Abstract
Dental pulp stem cells (DPSCs) are a new population of mesenchymal stem cells (MSCs) located in the oral cavity with potential capacities for tissue regeneration and immunomodulation. The purpose from this study was to determine effects of curcumin nanoparticle into phytosomal formulation (PC) on the relative expression of DSPP, VEGF-A, HLA-G5, VCAM1, RelA and STAT3 genes which are among the most important factors influencing processes of immunomodulatory and tissue regenerative by DPSCs. After isolation and culture of DPSCs, these cells were characterized according to predetermined criteria including flow cytometric analysis for detection of the most important cell surface markers and also evaluation of multilineage differentiation potential. Then, the MTT method was employed to check the cell viability in treatment with different concentrations of PC. Following DPSCs’ treatment with an optimal-non-toxic dose of this nanoparticle, quantification of expression of target genes was performed using real-time PCR procedure. According to results of immunophenotyping analysis and cell differentiation experiments, the isolated cells were confirmed as MSCs as more than 99% of them expressed specific mesenchymal markers while only about 0.5% of them were positive for hematopoietic marker. The real-time PCR results indicated that PC significantly reduced the expression of RelA, STAT3, VCAM1 and HLA-G5 genes up to many times over while optimally enhanced the expression of DSPP and VEGF-A genes, although this enhance was statistically significant only for VEGF-A (all P < 0.001). The study suggests that PC affects the stemness capabilities of DPSCs and it may facilitate the development of MSCs-based therapeutics in regenerative dentistry.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dental pulp mesenchymal stem cells (DPSCs/DP-MSCs) are a heterogeneous and non-hematopoietic population of mesenchymal stem cells (MSCs) that were first isolated in 2000 from dental pulp tissue by Gronthos and coworkers [1]. According to the ISCT (International Society for Cell & Gene Therapy) criteria, DPSCs are characterized by high proliferation capacity, multilineage differentiation ability, expression of specific mesenchymal markers (CD90, CD73 and CD105) and non-expression of haematopoietic markers (CD19, CD34 and CD45) [2, 3].
It had been reported that these cells compared to other sources of MSCs possess higher capabilities in terms of proliferation, immunomodulation, tissue repair and regeneration [4,5,6,7,8]. The immunomodulation potential is due to direct interaction with immune cells by PD-L1 and -L2 (programmed cell death ligand -1 and -2), HLA-G1 (human leukocyte antigen-G1) and also secretion of cytokines and growth factors including IDO (indolamine 2,3-dioxygenase), HLA-G5 (human leukocyte antigen-G5), IL-10 (interleukin 10), TGF-β (transforming growth factor beta) and PGE2 (prostaglandin E2) [9,10,11].
Dental pulp is a crucial tissue for longevity and homeostasis of teeth. Tooth decay as one of the most common health problems can lead to pulp damage and necrosis and eventually tooth loss. For this reason, one of the important goals in regenerative dentistry is maintenance and repair of inflamed and damaged pulp and also avoid invasive treatments such as pulpectomy [12]. Increasing the expression of DSPP (dentin sialophosphoprotein), ALP (alkaline phosphate), DMP-1(dentin matrix protein 1) and VEGF (Vascular endothelial growth factor) genes which involve in regeneration or repair dental pulp-dentin complex, is a new opportunity to improve the stemness status of these cells for treatment of the damaged pulp in patients with pulpitis [1, 12,13,14,15]. Furthermore, researches have proposed that the high expression of CD106/VCAM1 (vascular cell adhesion molecule-1) and STRO-1 (stromal precursor antigen-1) biomarkers as well as STAT (signal transducer and activator of transcription) family signaling molecules and also low/non-expression of the RelA (nuclear factor-kB p65) transcription factor enhance migration, differentiation and immune modulation properties of MSC cells [16,17,18,19,20,21,22,23,24,25,26].
In this regard, the usage of herbal extracts or compounds have been introduced as ideal gene stimulators [27, 28]. Curcumin is a natural compound obtained from Curcuma longa plant with numerous therapeutic and biological activities, including anti-inflammatory and tissue repair and regenerative activities. However, poor solubility and instability of curcumin, plus its rapid metabolism profile, limit its therapeutic properties [29,30,31,32].
Nano-material technology is a growing field with very promising potentials and applications in modern medicine. Recently, phytosomal curcumin (PC), a nanoparticle type of curcumin into phytosomal formulation has been considered as novel candidate for overcoming some limitations of curcumin, improving the drug delivery into the cell and crossing unavailable different barriers. It was observed that phytosomes are highly stable compared to liposomes and other nanoparticles due to their strong covalent bonds between phosphatidylcholine and curcumin [33,34,35,36].
Because of the precise molecular mechanisms of immunomodulation and regeneration in DP-MSCs are still uncertain, more investigation is needed to support clinical applications of DP-MSCs and optimize transplantation conditions. Based on the above, it was the aim of this study to evaluate the role of PC on DSPP, VEGF-A, HLA-G5, VCAM1, RelA and STAT3 gene expression levels of DP-MSCs, targeting their application as inducers/modifiers of their immunomodulatory and regenerative potential in dental pulp tissue regeneration.
Materials and methods
Isolation and culture of DP-MSCs
Healthy and non-carious third molars (n = 3) were taken with informed consent from patients (20–25 years old) at Dental center of Imam Reza Hospital in Birjand (Iran) according to the guidelines of ethics committee of the Birjand University of medical sciences (ethical number: IR.BUMS.REC.1399.090). DP-MSCs from pulp organ tissues were isolated as described previously [37]. Briefly, after the separation of pulp tissue from dental root and crown parts, the pulp digestion process was performed with collagenase enzyme type I (Gibco, USA). Then, digested tissue suspension was centrifuged and resulted pellet was cultured in complete medium including the Dulbecco’s modified Eagle’s medium mixture F12 (DMEM/F12) with 50 mg/ml streptomycin, 50 unit/ml penicillin and 15% fetal bovine serum (all from Gibco, USA). Cells were incubated at 37 °C under water-saturated atmosphere plus 5% CO2. The medium was refreshed two times in week until cells confluence was obtained. All subsequent analyses were done with cells in passages of 3–5.
Multilineage differentiation potential of DP-MSCs
To induce DP-MSCs osteogenic differentiation, 105 cells were implanted in each well of 6-well plates. When the cells achieved 100% confluency, they were induced with an osteogenic medium in DMEM containing 15% FBS, 10 mM β-glycerol phosphate, 0.1 µM dexamethasone, and 50 µM ascorbic acid (all from Sigma-Aldrich, USA) for 21 days. The differentiated cells were then fixed in 4% paraformaldehyde and stained for Alizarin Red [37]. In addition, to induce adipogenesis, the cells were treated with an inductive medium contain 0.5 mM isobutyl-methyl-xanthine, 1 µM dexamethasone, 200 µM indomethacin (all from Sigma-Aldrich, USA), for 14 days. Then, the cultures were stained using Oil Red solution after cell fixation with paraformaldehyde [37]. Cell staining results were investigated under a phase-contrast microscope (Olympus Microsystems).
Immunophenotyping of DP-MSCs by flow cytometric analysis
Immunophenotyping analysis was performed according to the optimized protocol by Al-Habib et al. with a slight change [38]. Briefly, 106 cells were harvested and washed with PBS solution and subsequently stained with human conjugated antibodies at 4 °C in the dark. Finally, after washing the cells with PBS, they were used for flow cytometric analysis by CyFlow Cube 6 cytometer (Sysmex Partec, Germany). The following antibodies were used to identify cell-specific markers: CD105-PE, CD90-APC, CD73-PE CY7 and CD45-FITC (all from eBioscience, USA).
Cell treatments
Stock solutions in dimethyl sulfoxide (DMSO, Sigma) were prepared, according to the molecular mass and solubility of the PC. The stock solutions were then diluted with complete medium to obtain the different concentrations PC (i.e., 10–100 μM). In addition, as a control group, the cells were incubated in a complete medium containing 1% DMSO. Therefore, there were two groups of control samples: DP-MSCs + DMSO and un-treated DP-MSCs. PC was received from Sami Labs Ltd (Bangalore, India).
Evaluation of cell viability
The optimum and highest non-toxic concentration of PC on the DP-MSCs was determined by MTT procedure [39]. Briefly, the cells were seeded in 96-well microplates (5 × 103 cells in each well) and allowed to adhere for 24 h. First, the cells were treated to PC media as described earlier at a broad spectrum of concentrations for 24 h to obtain approximately the appropriate non-toxic dose. Then, 30 μL of MTT (Sigma-Aldrich, USA) solution with 5 mg/mL concentration was subjected to every well 24, 48, and 72 h after the PC treatment in various concentrations (30, 35, and 40 μM) and the plates were maintained in the dark for 4 h at 37 °C. The medium was slowly discarded and DMSO was subjected to the wells. Absorbance was assessed at 570 nm by the Epoch Microplate spectrophotometer (BioTek Instrument, USA).
Quantitative real-time PCR analysis
Total RNA extraction and cDNA synthesis were done according to the manufacturer’s guidelines (both from Pars Tous, Iran), at 24 and 48 h after DP-MSCs treatment with PC. SYBR Green-based quantitative real-time PCR by the 2−ΔΔCt method was utilized for investigation of the relative expression of DSPP, VEGF-A, HLA-G5, VCAM1, RelA and STAT3 genes. In the analysis, target genes were normalized to the internal control gene GAPDH and each assay was done in triplicate (n = 3). The sequence of primers used were as follows: DSPP, forward: 5′-TTCCGATGGGAGTCCTAGTG-3′ and reverse: 5′-TCTTCTTTCCCATGGTCCTG-3′; VEGF-A, forward: 5′-AGGGCAGAATCATCACGAAGT-3′ and reverse: 5′-AGGGTCTCGATTGGATGGCA-3′; HLA-G5, forward: 5′ CTGAGATGGAAGCAGTCTT-3′ and reverse: 5′-GCTCCCTCCTTTTCAATCT-3′; STAT3, forward: 5′-GAAGAATCCAACAACGGCAG-3′ and reverse: 5′-TCACAATCAGGGAAGCATCAC-3′; RelA, forward: 5′-AGGCTTCTGGGCCTTATGTG-3′ and reverse: 5′-TGCTTCTCTCGCCAGGAATAC-3′; VCAM1, forward: 5′-ATGTCAATGTTGCCCCCAGA-3′ and reverse: 5′-ACAGGATTTTCGGAGCAGGA-3′; GAPDH, forward: 5′-CGAACCTCTCTGCTCCTCCTGTTCG-3′ and reverse: 5′-CATGGTGTCTGAGCGATGTGG-3′.
Statistical analysis
Statistical significance of the cell viability test data was checked using SPSS Ver. 26 software and one-way ANOVA variance (Tukey’s test). The real-time PCR data were analyzed with the Rest software (T-test). In this study, all data were indicated as the mean ± standard deviation at statistical power of P < 0.05.
Results
Culture and characterization of DP-MSCs
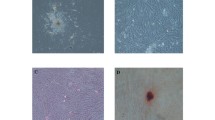
Following 5–6 days of digested pulp incubation, DP-MSCs around pulp tissue along with non-adherent small cells were observed. Almost after three cell passages, a pure population of fibroblast-like, spindle-shaped MSCs was obtained (Fig. 1a, b). The multi-differentiation capacity of extracted cells was ascertained by adipogenesis and osteogenesis induction. Following 14 days adipogenic induction, fat droplets were observable which were acknowledged using Oil Red staining (Fig. 1c). Moreover, DP-MSCs in the osteogenic condition established cellular aggregates that were distinguished by the existence of calcium material deposits. The calcium deposits were stained by Alizarin Red on 21st day of osteo-differentiation (Fig. 1d). The expression of DP-MSCs markers was evaluated by flow cytometry which demonstrated that these cells were positive for CD105, CD90, and CD73 and negative for CD45 (Fig. 2).
Isolation, culture and multipotential differentiation of DP-MSCs in vitro. a, b Migration of MSCs from pulp tissue and change morphologically into pure fibroblast-like, spindle-shaped cells after 3 passages; c adipocyte differentiation and lipid vacuole formation as manifested using Oil Red stain solution after stimulation for 14 days; d osteocyte differentiation and calcium deposit formation as manifested using Alizarin Red stain solution after stimulation for 21 days. DP-MSC, dental pulp mesenchymal stem cell; MSC, mesenchymal stem cell
Immunophenotyping of DP-MSCs isolated from freshly extracted, non-carious human third molar tooth. Flow cytometry histograms display expression of specific mesenchymal markers and no-expression of hematopoietic markers by the DP-MSC population. The isolated and cultured cell phenotype was CD90 + /CD73 + /CD105 + /CD45−. DP-MSC dental pulp mesenchymal stem cell
The lethal effects of PC at high doses and times on DP-MSCs
According to MTT results, 45–60 μM concentrations of PC had significant cell toxicity effects on the DP-MSCs after 24 h (P < 0.05), while no statistically considerable effects were detected in this regard for the concentrations less than or equal to 40 μM (P > 0.05). In addition, the results revealed that there were remarkable differences in cell viability between PC treatment and control groups at concentrations above 30 μM after 72 h (P < 0.05). Therefore, for next experiments, it was selected the concentration of 30 μM in 24 and 48 h based on the results where PC had no effect on hDPSC viability (P > 0.05). Moreover, cell treatment with 1% DMSO did not represent any significant effects (Fig. 3).
Effects of PC on the DP-MSCs viability and proliferation were determined by MTT assay. The column charts show the viable cell percentage with respect to control group (Ctrl, 100%) after the treatment with various concentrations of PC. Data are mean ± SD at least three independent triplicated experiments. *P < 0.05; NS no-significant, PC phytosomal curcumin, DP-MSC dental pulp mesenchymal stem cell, SD standard deviation
The effects of PC on the gene expression of regenerative and immunomodulatory markers in DP-MSCs
Relative expression of DSPP, VEGF-A, HLA-G5, VCAM1, RelA and STAT3 genes were evaluated in the cells cultured in complete medium with or without PC at 24 and 48 h. According to the real-time PCR analysis (Fig. 4), cell treatment with PC resulted in the great up-regulation of VEGF-A gene up to 3.01-fold and 1.61-fold, respectively, in 24 and 48 h (P < 0.001). In addition, significant down-regulation of HLA-G5 (24 h = 0.438-fold, 48 h = 0.674-fold), VCAM1 (24 h = 0.543-fold, 48 h = 0.258-fold), RelA (48 h = 0.376-fold) and STAT3 (24 h = 0.23-fold, 48 h = 0.57-fold) genes was observed after treatment with the nanoparticle (all P < 0.001). In this study, the expression of DSPP mRNA increased in the treatment groups, but this increase was not statistically significant compared to the un-treated groups (24 h = 1.12-fold, 48 h = 1.3-fold and P > 0.05).
Quantitative real-time PCR analysis for DSPP, VEGF-A, HLA-G5, VCAM1, RelA and STAT3 genes in DP-MSCs after the treatment with 30 μM PC. Data are mean ± SD of triplicate experiments. *P < 0.05; NS no-significant, PC phytosomal curcumin, DP-MSC dental pulp mesenchymal stem cell, SD standard deviation, DSPP dentin sialophosphoprotein, VEGF-A vascular endothelial growth factor-A, HLA-G5 human leukocyte antigen-G5, VCAM1 vascular cell adhesion molecule-1, RelA nuclear factor-kB p65, STAT3 signal transducer and activator of transcription-3
Discussion
Pulp regeneration is a new and promising biological strategy in regenerative dentistry for treating the damaged dental pulp with irreversible inflammation. DP-MSCs have been introduced as a prime candidate for the treatment of inflammatory and destructive diseases, including pulpitis [15, 40, 41]. The role of DP-MSCs in pulp regeneration is due to their ability to modulation of immune responses and also multi-differentiation especially into osteo/odontogenic lineages [40, 42]. Change in the gene expression profile of progenitor cells is a widely researched strategy in regenerative medicine. This strategy aims to facilitate cell growth, improve cell survival as well as promote therapeutic effectiveness and stemness status of the cells [27, 40, 43].
The current study was conducted to assess the immunoregulation and repair/regeneration capabilities of DPSCs after treatment with PC on the premise that these cells can potentially be optimized by the nanoparticle. Therefore, DP-MSCs were isolated from pulp tissue using an enzymatic method. The isolated cell biological characteristics including cell surface markers expression and differentiation into various tissues demonstrated that these cells were of mesenchymal stem cell lineage. According to the results of the cell viability test, PC had the time/dose-dependent lethal effects on DP-MSCs. In this study, we performed the real-time PCR analysis on the cells under the condition of treatment with 30 μM of PC.
Our results showed that PC can enhance the expression of DSPP and VEGF-A genes up to many times over, although this enhance was statistically significant only for VEGF-A. In confirmation of the importance of improving these genes by PC, it has been reported that DSPP protein expression and then proteolytic processing of it to little fragments are key steps in dentin formation. So that inhibition of DSPP processing caused hypomineralization defects of dentin in mice and its expression perfectly relieved the dentin weakness in DSPP-deficient mouse model [13].
On the other hand, recent studies have demonstrated that angiogenesis is necessary for maintain pulp homeostasis and survival of transplanted DPSCs to pulp regeneration in vivo and modulation of the immune system in vitro [15, 24, 41, 44]. In this regard, Nakashima and his colleagues [12] introduced DPSC cells as a potential candidate for pulp regeneration and cerebral and limb ischemia treatment due to the high properties of angiogenic and neurogenic.
In accordance with our goals, cell treatment with PC decreased significantly the expression of the RelA molecule which its inhibition may help to improve bone regeneration [45, 46]. More recently, at the ex vivo level, Shuxiang et al. clearly demonstrated that RelA deletion can promote osteogenesis and chondrogenesis in bone marrow-MSCs [23] and it was agreed with the results published by Wang and coworkers [47].
Results of our study also indicated that PC significantly reduces the expression of STAT3, VCAM1 and HLA-G5 genes in DPSCs. In a study conducted by Demircan, it was reported that DP-MSCs exert potent immunoregulatory functions through the increased expression of HLA-G, VCAM1 and VEGF markers in co-culturing with T lymphocyte cells [24]. Although previous studies have shown the role of STAT3 in improving the differentiation and immune modulation potentials of MSCs [25, 26], but similar to our results, it was reported that curcumin exhibits significant effects in promoting the proliferation of neural stem cell (NSC) by down-regulation of STAT3 and glucocorticoid receptor (GR) [48]. In addition, in this regard, Cao et al. suggested that elimination of STAT3 stimulates neural-differentiation in NSCs isolated from mouse embryos [49]. On the other hand, researchers in various research fields have attributed the protective effects of curcumin to the inhibition of signaling pathways associated with STAT3 molecule [50, 51].
A recent article revealed that treatment with liposomal curcumin (CurLIP) causes the improvement in proliferation and immunomodulatory capacities of DPSCs through the negative regulation of NF-kB factor-associated signaling pathways [52]. Another study supported that curcumin therapy can enhance osteo-differentiation in MSCs and optimize them for bone tissue regeneration [53].
Therefore, from these results, it may be inferred that although, PC reduces expression of the HLA-G5 and VCAM1 genes which are important in modulating immunity by MSCs but probably is able to improve stemness capacities of DPSC via up-regulation of DSPP and VEGF-A as well as down-regulation of RelA and STAT3.
Conclusion
These findings reveal that PC can affect the stemness capability of DPSCs in the in vitro level and it may facilitate the development of MSCs-based therapeutics for the degenerative and inflammatory diseases especially maintenance and treatment of inflamed and damaged pulp. However, more studies are required to exactly verify the efficiency PC as a novel strategy to promote tissue regeneration and immunomodulatory properties of MSCs.
Data availability
Data are available from the corresponding author upon reasonable request.
Abbreviations
- DPSC:
-
Dental pulp stem cell
- DP-MSCs:
-
Dental pulp mesenchymal stem cells
- PC:
-
Phytosomal curcumin
- DSPP:
-
Dentin sialophosphoprotein
- VEGF:
-
Vascular endothelial growth factor
- HLA-G5:
-
Human leukocyte antigen-G5
- STAT:
-
Signal transducer and activator of transcription
- VCAM:
-
Vascular cell adhesion molecule
References
Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci. 2000;97(25):13625–30.
Lin C-S, Xin Z-C, Dai J, Lue TF. Commonly used mesenchymal stem cell markers and tracking labels: limitations and challenges. Histol Histopathol. 2013;28(9):1109.
Dominici ML, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini FC, Krause DS, Deans RJ, Keating A, Prockop DJ, Horwitz EM. Minimal criteria for defining multipotent mesenchymal stromal cells. Int Soc Cell Therapy Position Statement. 2006;8(4):315–7.
Siew Ching H, Luddin N, Ab Rahman I, Thirumulu PK. Expression of odontogenic and osteogenic markers in DPSCs and SHED: a review. Curr Stem Cell Res Ther. 2017;12(1):71–9.
Sui B, Wu D, Xiang L, Fu Y, Kou X, Shi S. Dental pulp stem cells: from discovery to clinical application. J Endod. 2020;46(9):S46-55.
Ishizaka R, Iohara K, Murakami M, Fukuta O, Nakashima MJB. Regeneration of dental pulp following pulpectomy by fractionated stem/progenitor cells from bone marrow and adipose tissue. Biomaterials. 2012;33(7):2109–18.
Iohara K, Utsunomiya S, Kohara S, Nakashima M. Allogeneic transplantation of mobilized dental pulp stem cells with the mismatched dog leukocyte antigen type is safe and efficacious for total pulp regeneration. Stem Cell Res Therapy. 2018;9(1):1–6.
Pierdomenico L, Bonsi L, Calvitti M, Rondelli D, Arpinati M, Chirumbolo G, Becchetti E, Marchionni C, Alviano F, Fossati V, Staffolani N. Multipotent mesenchymal stem cells with immunosuppressive activity can be easily isolated from dental pulp. Transplantation. 2005;80(6):836–42.
Hossein-Khannazer N, Hashemi SM, Namaki S, Ghanbarian H, Sattari M, Khojasteh A. Study of the immunomodulatory effects of osteogenic differentiated human dental pulp stem cells. Life Sci. 2019;216:111–8.
Andrukhov CB, Blufstein A, Rausch-Fan X. Immunomodulatory properties of dental tissue-derived mesenchymal stem cells: implication in disease and tissue regeneration. World J Stem Cells. 2019;11(9):604.
Li Y, Zhang D, Xu L, Dong L, Zheng J, Lin Y, Huang J, Zhang Y, Tao Y, Zang X, Li D. Cell–cell contact with proinflammatory macrophages enhances the immunotherapeutic effect of mesenchymal stem cells in two abortion models. Cell Mol Immunol. 2019;16(12):908–20.
Nakashima M, Iohara K, Sugiyama M. Human dental pulp stem cells with highly angiogenic and neurogenic potential for possible use in pulp regeneration. Cytokine Growth Factor Rev. 2009;20(5–6):435–40.
Zhu Q, Gibson MP, Liu Q, Liu Y, Lu Y, Wang X, Feng JQ, Qin C. Proteolytic processing of dentin sialophosphoprotein (DSPP) is essential to dentinogenesis. J Biol Chem. 2012;287(36):30426–35.
Jin Q, Yuan K, Lin W, Niu C, Ma R, Huang Z. Comparative characterization of mesenchymal stem cells from human dental pulp and adipose tissue for bone regeneration potential. Artif Cells Nanomed Biotechnol. 2019;47(1):1577–84.
Dissanayaka WL, Hargreaves KM, Jin L, Samaranayake LP, Zhang C. The interplay of dental pulp stem cells and endothelial cells in an injectable peptide hydrogel on angiogenesis and pulp regeneration in vivo. Tissue Eng Part A. 2015;21(3–4):550–63.
Yu J, He H, Tang C, Zhang G, Li Y, Wang R, Shi J, Jin Y. Differentiation potential of STRO-1+ dental pulp stem cells changes during cell passaging. BMC Cell Biol. 2010;11(1):1–7.
Yang X, Zhang W, van den Dolder J, Walboomers XF, Bian Z, Fan M, Jansen JA. Multilineage potential of STRO-1+ rat dental pulp cells in vitro. J Tissue Eng Regen Med. 2007;1(2):128–35.
Yang X, Walboomers XF, van den Beucken JJ, Bian Z, Fan M, Jansen JA. Hard tissue formation of STRO-1–selected rat dental pulp stem cells in vivo. Tissue Eng Part A. 2009;15(2):367–75.
Yang X, Van der Kraan PM, Dolder JV, Walboomers XF, Bian Z, Fan M, Jansen JA. STRO-1 selected rat dental pulp stem cells transfected with adenoviral-mediated human bone morphogenetic protein 2 gene show enhanced odontogenic differentiation. Tissue Eng. 2007;13(11):2803–12.
Fukiage K, Aoyama T, Shibata KR, Otsuka S, Furu M, Kohno Y, Ito K, Jin Y, Fujita S, Fujibayashi S, Neo M. Expression of vascular cell adhesion molecule-1 indicates the differentiation potential of human bone marrow stromal cells. Biochem Biophys Res Commun. 2008;365(3):406–12.
Mabuchi Y, Morikawa S, Harada S, Niibe K, Suzuki S, Renault-Mihara F, Houlihan DD, Akazawa C, Okano H, Matsuzaki Y. LNGFR+ THY-1+ VCAM-1hi+ cells reveal functionally distinct subpopulations in mesenchymal stem cells. Stem Cell Rep. 2013;1(2):152–65.
Hollands P, Aboyeji D, Orcharton M. Dental pulp stem cells in regenerative medicine. Br Dent J. 2018;224(9):747.
Yu S, Li P, Li B, Miao D, Deng Q. RelA promotes proliferation but inhibits osteogenic and chondrogenic differentiation of mesenchymal stem cells. FEBS Lett. 2020;594(9):1368–78.
Demircan PC, Sariboyaci AE, Unal ZS, Gacar G, Subasi C, Karaoz E. Immunoregulatory effects of human dental pulp-derived stem cells on T cells: comparison of transwell co-culture and mixed lymphocyte reaction systems. Cytotherapy. 2011;13(10):1205–20.
Xu K, Xiao J, Zheng K, Feng X, Zhang J, Song D, Wang C, Shen X, Zhao X, Wei C, Huang D. MiR-21/STAT3 signal is involved in odontoblast differentiation of human dental pulp stem cells mediated by TNF-α. Cell Reprogram. 2018;20(2):107–16.
Vigo T, La Rocca C, Faicchia D, Procaccini C, Ruggieri M, Salvetti M, Centonze D, Matarese G, Uccelli A. IFNβ enhances mesenchymal stromal (Stem) cells immunomodulatory function through STAT1-3 activation and mTOR-associated promotion of glucose metabolism. Cell Death Dis. 2019;10(2):1–8.
Kornicka K, Kocherova I, Marycz K. The effects of chosen plant extracts and compounds on mesenchymal stem cells—a bridge between molecular nutrition and regenerative medicine-concise review. Phytother Res. 2017;31(7):947–58.
Das U, Behera SS, Pramanik K. Ethno-herbal-medico in wound repair: an incisive review. Phytotherapy Res. 2017;31(4):579–90.
Mohammadi A, Blesso CN, Barreto GE, Banach M, Majeed M, Sahebkar A. Macrophage plasticity, polarization and function in response to curcumin, a diet-derived polyphenol, as an immunomodulatory agent. J Nutr Biochem. 2019;1(66):1–6.
Kahkhaie KR, Mirhosseini A, Aliabadi A, Mohammadi A, Mousavi MJ, Haftcheshmeh SM, Sathyapalan T, Sahebkar A. Curcumin: a modulator of inflammatory signaling pathways in the immune system. Inflammopharmacology. 2019;27(5):885–900.
Hassan FU, Rehman MS, Khan MS, Ali MA, Javed A, Nawaz A, Yang C. Curcumin as an alternative epigenetic modulator: mechanism of action and potential effects. Front Genet. 2019;4(10):514.
Ahangari N, Kargozar S, Ghayour-Mobarhan M, Baino F, Pasdar A, Sahebkar A, Ferns GA, Kim HW, Mozafari M. Curcumin in tissue engineering: a traditional remedy for modern medicine. BioFactors. 2019;45(2):135–51.
Tuyaerts S, Rombauts K, Everaert T, Van Nuffel AM, Amant F. A phase 2 study to assess the immunomodulatory capacity of a lecithin-based delivery system of curcumin in endometrial cancer. Front Nutr. 2019;11(5):138.
Pastorelli D, Fabricio AS, Giovanis P, D’Ippolito S, Fiduccia P, Soldà C, Buda A, Sperti C, Bardini R, Da Dalt G, Rainato G. Phytosome complex of curcumin as complementary therapy of advanced pancreatic cancer improves safety and efficacy of gemcitabine: results of a prospective phase II trial. Pharmacol Res. 2018;1(132):72–9.
Marjaneh RM, Rahmani F, Hassanian SM, Rezaei N, Hashemzehi M, Bahrami A, Ariakia F, Fiuji H, Sahebkar A, Avan A, Khazaei M. Phytosomal curcumin inhibits tumor growth in colitis-associated colorectal cancer. J Cell Physiol. 2018;233(10):6785–98.
Mirzaei H, Shakeri A, Rashidi B, Jalili A, Banikazemi Z, Sahebkar A. Phytosomal curcumin: a review of pharmacokinetic, experimental and clinical studies. Biomed Pharmacother. 2017;1(85):102–12.
Gronthos S, Arthur A, Bartold PM, Shi SA. method to isolate and culture expand human dental pulp stem cells. In: Mesenchymal stem cell assays and applications. Totowa: Humana Press; 2011. p. 107–21.
Al-Habib M, Huang GT. Dental mesenchymal stem cells: dental pulp stem cells, periodontal ligament stem cells, apical papilla stem cells, and primary teeth stem cells—isolation, characterization, and expansion for tissue engineering. Odontogenesis. 2019;1922:59–76.
Ayadilord M, Nasseri S, Emadian Razavi F, Saharkhiz M, Rostami Z, Naseri M. Immunomodulatory effects of phytosomal curcumin on related-micro RNAs, CD200 expression and inflammatory pathways in dental pulp stem cells. Cell Biochem Funct. 2021. https://doi.org/10.1002/cbf.3659.
Zhu L, Dissanayaka WL, Zhang C. Dental pulp stem cells overexpressing stromal-derived factor-1α and vascular endothelial growth factor in dental pulp regeneration. Clin Oral Invest. 2019;23(5):2497–509.
Dissanayaka WL, Zhu L, Hargreaves KM, Jin L, Zhang C. Scaffold-free prevascularized microtissue spheroids for pulp regeneration. J Dent Res. 2014;93(12):1296–303.
Nakashima M, Iohara K, Murakami M, Nakamura H, Sato Y, Ariji Y, Matsushita K. Pulp regeneration by transplantation of dental pulp stem cells in pulpitis: a pilot clinical study. Stem Cell Res Therapy. 2017;8(1):1–3.
Fierro FA, Kalomoiris S, Sondergaard CS, Nolta JA. Effects on proliferation and differentiation of multipotent bone marrow stromal cells engineered to express growth factors for combined cell and gene therapy. Stem Cells. 2011;29(11):1727–37.
Zimta AA, Baru O, Badea M, Buduru SD, Berindan-Neagoe I. The role of angiogenesis and pro-angiogenic exosomes in regenerative dentistry. Int J Mol Sci. 2019;20(2):406.
Hirata-Tsuchiya S, Fukushima H, Katagiri T, Ohte S, Shin M, Nagano K, Aoki K, Morotomi T, Sugiyama G, Nakatomi C, Kokabu S. Inhibition of BMP2-induced bone formation by the p65 subunit of NF-κB via an interaction with Smad4. Mol Endocrinol. 2014;28(9):1460–70.
Tarapore RS, Lim J, Tian C, Pacios S, Xiao W, Reid D, Guan H, Mattos M, Yu B, Wang CY, Graves DT. NF-κB has a direct role in inhibiting Bmp-and Wnt-induced matrix protein expression. J Bone Miner Res. 2016;31(1):52–64.
Wang N, Zhou Z, Wu T, Liu W, Yin P, Pan C, Yu X. TNF-α-induced NF-κB activation upregulates microRNA-150–3p and inhibits osteogenesis of mesenchymal stem cells by targeting β-catenin. Open Biol. 2016;6(3): 150258.
Ma XX, Liu J, Wang CM, Zhou JP, He ZZ, Lin H. Low-dose curcumin stimulates proliferation of rat embryonic neural stem cells through glucocorticoid receptor and STAT 3. CNS Neurosci Ther. 2018;24(10):940–6.
Cao F, Hata R, Zhu P, Nakashiro KI, Sakanaka M. Conditional deletion of Stat3 promotes neurogenesis and inhibits astrogliogenesis in neural stem cells. Biochem Biophys Res Commun. 2010;394(3):843–7.
Zhang DM, Li YC, Xu D, Ding XQ, Kong LD. Protection of curcumin against fructose-induced hyperuricaemia and renal endothelial dysfunction involves NO-mediated JAK–STAT signalling in rats. Food Chem. 2012;134(4):2184–93.
Liu L, Liu YL, Liu GX, Chen X, Yang K, Yang YX, Xie Q, Gan HK, Huang XL, Gan HT. Curcumin ameliorates dextran sulfate sodium-induced experimental colitis by blocking STAT3 signaling pathway. Int Immunopharmacol. 2013;17(2):314–20.
Sinjari B, Pizzicannella J, D’Aurora M, Zappacosta R, Gatta V, Fontana A, Trubiani O, Diomede F. Curcumin/liposome nanotechnology as delivery platform for anti-inflammatory activities via NFkB/ERK/pERK pathway in human dental pulp treated with 2-hydroxyethyl methacrylate (HEMA). Front Physiol. 2019;11(10):633.
Wang N, Wang F, Gao Y, Yin P, Pan C, Liu W, Zhou Z, Wang J. Curcumin protects human adipose-derived mesenchymal stem cells against oxidative stress-induced inhibition of osteogenesis. J Pharmacol Sci. 2016;132(3):192–200.
Acknowledgements
The writers appreciate Birjand University of Medical Sciences for supporting this research and also Dr. Seyed Mohammad Riahi, Cardiovascular Diseases Research Center, Department of Epidemiology and Biostatistics, School of Medicine, Birjand University of Medical Sciences, Birjand, Iran, for providing assistance in the statistical analysis.
Funding
This research was financed by Birjand University of Medical Sciences, Iran (Grant no.456182).
Author information
Authors and Affiliations
Contributions
MS study design, executor of plan, analysis and interpretation of data and drafting of the manuscript. MA study design, executor of plan, analysis and interpretation of data and drafting of the manuscript. FER study design, edit and critical revision of the manuscript for important intellectual content. MN material support, study design, executor of plan, supervision and interpretation and analysis of data and edit of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest to declare.
Ethical approval
The research adheres to the guidelines of ethics committee of the Birjand University of medical sciences, Iran (ethical number: IR.BUMS.REC.1399.090).
Informed consent
Informed consent was obtained from participants included in the study.
Consent for publication
The participants consented to the publication of these data.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Saharkhiz, M., Ayadilord, M., Emadian Razavi, F. et al. Effects of phytosomal curcumin treatment on modulation of immunomodulatory and pulp regeneration genes in dental pulp mesenchymal stem cells. Odontology 110, 287–295 (2022). https://doi.org/10.1007/s10266-021-00659-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10266-021-00659-4