Abstract
In recent years, the treatment of periodontal bone defect has been a major challenge. Cell-based bone tissue engineering provides an advanced way for bone regeneration. Bone formation hinges on the potential of osteogenesis in bone marrow stromal cells (BMSCs). Shikonin (SHI), an active principle of Radix Lithospermi, has shown a striking role to mitigate osteoporosis of ovariectomized mice, whereas its effects on periodontal bone defect are vague. Herein, we explored the impact of SHI on osteogenic differentiation of BMSCs in vitro and further analyzed the potential mechanisms using an inhibitor of p38 MAPK (SB203580). A rat periodontal bone defect model was built to assess its effects on bone formation in vivo by micro-CT and immunofluorescence. Our results showed SHI with no cytotoxicity could conspicuously enhanced alkaline phosphatase (ALP) activity, calcium accumulation and the expression of runt-related transcription factor 2 (Runx2) and osteocalcin (OCN) of BMSCs in vitro. Increased bone volume/tissue volume (BV/TV) and osteopontin (OPN) expression after SHI administration further demonstrated the capacity of promoting osteogenesis of SHI in vivo. Furthermore, SHI could also increase the phosphorylation of p38. However, the phosphorylation of p38 and expression of osteogenic indicators promoted by SHI were reversed by SB203580, thereby illustrating the positive regulatory relationship between p38 MAPK and SHI-mediated osteogenesis. This finding may help SHI become a promising agent with respect to the therapy of periodontal bone defect.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Severe periodontal bone defect secondary to periodontitis has been the primary challenge of implant restoration. Bone grafting from the patient’s mandibular ramus or iliac crest is a routine strategy for the treatment of periodontal bone defects [1], which also has obvious limitations such as injury to the donor site, limited amount of donor sites and infection [2]. Due to its extensive sources and multidirectional differentiation ability, bone marrow stromal cells (BMSCs) are widely used for bone regeneration in tissue engineering [3,4,5,6]. Therefore, enhancing osteogenesis of BMSCs may be a latent therapeutic method to achieve bone regeneration. At present, gradually increasing traditional Chinese medicine with few side effects is chosen to promote osteogenesis of BMSCs and bone repairs, so that it has attracted more and more scholars’ attention in this field.
Shikonin (SHI), an active principle obtained from tradition Chinese medicine Radix Lithospermi, has been identified to have anti-inflammatory and anti-tumor properties [7, 8]. It was recently reported that SHI could suppress differentiation of osteoclasts and diminish the bone loss of ovariectomized mice obviously [9]. Furthermore, SHI could also augment the gene expression and protein abundance of BMP-2 signal and phosphorylate a downstream factor Smad5 to activate Runx2 and further promote osteoblast osteogenesis [10]. Previous evidence found that the phosphorylation and activation of p38 occurred through the Smad-independent pathway for brief periods after BMP stimulation [11], suggesting that p38 MAPK might be involved in SHI-induced osteogenesis.
Mitogen-activated protein kinase (MAPK) is the main carrier for signaling through the cell surface to the nucleus and can be expressed by all eukaryotes [12]. As one of the main MAPK subfamilies, p38 MAPK can be activated by phosphorylation through a tertiary cascade reaction and participate in basic biological processes such as cell growth, development and differentiation [13, 14]. Due to its close relationship with bone development and metabolism [15,16,17], it has long been widely studied in the field of bone regeneration.
Herein, we investigated whether SHI could promote osteogenesis of BMSCs in vitro and in vivo and verified the influence of p38 MAPK in SHI-mediated osteogenesis, providing a promising therapeutic method for bone regeneration.
Materials and methods
Reagents
Shikonin (Cell culture grade) was purchased from Solarbio (Beijing, China) and stored at – 80 ℃. α-Minimum essential medium (α-MEM) and fetal bovine serum (FBS) were purchased from BioInd (Israel). SB203580 was purchased from Topscience (Shanghai, China). Antibodies were purchased from BIOSS Biotechnology (Beijing, China). PCR-related reagents were purchased from Vazyme (Nanjing, China). Primers were synthesized by Azenta (Chelmsford, MA, USA).
Cell culture and identification
BMSCs were extracted from 4-week-old male Wistar rats [18], for which use of animals was ratified by Ethics Committee of Jinan Stomatological Hospital (No. JNSKQYY-2022167). Primary cells were maintained in α-MEM supplemented with 20% FBS in 5% CO2 at 37 ℃. When the density of BMSCs reached 80% confluence, they were passaged. Cells (passage 3 ~ 4) were used for subsequent experiments. To verify the multipotent differentiation potency, Cells were separately maintained in osteogenic induction medium (OIM) and adipose induction medium (Gibco, USA). After induction of 3 weeks, respectively, cells were dyed using Alizarin Red S (ARS) and Oil Red O dye (Solarbio, China), and taken at a microscope (Olympus, Japan) in time.
Cytotoxicity assay
BMSCs (5 × 103) were cultured in 96-well plates supplemented with various concentrations of SHI (0, 0.05, 0.1, 0.5, 1 and 2 μg/ml) within 4 days. According to the manufacturer's instructions, the cytotoxicity of SHI was analyzed by a CCK-8 kit (Biosharp, China) and the absorbance was recorded at 450 nm by a microplate reader (Thermo, USA).
ALP staining and activity assay
BMSCs were maintained in OIM and various concentrations of SHI (0, 0.05, 0.1, 0.5 μg/ml) for 7 days and fixed in 4% paraformaldehyde (PFA) for 30 min, rinse gently, and then dyed by ALP stains (Beyotime, China) for 20 min at room temperature. The ALP activity was assessed by an ALP assay kit (Beyotime) and the absorbance was recorded at 405 nm.
ARS staining
Cells were induced for 3 weeks as above, and then fixed with 4% PFA and stained using 1% ARS solution (Solarbio, China) until the expected color appeared. Calcium accumulation was visualized by a microscope. Then, calcium nodules were dissolved by 10% cetylpyridinium chloride (Solarbio, China), and the absorbance at 562 nm was recorded for quantification.
Real-time PCR
After induction for 7 and 14 days, cellular total RNA was extracted by an RNA Extraction Kit and used to synthesize cDNA with a Reverse Transcription Kit. Real-time PCR (20 μl total volume) was accomplished using a Universal SYBR qPCR Master Mix and the procedure was set up as previous description [19]. β-Actin was used as a standardized reference. The final results were presented indirectly via the 2−ΔΔCT method [20]. The designed primers are exhibited in Table 1.
Western blot assay
Protein of each group was extracted by a RIPA lysis buffer and quantified by a BCA kit. Then, each sample was fractionated on polyacrylamide gels and diverted to PVDF membranes. After that, membranes were sealed by 5% BSA supplemented with TBST and incubated with the specific primary antibodies against ALP, Runx2, OCN, p38, p-p38 and β-actin (identical dilution 1: 1000) at 4 ˚C overnight, followed by maintaining with secondary antibody (dilution 1: 5000) for 1 h. Protein band abundance was visualized with an enhanced chemiluminescence (ECL) kit (Biosharp, Anhui, China) at a Amersham ImageQuant 800 imager (GE, Inc., USA) and quantified by Image J software (Nation Institutes of Health, USA).
Rat model of periodontal bone defect
Eight male Wistar rats (age, 8 weeks; weight, 300 ± 20 g) were derived from Shandong University Laboratory Animal Center (Shandong, China) and fed with free access to fodder and water. The rats with bilateral periodontal bone defect of 5 × 2 × 1 mm (model establishment was based on a previous study [21]) were randomly allocated to 2 groups: SHI group (2 mg/kg SHI [9] was injected subcutaneously once every 3 days after 3 days of rats modeling), control group (equal amount of 0.9% sodium chloride was injected subcutaneously). 16 mandibles with periodontal bone defects were harvested after 4 weeks of treatment. 6 mandibles in each group were used for Micro-CT, the rest were used for immunofluorescence.
Micro-computed tomography (Micro-CT)
To assess the alveolar bone repair, fixed mandible samples were scanned by a Micro-CT (PerkinElmer, Inc., MA, USA) at 80 kV and 88 μA. Built-in software was used to analyze bone volume/tissue volume (BV/TV), two-dimensional (2D) and three-dimensional (3D) images of bone defects according to the manufacturer’s instructions.
Immunofluorescence
Separate new bone tissues were fixed in 4% PFA and embedded in paraffin, and then cut into slices subsequently. Sections were treated with 0.1% Tritonx-100 for 20 min, blocked with 5% bovine serum albumin (BSA) for 1 h at room temperature (RT) and incubated successively with primary antibody against osteopontin (OPN; dilution 1: 200, 4 °C, overnight) and secondary antibody (RT, 1 h). Nuclei were stained with a DAPI solution (Sigma, USA). Fluorescent images were observed and recorded by a fluorescence microscopy (Olympus, Japan).
Statistical analysis
All data were presented as the mean ± standard deviation of three independent experiments and analyzed by Student’s t test (two groups) or one-way ANOVA (multiple groups) at GraphPad Prism 8.0 software (San Diego, CA, USA). Statistical significance was accepted with a P value less than 0.05.
Results
Culture and characteristics of BMSCs
The isolated primary cells initially grew like colonies, and the morphology was irregular and mainly short spindle shape. Cell appearance steadily exhibited long spindle shape after three passages (Fig. 1a). BMSCs in this period showed uniform morphology and higher cell vitality, which could be used in subsequent experiments. As shown in Fig. 1b, the deposition of calcium ions and the generation of massive lipid droplets could be distinctly seen after ARS staining and oil red O staining, respectively. The multiple differentiation capacities manifested the stem cell traits of isolated cells.
Identification of BMSCs and cytotoxicity assay of SHI. a The typical MSCs morphology of BMSCs. b Calcium nodules and lipid droplets formation were showed by Alizarin Red S and Oil Red O staining separately. c The cytotoxicity of SHI was detected by CCK-8. SHI at concentration between 0.05 and 0.5 μg/ml was not to be cytotoxic (p > 0.05 compared with the 0 group)
SHI enhanced ALP activity and mineralization of BMSCs at non-cytotoxic concentrations
To detect the cytotoxicity of SHI in vitro, BMSCs were cultured with diverse concentrations of SHI at first to the fourth day. The CCK-8 results suggested SHI at concentration between 0.05 and 0.5 μg/ml was not to be cytotoxic (Fig. 1c). The results of ALP activity assay suggested SHI elicits preferable ALP activity when contrast to the control, especially prominent in the 0.1 μg/ml group (Fig. 2c). In addition, the results of ALP (Fig. 2a) and ARS (Fig. 2b, d) staining also demonstrated that the degree of staining was deeper in the SHI-induced group. Thus, 0.1 μg/ml SHI was picked out for the subsequent tests.
SHI promoted osteogenic differentiation of BMSCs in vitro. a ALP staining and c ALP activity were implemented on day 7. b Alizarin Red S staining on day 21 (scale bars, 100 μm). d The ARS semiquantitative detection by absorbance at 562 nm. e The gene expression of osteogenesis markers (ALP, Runx2, and OCN) after osteogenic induction for 7 and 14 days. (f, g) The protein expression of ALP, Runx2, and OCN after osteogenic induction for 7 days. *p < 0.05, **p < 0.01, ***p < 0.001 compared with the OIM group
SHI accelerated osteogenic markers expression in BMSCs
Real-time PCR was to detect the expression of osteogenic genes (ALP, Runx2 and OCN) at days 7 and 14. Compared with the control (OIM group), the mRNA expression of osteogenic genes was conspicuously enhanced in the OIM + SHI group (Fig. 2e). At the same time, the results of western blot reveled the augmented levels of ALP, Runx2 and OCN protein expression might also be associated with the SHI-induced (Fig. 2f, g).
SHI promoted osteogenesis in vivo
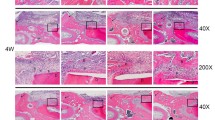
Rat periodontal bone defect repair was detected at 4 weeks post-operation by micro-CT. The bone defects had been filled to a large extent with new bone. Increased BV/TV was observed after SHI administration. More new bone was observed in the SHI group compared with the control group (Fig. 3). Consistently, the expression of OPN protein was enhanced in the SHI group (Fig. 4b1, b2) compared with the control group (Fig. 4a1, a2), according to immunofluorescence results.
SHI enhanced phosphorylation of p38 in its early differentiation stage
Whether the p38 signal was activated by SHI in BMSCs was assessed by testing the level of phosphorylated p38 (p-p38) in BMSCs. After the addition of SHI in OIM, the initial phosphorylation level of the p38 signal was lowest. Over time, the phosphorylation level of p38 augmented expressly, especially prominent in the 1 h (Fig. 5a, b).
SB inhibited the effect of SHI on osteogenic markers in BMSCs. (a, b) Over time, the phosphorylation level of p38 augmented expressly, especially prominent in the 1 h. c The gene pression of ALP, Runx2 and OCN in BMSCs pretreated with SB (10 μM) for 0.5 h, and incubated with SHI and OIM for 14 days. d, e The protein expression of ALP, Runx2 and OCN with the same treatment. *p < 0.05, **p < 0.01, ***p < 0.001
SB203580 diminished the expression of osteogenic markers in SHI-induced BMSCs
SB203580 (SB) is a specific inhibitor of p38 MAPK pathway. To prove the role of p38 during its differentiation stage, cells were pretreated with SB (10 μM) for 30 min prior to SHI induce. As shown in Fig. 5c, d, e, SHI expressly enhanced the expression of osteogenic markers, whereas blocking of p38 completely reversed the situation. Therefore, the p38 MAPK signaling pathway seems to participate in osteogenic response of SHI-induced BMSCs.
Discussion
In recent years, the treatment of periodontal bone defect has been a major challenge. Cell-based bone tissue engineering provides an advanced way for bone defects reconstruction. BMSCs have been widely applied to the research of bone regeneration. Because of its wide range of sources, ability of strong proliferation and differentiate into osteoblasts under specific conditions has become the most widely applied cells for bone tissue engineering [22, 23]. Increasing traditional Chinese medicine with few side effects was selected to promote osteogenic differentiation of BMSCs [24]. Shikonin, the main active principle of Radix Lithospermi, has been reported to have a wide range of pharmacological effects in different diseases. For example, SHI could suppress the proliferation and migration of lung carcinomas in an inflammatory microenvironment [25]. Similarly, SHI has inhibitory effects on breast cancer and osteosarcoma [26, 27]. Moreover, SHI could also inhibit the inflammatory response, such as periodontitis and rheumatoid arthritis [28, 29]. Meanwhile, SHI is a differentiation-inducing agent, which modulates the intercellular redox homeostasis, thus facilitating differentiation of HL-60 cell by Nrf2/ARE pathway [30]. SHI induces odontoblastic differentiation of dental pulp stem cells via AKT-mTOR signaling and effectively inhibits adipogenesis in 3T3-L1 cells by diminishing ERK 1/2 phosphorylation [31, 32]. SHI also has a good therapeutic effect on osteoporosis by inhibiting the formation of osteoclasts [9]. However, the impact of SHI on osteogenic differentiation of BMSCs has not reported.
Here, we explored whether SHI could stimulate ossification of BMSCs and its preliminary mechanism involved. During the phase of cell culture, the addition of appropriate SHI did not affect cell viability (Fig. 1c). ALP activity, as a marker of early osteogenesis [33, 34], peaked in response to 0.1 µg/ml SHI on day 7 and attenuated thereafter (Fig. 2c). Moreover, similar results were also obtained in subsequent mineralization assay (Fig. 2d). Thus, the result implied us that the trend of 0.1 μg/ml SHI promoting osteogenesis was more prominent. Runx2 is a capital transcription factor in regulating osteogenesis [35]. OCN is a specific marker of bone formation and one of the main indicators to evaluate bone metabolism [36]. In this report, the up-regulation of these above markers was associated with the SHI-induced closely (Fig. 2e, f, g). Furthermore, in vivo, we needed to observe the effect of SHI on the repair of bone defects, so we established the model of periodontal bone defect with reference to the previous study. Increased BV/TV and OPN expression after SHI administration further demonstrated the capacity of promoting osteogenesis (Figs. 3 and 4).
Foregone studies have covered the capital role of p38 MAPK in osteogenesis [37,38,39]. Diminution of p38 phosphorylation could notably attenuated osteoblast differentiation and migration [40]. Cytochalasin D was able to activate the p38 pathway to promote osteoblastic differentiation [19]. Asarylaldehyde could induce periodontal ligament stem cells osteogenic differentiation through the same pathway [20]. Our results showed that SHI notably augmented p-p38 level in a short time. In addition, inhibition of the p38 signal activation completely reversed the osteogenesis effect of SHI to BMSCs (Fig. 5). These results definitely reveal that p38 MAPK pathway positively regulates the osteogenic process induced by SHI. In addition, this finding may help SHI become a promising agent with respect to the therapy of periodontal bone defects. In the future research, we will further explore the combination of SHI with appropriate sustained-release system and biological scaffold to ensure local administration and sustained release of SHI which will achieve faster bone healing.
Data availability
All data included in this study are available upon request by contact with the corresponding author.
References
Chavda S, Levin L. Human studies of vertical and horizontal alveolar ridge augmentation comparing different types of bone graft materials: a systematic review. J Oral Implantol. 2018;44(1):74–84.
Chatelet M, Afota F, Savoldelli C. Review of bone graft and implant survival rate: a comparison between autogenous bone block versus guided bone regeneration. J Stomatol Oral Maxillofac Surg. 2022;123(2):222–7.
Dimitriou R, Jones E, McGonagle D, Giannoudis PV. Bone regeneration: current concepts and future directions. BMC Med. 2011;31(9):66–75.
Taschieri S, Bruno GA, Grecchi E, Paolo S, Girolamo D, Del Fabbro M. Nanotechnology scaffolds for alveolar bone regeneration. Materials (Basel). 2020;13(1):201–20.
Deng W, Obrocka M, Fischer I, Prockop DJ. In vitro differentiation of human marrow stromal cells into early progenitors of neural cells by conditions that increase intracellular cyclic AMP. Biochem Biophys Res Commun. 2001;282(1):148–52.
Guan M, Yao W, Liu R, Lam KS, Nolta J, Jia J, Panganiban B, Meng L, Zhou P, Shahnazari M, Ritchie RO, Lane NE. Directing mesenchymal stem cells to bone to augment bone formation and increase bone mass. Nat Med. 2012;18(3):456–62.
Fan C, Zhang X, Upton Z. Anti-inflammatory effects of shikonin in human periodontal ligament cells. Pharm Biol. 2018;56(1):415–21.
Tsai MF, Chen SM, Ong AZ, Chung YH, Chen PN, Hsieh YH, Kang YT, Hsu LS. Shikonin induced program cell death through generation of reactive oxygen species in renal cancer cells. Antioxidants (Basel). 2021;10(11):1831–44.
Chen K, Yan Z, Wang Y, Yang Y, Cai M, Huang C, Li B, Yang M, Zhou X, Wei X, Yang C, Chen Z, Zhai X, Li M. Shikonin mitigates ovariectomy-induced bone loss and RANKL-induced osteoclastogenesis via TRAF6-mediated signaling pathways. Biomed Pharmacother. 2020;126: 110067.
Fang T, Wu Q, Mu S, Yang L, Liu S, Fu Q. Shikonin stimulates MC3T3-E1 cell proliferation and differentiation via the BMP-2/Smad5 signal transduction pathway. Mol Med Rep. 2016;14(2):1269–74.
Moustakas A, Heldin CH. Non-smad TGF-beta signals. J Cell Sci. 2005;118(16):3573–84.
Cargnello M, Roux PP. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol Mol Biol Rev. 2011 Mar;75(1):50–83.
Roux PP, Blenis J. ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol Mol Biol Rev. 2004;68(2):320–44.
Cuadrado A, Nebreda AR. Mechanisms and functions of p38 MAPK signalling. Biochem J. 2010;429(3):403–17.
Chan YH, Ho KN, Lee YC, Chou MJ, Lew WZ, Huang HM, Lai PC, Feng SW. Melatonin enhances osteogenic differentiation of dental pulp mesenchymal stem cells by regulating MAPK pathways and promotes the efficiency of bone regeneration in calvarial bone defects. Stem Cell Res Ther. 2022;13(1):73–90.
Jiang Y, Wu W, Jiao G, Chen Y, Liu H. LncRNA SNHG1 modulates p38 MAPK pathway through Nedd4 and thus inhibits osteogenic differentiation of bone marrow mesenchymal stem cells. Life Sci. 2019;1(228):208–14.
Kang MA, Lee J, Park SH. Cannabidiol induces osteoblast differentiation via angiopoietin1 and p38 MAPK. Environ Toxicol. 2020;35(12):1318–25.
Pieróg J, Tamo L, Fakin R, Kocher G, Gugger M, Grodzki T, Geiser T, Gazdhar A, Schmid RA. Bone marrow stem cells modified with human interleukin 10 attenuate acute rejection in rat lung allotransplantation. Eur J Cardiothorac Surg. 2018;53(1):194–200.
Liu Q, Zhuang Y, Ouyang N, Yu H. Cytochalasin D promotes osteogenic differentiation of MC3T3-E1 cells via p38-MAPK signaling pathway. Curr Mol Med. 2019;20(1):79–88.
Hwang JW, Park WJ, Han Y. Asarylaldehyde enhances osteogenic differentiation of human periodontal ligament stem cells through the ERK/p38 MAPK signaling pathway. Biochem Biophys Res Commun. 2021;19(545):27–32.
Lv L, Wang Y, Zhang J, Zhang T, Li S. Healing of periodontal defects and calcitonin gene related peptide expression following inferior alveolar nerve transection in rats. J Mol Histol. 2014;45(3):311–20.
Caplan AI, Bruder SP. Mesenchymal stem cells: building blocks for molecular medicine in the 21st century. Trends Mol Med. 2001;7(6):259–64.
Uder C, Brückner S, Winkler S, Tautenhahn HM, Christ B. Mammalian MSC from selected species: Features and applications. Cytometry A. 2018;93(1):32–49.
Zhou H, Wang S, Xue Y, Shi N. Regulation of the levels of Smad1 and Smad5 in MC3T3-E1 cells by Icariine in vitro. Mol Med Rep. 2014;9(2):590–4.
Pan T, Zhang F, Li F, Gao X, Li Z, Li X, Ren X. Shikonin blocks human lung adenocarcinoma cell migration and invasion in the inflammatory microenvironment via the IL-6/STAT3 signaling pathway. Oncol Rep. 2020;44(3):1049–63.
Yang Y, Gao W, Tao S, Wang Y, Niu J, Zhao P, Rao C, Yang L. ER-mediated anti-tumor effects of shikonin on breast cancer. Eur J Pharmacol. 2019;15(863): 172667.
Deng B, Qiu B. Shikonin inhibits invasiveness of osteosarcoma through MMP13 suppression. Tumour Biol. 2015;36(12):9311–7.
Shindo S, Hosokawa Y, Hosokawa I, Ozaki K, Matsuo T. Shikonin inhibits inflammatory cytokine production in human periodontal ligament cells. Inflammation. 2016;39(3):1124–9.
Li J, Pang J, Liu Z, Ge X, Zhen Y, Jiang CC, Liu Y, Huo Q, Sun Y, Liu H. Shikonin induces programmed death of fibroblast synovial cells in rheumatoid arthritis by inhibiting energy pathways. Sci Rep. 2021;11(1):18263.
Zhang B, Chen N, Chen H, Wang Z, Zheng Q. The critical role of redox homeostasis in shikonin-induced HL-60 cell differentiation via unique modulation of the Nrf2/ARE pathway. Oxid Med Cell Longev. 2012;15: 781516.
Kajiura K, Umemura N, Ohkoshi E, Ohta T, Kondoh N, Kawano S. Shikonin induces odontoblastic differentiation of dental pulp stem cells via AKT-mTOR signaling in the presence of CD44. Connect Tissue Res. 2021;62(6):689–97.
Gwon SY, Ahn JY, Jung CH, Moon BK, Ha TY. Shikonin suppresses ERK 1/2 phosphorylation during the early stages of adipocyte differentiation in 3T3-L1 cells. BMC Complement Altern Med. 2013;6(13):207–14.
Whyte MP. Hypophosphatasia and the role of alkaline phosphatase in skeletal mineralization. Endocr Rev. 1994;15(4):439–61.
Moss DW. Aspects of the relationship between liver, kidney and bone alkaline phosphatases. Prog Clin Biol Res. 1984;166:79–86.
Komori T. Runx2, an inducer of osteoblast and chondrocyte differentiation. Histochem Cell Biol. 2018;149(4):313–23.
Al Rifai O, Chow J, Lacombe J, Julien C, Faubert D, Susan-Resiga D, Essalmani R, Creemers JW, Seidah NG, Ferron M. Proprotein convertase furin regulates osteocalcin and bone endocrine function. J Clin Invest. 2017;127(11):4104–17.
Song Y, Wu H, Gao Y, Li J, Lin K, Liu B, Lei X, Cheng P, Zhang S, Wang Y, Sun J, Bi L, Pei G. Zinc Silicate/nano-hydroxyapatite/collagen scaffolds promote angiogenesis and bone regeneration via the p38 MAPK pathway in activated monocytes. ACS Appl Mater Interfaces. 2020;12(14):16058–75.
Yang X, Yang Y, Zhou S, Gong X, Dai Q, Zhang P, Jiang L. Puerarin stimulates osteogenic differentiation and bone formation through the ERK1/2 and p38-MAPK signaling pathways. Curr Mol Med. 2018;17(7):488–96.
Choi H, Jeong BC, Kook MS, Koh JT. Betulinic acid synergically enhances BMP2-induced bone formation via stimulating Smad 1/5/8 and p38 pathways. J Biomed Sci. 2016;23(1):45–53.
Ran G, Fang W, Zhang L, Peng Y, Wu A, Li J, Ding X, Zeng S, He Y. Polypeptides IGF-1C and P24 synergistically promote osteogenic differentiation of bone marrow mesenchymal stem cells in vitro through the p38 and JNK signaling pathways. Int J Biochem Cell Biol. 2021;141: 106091.
Acknowledgements
Natural Science Foundation of Shandong Province (ZR2020MH182, ZR2021MH230), Clinical medicine science and technology innovation plan of Jinan (202019068, 2022-2-167).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lin, X., Wang, Y., Guo, X. et al. Shikonin promotes rat periodontal bone defect repair and osteogenic differentiation of BMSCs by p38 MAPK pathway. Odontology 111, 649–657 (2023). https://doi.org/10.1007/s10266-022-00774-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10266-022-00774-w