic reflex testing by providing additional measures of autonomic function that are not related to cardiovascular function. Pupillometry can potentially identify both parasympathetic and sympathetic abnormalities. Another important observation from our study is that BPD, which can be easily measured during routine clinical evaluation, was not significantly different in the three groups when corrected for age. Thus, assessment of pupil autonomic function requires a dynamic assessment of the pupil light reflex. We also showed that most indices of pupil function decrease with age, similar to the measures of parasympathetic cardiovagal autonomic function (HRDB range and Valsalva ratio) [14]. Recognizing this finding is critically important, as degenerative autonomic disorders are more common in older age. However, after appropriate adjustment for age, pupillary indices were still significantly different in patients with moderate to severe autonomic dysfunction compared to controls. Pupillary parasympathetic markers (MCV, ACA, and RCA) were significantly different in the high CASS group compared to others. Indices obtained from early pupillary dilation (DV1 and MDV) did not differ between the three groups.
Other investigators have studied pupillometry as a way to assess autonomic function in various disorders [3] and as a screening tool in diagnosing autonomic neuropathy in diabetes [5]. Our study provides further evidence that pupillary function can be objectively determined [2], and that the pupillary indices are consistently different in patients with moderate to severe autonomic failure compared to healthy controls. In contrast to previous studies of pupillometry, to our knowledge, we are the first to correlate pupil function with autonomic severity score rather than specific disease entities. This is an important distinction, as pupillary dysfunction seems to correlate with the presence of diffuse global autonomic dysfunction.
Our study has many limitations. We attempted to identify multiple indices from the controls, and it is likely that some of the indices correlate very strongly with each other and do not add additional information [15]. For example, MCV, ACA, and RCA are considered solely parasympathetic measures, but it is unclear if calculation of RCA provides any additional information compared to ACA. Also, the indices from the initial part of the upslope (Fig. 1), MDV and DV1, have a high correlation with the parasympathetic indices and probably represent a withdrawal of parasympathetic drive to the pupil rather than a true sympathetic measure. Evaluation of the later part of pupillary dilation, T¾ and DV¾, was perhaps a better measure of sympathetic function, but T¾ and DV¾ were not significantly different between the groups. However, when T¾ was corrected for the magnitude of pupillary dilation (T¾:ACA), there was a significant difference between the groups. Currently, we use MCV and ACA as parasympathetic markers in our clinic and plan to incorporate late pupillary redilation indices, T¾, DV¾, and T¾:ACA as markers of sympathetic function after further studies.
While the pupil responses were quite stable on repeated measurements during a single test session, all of our testing was done in the morning and we did not perform extensive test–retest reliability studies over several days or at different times of day. It is potentially possible that pupil reflex measures will vary during the day in the same individual with mild variation in physiological states [16]. However, our data suggest that such variation will be smaller than the differences between the patients with autonomic failure and normal autonomic function. Most other measures of autonomic reflex testing such as HRDB range vary minimally with repeated testing. Also, our group of patients with moderate to severe autonomic dysfunction included patients with different causes of dysautonomia. This lack of uniformity in the disease group is a limitation, but also points to the fact that pupillometry can be of potential use irrespective of the cause of dysautonomia. In high CASS group, subset scores from CASS (sudomotor, adrenergic, and cardiovascular HR index) were not individually correlated with pupillary dysfunction because of the small sample size. This limits our ability to compare the parasympathetic and sympathetic indices of pupillary function to other standard measures of parasympathetic and sympathetic function. Finally, sensitivity/specificity analysis and development of pupillary indices as a screening test for autonomic dysfunction would have been useful, while small sample size, wide confidence intervals, and methodology were limiting factors and these were not performed.
In summary, we have examined several indices of infrared pupillometry that reflect parasympathetic and sympathetic dysfunction and found that these differ significantly in patients with diffuse autonomic failure compared to subjects with normal autonomic function. Studies comparing pupil function with global autonomic dysfunction, especially cardiac autonomic dysfunction, need to be completed. In addition, studies on individual diseases with known pupillary involvement such as diabetes [17] might provide valuable data resulting in earlier diagnosis and prevention of the progression of autonomic dysfunction.
References
Ravits JM (1997) AAEM minimonograph #48: autonomic nervous system testing. Muscle Nerve 20(8):919–937. doi:10.1002/(SICI)1097-4598(199708)20:8<919:AID-MUS1>3.0.CO;2-9
Bremner F (2009) Pupil evaluation as a test for autonomic disorders. Clin Auton Res 19(2):88–101. doi:10.1007/s10286-009-0515-2
Bremner F, Smith S (2006) Pupil findings in a consecutive series of 150 patients with generalised autonomic neuropathy. J Neurol Neurosurg Psychiatry 77(10):1163–1168. doi:jnnp.2006.09283310.1136/jnnp.2006.092833
Bremner F, Smith S (2008) Pupillographic findings in 39 consecutive cases of Harlequin syndrome. J Neuroophthalmol 28(3):171–177. doi:10.1097/WNO.0b013e318183c88500041327-200809000-00002
Ferrari GL, Marques JL, Gandhi RA, Heller SR, Schneider FK, Tesfaye S, Gamba HR (2010) Using dynamic pupillometry as a simple screening tool to detect autonomic neuropathy in patients with diabetes: a pilot study. Biomed Eng Online 9:26. doi:1475-925X-9-2610.1186/1475-925X-9-26
Mukherjee S, Vernino S (2007) Dysfunction of the pupillary light reflex in experimental autoimmune autonomic ganglionopathy. Auton Neurosci 137(1–2):19–26. doi:S1566-0702(07)00116-610.1016/j.autneu.2007.05.005
Muppidi S, Scribner M, Gibbons CH, Adams-Huet B, Spaeth EB, Vernino S (2012) A unique manifestation of pupillary fatigue in autoimmune autonomic ganglionopathy. Arch Neurol. doi:archneurol.2011.214310.1001/archneurol.2011.2143
Giza E, Fotiou D, Bostantjopoulou S, Katsarou Z, Karlovasitou A (2011) Pupil light reflex in Parkinson’s disease: evaluation with pupillometry. Int J Neurosci 121(1):37–43. doi:10.3109/00207454.2010.526730
Giza E, Fotiou D, Bostantjopoulou S, Katsarou Z, Gerasimou G, Gotzamani-Psarrakou A, Karlovasitou A (2012) Pupillometry and 123I-DaTSCAN imaging in Parkinson’s disease: a comparison study. Int J Neurosci 122(1):26–34. doi:10.3109/00207454.2011.619285
Lowenstein O, Loewenfeld IE (1950) Mutual role of sympathetic and parasympathetic in shaping of the pupillary reflex to light: pupillographic studies. Arch Neurol Psychiatry 64(3):341–377
Smith SA, Smith SE (1999) Bilateral Horner’s syndrome: detection and occurrence. J Neurol Neurosurg Psychiatry 66(1):48–51
Low PA (1993) Composite autonomic scoring scale for laboratory quantification of generalized autonomic failure. Mayo Clin Proc 68(8):748–752
Low PA, Benrud-Larson LM, Sletten DM, Opfer-Gehrking TL, Weigand SD, O’Brien PC, Suarez GA, Dyck PJ (2004) Autonomic symptoms and diabetic neuropathy: a population-based study. Diabetes Care 27(12):2942–2947. doi:27/12/2942
Low PA, Denq JC, Opfer-Gehrking TL, Dyck PJ, O’Brien PC, Slezak JM (1997) Effect of age and gender on sudomotor and cardiovagal function and blood pressure response to tilt in normal subjects. Muscle Nerve 20(12):1561–1568. doi:10.1002/(SICI)1097-4598(199712)20:12<1561:AID-MUS11>3.0.CO;2-3
Bremner FD (2012) Pupillometric evaluation of the dynamics of the pupillary response to a brief light stimulus in healthy subjects. Invest Ophthalmol Vis Sci 53(11):7343–7347. doi:10.1167/iovs.12-10881
Koelewijn T, Zekveld AA, Festen JM, Kramer SE (2012) Pupil dilation uncovers extra listening effort in the presence of a single-talker masker. Ear Hear 33(2):291–300. doi:10.1097/AUD.0b013e3182310019
Vinik AI, Maser RE, Mitchell BD, Freeman R (2003) Diabetic autonomic neuropathy. Diabetes Care 26(5):1553–1579
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Muppidi, S., Adams-Huet, B., Tajzoy, E. et al. Dynamic pupillometry as an autonomic testing tool. Clin Auton Res 23, 297–303 (2013). https://doi.org/10.1007/s10286-013-0209-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10286-013-0209-7