Abstract
Objective
Postural tachycardia syndrome (PoTS) is an important cause of orthostatic intolerance resulting from cardiovascular autonomic dysfunction. In addition to postural symptoms, PoTS patients may have allied features, including gastrointestinal (GI) symptoms, which have not yet been thoroughly investigated. We evaluated gastric myoelectrical activity in PoTS patients.
Methods
Using cutaneous electrogastrography (EGG), we recorded gastric myoelectrical activity before and after standard liquid meal ingestion in 15 PoTS patients (age 27 ± 4 years); including 7 with and 8 without GI symptoms, and in 11 healthy individuals (age 23 ± 7 years). We performed spectral analysis of EGG recordings to obtain the dominant frequency of gastric pacemaker rhythm (DF), instability coefficient of DF (ICDF), and low (LFR%), normal (NFR%), and high (HFR%) range power percentages of the total power.
Results
Instability coefficient of DF, an index of variability of gastric pacemaker rhythm, was significantly elevated both pre- and post-prandially (30–45 min after the meal) in the PoTS group (8.8 ± 6, 10.0 ± 8 %) compared with controls (4.0 ± 3, 4.0 ± 3 %; both p < 0.05). Patients with GI symptoms had significantly higher post-prandial ICDF (15.0 ± 5 %) than those without GI symptoms (5.6 ± 4 %; p < 0.05). There were no significant differences in DF, LFR%, NFR% and HFR% before and after the meal between the PoTS and control groups, or between PoTS patients with and without GI symptoms.
Interpretation
Our study revealed increased variability of gastric pacemaker rhythm in PoTS, and these findings might be related to pathophysiology of functional GI symptoms in PoTS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Postural tachycardia syndrome (PoTS) is a relatively recently recognised syndrome, characterised by excessive tachycardia on standing (orthostatic tachycardia) in the absence of postural hypotension [1]. PoTS patients predominantly report symptoms of orthostatic intolerance, e.g., light-headedness, palpitations, sweating, shortness of breath, chest pain and fatigue. In addition, PoTS patients may also report symptoms that are not related to posture [1]. For instance, PoTS is also associated with migraine, bladder dysfunction and with gastrointestinal (GI) symptoms, e.g., nausea, bloating, diarrhoea, constipation and abdominal pain [2, 3]. In studies on children, functional GI disorders, such as functional dyspepsia and irritable bowel syndrome, have been associated with orthostatic intolerance and, in particular, PoTS [4]. Although the pathological mechanisms behind possible functional GI disorders and GI symptoms in PoTS remain unclear, it is possible that the sympathetic nervous system dysfunction that can be evident in PoTS [5], e.g., as seen in the emesis of catecholamines in hyper-adrenergic PoTS [1], may directly disturb GI function. Abnormal modulatory monoamine transmission or vagal dysfunction could also be implicated since both have been reported in PoTS [6, 7]. Alternatively, because somatisation, depression and anxiety have all been observed in both patients with PoTS [3] and in patients with functional GI disorders [8], psychosomatic factors may be, at least in part, responsible for the GI symptoms that are seen in many patients with PoTS.
Gastric motility is critical for digestion and is controlled, in part, by the autonomic nervous system. Motility can be indirectly assessed non-invasively in vivo in humans using cutaneous electrogastrography (EGG) to record gastric myoelectrical activity. In healthy subjects, gastric myoelectrical activity is composed of ‘gastric slow waves’ and spike/second potentials at a frequency of 3 cycles per min (cpm) [9]. Gastric slow waves originate from the pacemaker cells on the major curvature of the stomach [9]. Cutaneous EGG, which can detect gastric slow waves, may help to reveal and to characterise abnormal gastric myoelectrical activity in patients with PoTS, but as yet there have not been any studies that have compared EGG activity between PoTS patients and healthy subjects before and after food ingestion.
In order to develop a better understanding of the aetiology of functional GI symptoms in PoTS, the aim of this study was to evaluate gastric myoelectrical activity before and after standard meal ingestion in PoTS patients and normal healthy controls. Based on previous research [10] showing associations between acute psychological stressors (e.g., shock avoidance tasks), heightened sympathetic nerve activity and gastric dysrhythmia, we hypothesised that PoTS patients, some of whom are also known to have elevated sympathetic activity [5], would also have gastric dysrhythmia.
Subjects and methods
Subjects
Fifteen patients with PoTS (1 male and 14 females, aged 27 ± 4 years) were enrolled in the study. A diagnosis of PoTS was based on the following criteria: (1) history of symptoms of orthostatic intolerance for at least 6 months; (2) sustained increase in heart rate while standing or on head-up tilt with a minimum change of at least 30 beats per minute or a heart rate in excess of 120 beats per minute; (3) absence of orthostatic hypotension (a fall in systolic/diastolic blood pressure greater than 20/10 mmHg, respectively). Patients were asked whether they had experienced the following GI symptoms (without an obvious cause) during the past 3 months: indigestion, early satiety, upper abdominal pain, nausea, vomiting, fullness, diarrhoea and constipation and were, on that basis, allocated to a “GI symptoms” or a “no GI symptoms” group. None of the participants had any organic neurologic disorders or clinically significant illnesses potentially affecting gastrointestinal motility or autonomic nervous function. Eleven of the 15 PoTS patients had been diagnosed or had features suggestive of joint hypermobility syndrome, or Ehlers Danlos III, according to the Beighton diagnostic criteria. Eleven healthy subjects (three males and eight females, aged 23 ± 7 years) from the general public and student populations of Imperial College London and the University of Oxford (recruited using posters and electronic advertisements), not on any medications and with no history of functional GI diseases or autonomic symptoms were also studied. Participants were excluded if they had a history of gastric, intestinal or colonic surgery. All patients and healthy participants were in the normal BMI range. Verbal and written informed consent was obtained from all participants. All procedures received institutional and local ethical approval.
Protocol
Each participant was asked to refrain from eating for at least 3 h before attending the laboratory. Regular medications were withdrawn 1 day before testing. None of the participants reported opiate or selective serotonin reuptake inhibitors (SSRI) use in their drug histories. All participants’ orthostatic cardiovascular autonomic function was assessed before and after the meal challenge test. Participants rested for 10 min in the supine position before undergoing an orthostatic challenge. Blood pressure and heart rate were recorded using upper arm sphygmomanometry (GE Medical Systems, Tampa, FL, USA). Mean arterial blood pressure was calculated as (1/3 × systolic blood pressure) + (2/3 × diastolic blood pressure). After the orthostatic challenge, all participants rested for 15 min before a liquid meal was ingested through a straw whilst supine. The meal consisted of 20 g glucose and 60 g Complan® made up to 300 ml with full cream milk. All participants then rested for 45 min before the orthostatic challenge was repeated. Gastric myoelectrical activity was recorded for 15 min before and for 45 min after the ingestion of the meal.
EGG measurement and analysis
Gastric myoelectrical activity was measured using a four-channel cutaneous EGG recorder (Nipro EG; Nipro, Japan) at a sampling rate of 1 Hz. Four surface recording electrodes and one reference electrode were placed on the abdominal skin surface as described previously in detail [12]. EGG data were analysed offline using EGG software (EGS2 Ver. 3.1 software, EG, Ram Co., Japan). Visual inspection of the raw EGG waves was used to select the channel with the highest amplitude from the four channels for further analysis, and to eliminate movement artefacts using the EGG software. Fast Fourier transformation (FFT) was applied to obtain EGG power spectra for 15 min before the meal (for baseline values), for 15 min immediately after the meal (early post-prandial segment) and for the period of 30–45 min after the meal (late post-prandial segment). We classified frequency ranges as low (1.6–2.0 cpm; LFR), normal (2.0–4.0 cpm; NFR) and high (4.0–9.0 cpm; HFR). The dominant frequency (DF) was defined as the frequency at which the overall power spectrum showed peak power in NFR [11]. The ratios of LFR (LFR%), NFR (NFR%) and HFR (HFR%) components were calculated as percentages of total power. Previous research indicated that, in healthy subjects, DF transiently decreases following ingestion of a meal, before returning to pre-prandial levels, if not exceeding them [12]. To detect the post-prandial DF dip, running spectral analysis was performed using FFT in the range 1.6–9.0 cpm (Fig. 2). FFT was applied to consecutive 512-s data periods with a 392-s overlap. Minimum post-prandial DF is defined as the nadir of the post-prandial DF dip. LFR%, NFR% and HFR% at the time of the minimum post-prandial DF were also recorded. Running FFT was also used to obtain the instability coefficient of DF (ICDF). ICDF is an indicator of the variability of DF; calculated as the ratio of the standard deviation and the mean of DF [11, 13]. ICDFs were obtained in three 15 min EGG segments; pre-prandial, and early (0–15 min) and late (30–45 min) post-prandial.
Statistical analysis
Data are presented as mean ± standard deviation or standard error where indicated. Independent t tests or repeated measures ANOVA were used to assess cardiovascular and EGG responses before and after the meal and between groups were appropriate. If a significant main effect was found after repeated measures ANOVA, Bonferroni correction for multiple comparisons was applied. Values of p = 0.05 were used to indicate statistical significance. All data were analysed using an online commercial software package (SPSS, PASW Statistics 18).
Results
GI symptoms
Seven of the PoTS patients met the inclusion criteria for the GI symptoms group (one male and six females; mean age = 29 ± 11 years) while eight patients did not (eight females; mean age = 25 ± 6 years).
Cardiovascular responses
Baseline supine blood pressure was not significantly different between the PoTS and healthy control groups (Table 1). Heart rate tended to be higher in the PoTS group (p = 0.05). Baseline blood pressure was not significantly different between PoTS patients with and without GI symptoms but the PoTS patients without GI symptoms had a significantly higher heart rate than PoTS with GI symptoms (p < 0.05). During pre-prandial orthostatic challenge, the elevation in heart rate was significantly higher in PoTS compared to healthy controls (p < 0.05) with no significant differences between PoTS patients with and without GI symptoms. Blood pressure was well maintained and was not significantly different relative to supine in any group.
After the meal, diastolic blood pressure and mean arterial pressure significantly decreased in the PoTS patients (both p < 0.05) whereas blood pressure was well maintained in the healthy controls. Supine heart rate did not change after the meal but was significantly higher in the PoTS group relative to the healthy control group (p < 0.05). There were no significant differences in the post-prandial blood pressure or heart rate responses in the patient groups with or without GI symptoms (p > 0.05). Heart rate remained significantly higher in the PoTS patients without GI symptoms relative to those patients with GI symptoms (p < 0.05). During post-prandial orthostatic challenge, PoTS patients had a significantly greater increase in heart rate than healthy subjects (p < 0.05) with no significant differences between PoTS patients with and without GI symptoms. Blood pressure was well maintained in all groups.
EGG responses
On visual inspection of the raw EGG waves, the gastric slow waves were of regular amplitude and frequency in the healthy subject group (Fig. 1a), whereas in the patient group participant’s waves were much less regular in both form and frequency (Fig. 1b). There were no significant differences in pre-prandial DF, LFR%, NFR% and HFR% between the PoTS patients and controls, whereas pre-prandial ICDF in the PoTS patients was significantly higher than that in the controls (p < 0.05; Table 2).
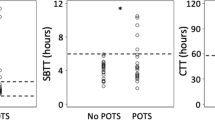
DF decreased transiently after the meal before rising to exceed pre-meal levels approximately 25 min after the meal in the control subjects and PoTS patients, but this response (post-prandial dip) appeared to be obscured in the PoTS patients (Fig. 2). DF and NFR% significantly decreased in the PoTS (both p < 0.05) and control (both p < 0.05) groups, whereas LFR% and HFR% significantly increased at the post-prandial dip compared with baseline in the PoTS (both p < 0.05) and control (p < 0.05, p < 0.05) groups. ICDF also significantly increased in the early post-prandial stage in the PoTS (p < 0.05) and control (p < 0.05) groups (Table 2). There were no significant differences in DF, LFR%, NFR% and HFR% at the post-prandial dip and late post-prandial stage between PoTS patients and healthy participants (Table 2). Although there was no significant difference in ICDF in the early post-prandial stage between the two groups, ICDF in the PoTS patients was significantly higher than that in the controls in the late post-prandial stage (p < 0.05; Fig. 3; Table 2).
Instability coefficient of dominant frequency (ICDF) throughout the meal challenge test (error bars represent one standard error of the mean). PoTS postural tachycardia syndrome, GI gastrointestinal. *p < 0.05 versus healthy subjects; $ p < 0.05 versus PoTS without GI symptoms; # p < 0.05 versus pre-meal
With regard to subgroup analysis in the PoTS patients, there were no significant differences in any pre-prandial parameters between patients with and without GI symptoms. There were no significant differences in DF, LFR%, NFR% and HFR% at the post-prandial dip (Table 2). Although ICDF also showed no significant difference in the early post-prandial stage, ICDF in the late post-prandial stage was significantly higher in the patients with GI symptoms compared with patients without GI symptoms (p < 0.05; Table 2; Fig. 3).
Discussion
Postural tachycardia syndrome is a syndrome characterised by excessive heart rate responses to orthostasis and orthostatic intolerance. Patients are typically young females who develop symptoms after a major event, e.g., pregnancy, viral illnesses, periods of intense stress or surgery. The cardiovascular features of the syndrome have been well described [14]. Many PoTS patients also describe non-posture-related symptoms, including functional GI symptoms, such as nausea, bloating, diarrhoea, constipation and abdominal pain [3], which have, as yet, not been thoroughly investigated. There has been no study that compared EGG findings between PoTS patients and healthy subjects. Our study evaluated EGG in PoTS patients and healthy subjects, and revealed that PoTS patients had fluctuating slow waves and high ICDF both before and after meal ingestion, particularly in patients with GI symptoms.
One explanation that could account for the coexistence of functional GI symptoms and PoTS is that of a vasomotor disturbance, since splanchnic hyperaemia has been reported as a major feature of certain PoTS patients during orthostasis [15] and postural symptoms are often exacerbated after food ingestion [16]. The excessive release of vasoactive intestinal peptides during orthostasis, resulting in thoracic hypovolaemia, may lead to the tachycardia [15]. Inappropriate secretion of intestinal peptides could also conceivably alter GI motility through increased delivery of hormonal regulators.
It is possible that the link between the GI symptoms in PoTS and functional GI disorders is predominantly psychosomatic. It is thought that depression and anxiety may be more common in PoTS patients [3, 17, 18]. Psychological disturbances, including excessive anxiety, are common in patients with functional GI disorders, and they strongly influence the clinical phenotype [19]. Interestingly, it has been shown that acute psychological stressors (e.g., shock avoidance tasks), which also increase sympathetic nerve activity, can evoke dysrhythmic gastric myoelectrical activity [10]. Fluctuating gastric myoelectrical activities in our PoTS patients may thus reflect their possible anxious phenotype, although anxiety sensitivity indices were not measured in this study. Furthermore, PoTS patients have been shown to have features suggestive of increased sympathetic nerve activity, e.g., a ‘hyperadrenergic tone’ [14, 20]. A heightened level of sympathetic nerve activity, either independent of or related to anxiety, could also contribute to impaired gastric myoelectrical activity [21]. Regardless of the potential cause of GI symptoms in PoTS, because gastric emptying reflects precise coordination between the fundus, antrum, pylorus and duodenum, a disruption of gastric myoelectrical activity, as observed in the PoTS patients in the present study, would very likely lead to delayed gastric emptying, bloating and nausea [22]; symptoms often reported in the same patient population. Gastric dysrhythmias have also been observed in individuals with nausea, vomiting, early satiety, anorexia, and dyspepsia including gastroparesis [23, 24].
Mechanistically, the high ICDF in PoTS patients in the present study may represent dysregulation of central and/or peripheral serotonergic and adrenergic pathways, both of which have been reported in PoTS patients [25, 26] and in patients with functional GI disorders [27, 28]. These pathways are both thought to be involved in modifying cardiovascular function [29, 30] and in regulating GI motility [31, 32]. It is thus interesting to note that 5-HT1A receptor agonists have been used in the treatment of functional GI disorders [33] and that SSRIs are capable, in some instances, of alleviating symptoms of both PoTS [34] and functional GI disorders. It is unclear whether the beneficial effects of these agents are mediated centrally or peripherally.
Interestingly, 11 of the 15 PoTS patients had been diagnosed or had features suggestive of joint hypermobility syndrome. It is well recognised that there is an association between joint hypermobility syndrome and functional GI disorders [35]. It is thus possible that the connective tissue matrix in the digestive tract is impaired in PoTS patients with joint hypermobility syndrome. The connective tissue matrix contributes to the passive mechanical properties of the gut [36] and these are likely to be important in the post-prandial gastric distension that we observed as a transient decrease in DF (post-prandial dip) in the healthy subjects in this study. Although the PoTS patients also demonstrated a similar quantitative decrease, it was qualitatively irregular and not as well defined. Meier-Ruge et al. described hypo-peristalsis in patients with colonic desmosis (a reduction of the connective tissue in the myenteric plexus region or within the circular or longitudinal muscle layers), providing evidence that changes in the extracellular matrix can affect GI motility and manifest GI symptoms [37]. Whether or not there are qualitative or quantitative changes in the extracellular matrix of patients with PoTS or with functional GI disorders has not yet been studied but would be important in order to corroborate this potential mechanism.
High ICDF may be also found in patients with Parkinson’s disease [11, 36], who have Lewy body pathology in the myenteric plexus. However, patients with Parkinson’s patients also exhibit reduced NFR% [36] or increased HFR% [11], unlike our PoTS patients. The gastric slow waves originate from the pacemaker on the major curvature of the stomach, and interstitial cells of Cajal and the myenteric plexus of Auerbach play an important role in generation and propagation of the gastric slow waves [11, 36]. The abnormal EGG findings in Parkinson’s disease most likely reflect arrhythmic or ectopic pacemaker discharge due to involvement of the myenteric plexus. Meanwhile, our PoTS patients showed preserved NFR% and yet had high ICDF. These findings suggest increased variability of the slow wave frequency rather than arrhythmia of the slow waves. Abnormal sympatho-vagal balance seems to increase fluctuations of the slow waves.
All patients stopped taking their medication the day before testing. However, long-term effects would not necessarily be abolished by short-term cessation of therapy. Another consideration is the small sample sizes of the groups. Although significant differences have been detected in a study with relatively small sample sizes, a larger study population would render the findings more robust.
In conclusion, our study showed that patients with PoTS have abnormal gastric myoelectrical activity, indicating increased variability of gastric slow wave frequency. The EGG abnormality suggests involvement of autonomic/enteric neural activities in PoTS, and might be related to pathophysiology of functional GI symptoms in these patients. Further investigations using additional assays, such as manometry or gastric emptying, are required in order to elucidate the mechanisms behind these findings.
References
Mathias CJ (2002) To stand on one’s own legs. Clin Med 2:237–245
Antiel RM, Risma JM, Grothe RM, Brands CK, Fischer PR (2008) Orthostatic intolerance and gastrointestinal motility in adolescents with nausea and abdominal pain. J Pediatr Gastroenterol Nutr 46:285–288
Low PA, Sandroni P, Joyner M, Win-Kuang S (2009) Postural tachycardia syndrome (POTS). J Cardiovasc Electrophysiol 20:352–358
Safder S, Chelimsky TC, O’Riordan MA, Chelimsky G. (2009) Autonomic testing in functional gastrointestinal disorders: implications of reproducible gastrointestinal complaints during tilt table testing. Gastroenterol Res Pract Article ID 868496
Furlan R, Jacob G, Snell M et al (1998) Chronic orthostatic intolerance—a disorder with discordant cardiac and vascular sympathetic control. Circulation 98:2154–2159
Stewart JM (2000) Autonomic nervous system dysfunction in adolescents with postural orthostatic tachycardia syndrome and chronic fatigue syndrome is characterized by attenuated vagal baroreflex and potentiated sympathetic vasomotion. Pediatr Res 48:218–226
Grubb BP, Karas BJ (1998) The potential role of serotonin in the pathogenesis of neurocardiogenic syncope and related autonomic disturbances. J Interv Card Electrophysiol 2:325–332
van Oudenhove L, Vandenberghe J, Geeraerts B et al (2007) Relationship between anxiety and gastric sensorimotor function in functional dyspepsia. Psychosom Med 69:455–463
Chang FY (2005) Electrogastrography: basic knowledge, recording, processing and its clinical applications. J Gastroenterol Hepatol 20:502–516
Muth ER, Koch KL, Stern RM, Thayer JF (1999) Effect of autonomic nervous system manipulations on gastric myoelectrical activity and emotional responses in healthy human subjects. Psychosom Med 61:297–303
Sakakibara Y, Asahina M, Suzuki AK, Hattori T (2009) Gastric myoelectrical differences between Parkinson’s disease and multiple system atrophy. Mov Disord 24:1579–1586
Kaneoke Y, Koike Y, Sakurai N et al (1995) Gastrointestinal dysfunction in Parkinson’s disease detected by electrogastroenterography. J Auton Nerv Syst 50:275–281
Suzuki A, Asahina M, Ishikawa C, Asahina KM, Honma K, Fukutake T, Hattori T (2005) Impaired circadian rhythm of gastric myoelectrical activity in patients with multiple system atrophy. Clin Auton Res 15:368–372
Mathias CJ, Low DA, Iodice V, Owens AP, Kirbis M, Grahame R (2011) The postural tachycardia syndrome (PoTS)—current experiences and concepts. Nat Neurol 8(1):22–34
Stewart JM, Medow MS, Glover JL, Montgomery LD (2006) Persistent splanchnic hyperemia during upright tilt in postural tachycardia syndrome. Am J Physiol Heart Circ Physiol 290:665–673
Thieben MJ, Sandroni P, Sletten DM et al (2007) Postural orthostatic tachycardia syndrome: the mayo clinic experience. Mayo Clin Proc 82:308–313
Masuki S, Eisenach JH, Johnson CP et al (2007) Excessive heart rate response to orthostatic stress in postural tachycardia syndrome is not caused by anxiety. J Appl Physiol 102:896–903
Raj SR (2006) The Postural Tachycardia Syndrome (POTS): pathophysiology, diagnosis & management. Indian Pacing Electrophysiol J 6:84–99
van Oudenhove L, Vandenberghe J, Geeraerts B et al (2007) Relationship between anxiety and gastric sensorimotor function in functional dyspepsia. Psychosom Med 69:455–463
Bonyhay I, Freeman R (2004) Sympathetic nerve activity in response to hypotensive stress in the postural tachycardia syndrome. Circulation 110:3193–3198
Mazur M, Furgała A, Jabłoński K, Madroszkiewicz D, Ciećko-Michalska I, Bugajski A, Thor PJ (2007) Dysfunction of the autonomic nervous system activity is responsible for gastric myoelectric disturbances in the irritable bowel syndrome patients. J Physiol Pharmacol 58(Suppl 3):131–139
Camilleri M, Hasler WL, Parkman HP et al (1998) Measurement of gastrointestinal motiliy in the GI laboratory. Gastroenterology 115:747–762
Koch KL, Stern RM, Stewart WR et al (1989) Gastric emptying and gastric myoelectric activity in patients with diabetic gastroparesis: effect of long-term domperidone treatment. Am J Gastroenterol 84:1069–1074
Abell TL, Camilleri M, Hench VS et al (1991) Gastric electromechanical function and gastric emptying in diabetic gastroparesis. Eur J Gastroenterol Hepatol 3:163–167
Esler M, Alvarenga M, Pier C et al (2006) The neuronal noradrenaline transporter, anxiety and cardiovascular disease. J Psychopharmacol 20:60–66
Grubb BP, Karas BJ (1998) The potential role of serotonin in the pathogenesis of neurocardiogenic syncope and related autonomic disturbances. J Interv Card Electrophysiol 2:325–332
Camilleri M, Atanasova E, Carlson PJ et al (2002) Serotonin-transporter polymorphism pharmacogenetics in diarrhea-predominant irritable bowel syndrome. Gastroenterology 123:425–432
Neal KB, Parry LJ, Bornstein JC (2009) Strain-specific genetics, anatomy and function of enteric neural serotonergic pathways in inbred mice. J Physiol 587:567–586
Jordan D (2005) Vagal control of the heart: central serotonergic (5-HT) mechanisms. Exp Physiol 90:175–181
Kasparov S, Teschemacher AG (2008) Altered central catecholaminergic transmission and cardiovascular disease. Exp Physiol 93:725–740
Nagata M, Osumi Y (1993) Central alpha 2-adrenoceptor-mediated inhibition of gastric motility in rats. Jpn J Pharmacol 62:329–330
O’Mahony S, Dinan TG, Keeling PW, Chua AS (2006) Central serotonergic and noradrenergic receptors in functional dyspepsia. World J Gastroenterol 12:2681–2687
Stanghellini V, De Ponti F, De Giorgio R, Barbara G, Tosetti C, Corinaldesi R (2003) New developments in the treatment of functional dyspepsia. Drugs 63:869–892
Russo V, De Crescenzo I, Ammendola E, Santangelo L, Calabro R (2007) Sympathovagal balance analysis in idiopathic postural orthostatic tachycardia syndrome. Acta Biomed 78:133–138
Zarate N, Farmer AD, Grahame R et al (2010) Unexplained gastrointestinal symptoms and joint hypermobility: is connective tissue the missing link? Neurogastroenterol Motil 22:252–278
Lu CL, Shan DE, Chen CY, Luo JC, Chang FY, Lee SD, Wu HC, Chen JD (2004) Impaired gastric myoelectrical activity in patients with Parkinson’s disease and effect of levodopa treatment. Dig Dis Sci 49:744–749
Meier-Ruge WA, Holschneider AM, Scharli AF (2001) New pathogenetic aspects of gut dysmotility in aplastic and hypoplastic desmosis of early childhood. Pediatr Surg Int 17:140–143
Acknowledgments
We thank Madeline Tippetts, Michael Peche and Vanessa Ponnusamy for their support with the clinical testing of the patients.
Conflict of interest
None declared.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Seligman, W.H., Low, D.A., Asahina, M. et al. Abnormal gastric myoelectrical activity in postural tachycardia syndrome. Clin Auton Res 23, 73–80 (2013). https://doi.org/10.1007/s10286-012-0185-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10286-012-0185-3