Abstract
This study assessed the antibacterial activity of BioRoot RCS in comparison with that of the Totalfill BC and AH Plus sealers against Enterococcus faecalis biofilms in dentinal tubules using confocal laser-scanning microscopy. Sixty-six root dentin halves were prepared and sterilized. Three sections were used to ensure sterilization. The remaining were inoculated with E. faecalis. Three specimens were examined to verify the viability of biofilms. The sixty specimens were randomly divided into four groups: AH Plus, BioRoot RCS, Totalfill BC sealer, and no sealer. The specimens were incubated for 1, 7, and 30 days. The specimens were stained and four corners of each disc were scanned. Statistical analysis was performed using two-way ANOVA and Tukey’s post hoc test. Almost half of the bacteria were dead in BioRoot RCS group on day 1 and in Totalfill BC group on day 7. All sealers killed significantly more bacteria than the control after 30 days (P < .05). On day 7, Totalfill BC showed a significantly higher percentage of dead bacteria than BioRoot RCS (P < .05). On day 30, the BioRoot RCS group registered the highest percentage of dead cells (61.75%), which was significantly higher than the percentages of the AH Plus and Totalfill BC groups (P < .05). Calcium silicate-based root canal sealers exerted antimicrobial effects against E. faecalis biofilms. The antibacterial activity of BioRoot RCS was significantly higher than that of the Totalfill BC and AH Plus sealers after 30 days of exposure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bacteria and their byproducts are the primary cause of pulpal and periapical pathosis [1]. Bacterial elimination from the root canal is achieved by both chemical disinfection and mechanical preparation of the root canal system. Despite the variety of available chemical irrigants and mechanical strategies, the complete elimination of microbes from the canal system in all cases is impossible. Therefore, the use of root canal filling materials with antibacterial activity is considered beneficial.
The root canal is classically filled using gutta-percha in combination with a root canal sealer [2]. Different sealers are constantly being developed and launched to the market. AH Plus (Dentsply DeTrey, Konstanz, Germany) is an epoxy resin-based sealer that has a good antibacterial effect against Enterococcus faecalis [3] and good adhesion to root dentin [4]. AH Plus is commonly used in endodontics and is used as a reference material for comparison [5, 6]. However, AH Plus exhibits a variable degree of cytotoxicity [5] and does not present any mineralization potential [7]. Totalfill BC sealer (FKG Dentaire, La-Chaux-de-Fonds, Switzerland also known as EndoSequence BC Sealer, Brasseler USA, Savannah, GA) is a premixed bioceramic sealer composed of zirconium oxide, calcium silicates, calcium phosphate monobasic, calcium hydroxide, filler, and thickening agents. The setting reaction of Totalfill BC sealer is triggered by the moisture present in the dentinal tubules. Totalfill BC sealer is a biocompatible sealer [6] that has the ability to release calcium ions [8]. BioRoot RCS (Septodont, Saint-Maur-des-Fosses, France) is another bioceramic sealer that has appeared on the market and is supplied as a powder and liquid. The powder is based on tricalcium silicate and zirconium oxide. The aqueous solution is composed mainly of calcium chloride. The BioRoot RCS sealer is prepared by hand-mixing one spoonful of powder with five drops of liquid using a spatula for 60 s. BioRoot RCS has low cytotoxicity in periodontal ligament cells and induces the secretion of osteogenic growth factors [9, 10]. It has excellent radiopacity [11] and has the ability to penetrate the dentinal tubules [12]. Furthermore, the penetration depth of BioRoot RCS is not affected by remnants of calcium hydroxide when used as an intracanal medicament [13].
Numerous studies have been performed to assess the antimicrobial activity of different endodontic sealers [14,15,16]. In earlier studies, the agar diffusion test was a commonly used technique [14, 15]. However, this technique is no longer recommended, because the results are dependent on the diffusion and physical properties of the tested materials. Therefore, the agar diffusion test has been replaced by the direct contact test (DCT) [17]. A disadvantage of the DCT is that it does not consider several factors in the experimental setting, such as the chemistry of the tooth and biofilm formation [18]. Furthermore, the presence of dentin reduces the bacterial killing of endodontic antimicrobial agents, which might be explained by the dentin-buffering effect [19, 20]. To overcome these limitations, Ma et al. developed a three-dimensional in vitro model for quantitative assessment of bacterial viability in dentin by confocal laser-scanning microscopy (CLSM) after infection and disinfection of the dentinal tubules [21].
Enterococcus faecalis, a gram-positive facultative anaerobe, is found in 4 to 40% of primary endodontic infections and in 24 to 77% of persistent endodontic infections [22]. It has the ability to survive in the root canal as a single organism [23] and to resist nutrient starvation for a long period of time [24]. Moreover, calcium hydroxide has a limited antimicrobial efficiency against E. faecalis [25]. Therefore, E. faecalis is often used as a model organism to evaluate the antimicrobial effectiveness of different irrigants, medicaments, and sealers [18, 21, 26]. The aim of this laboratory study was to assess the antibacterial activity of BioRoot RCS in comparison with the Totalfill BC and AH Plus sealers against E. faecalis biofilms in dentinal tubules using CLSM.
Materials and methods
Specimen preparation
Sixty-six semicylindrical root dentin halves were prepared from 33 extracted human single-rooted teeth according to the protocol described by Ma et al. [21], with some modifications. Teeth with one non-calcified canal, which was confirmed by radiographs taken from the buccolingual and mesiodistal views, were selected. Roots with caries, cracks, anatomic irregularities, or previous endodontic treatment were excluded. The teeth were stored in normal saline solution at 5 °C until use. For preparation, the roots were embedded vertically at the cementoenamel junction (CEJ) in a rubber mould containing epoxy resin (Vertex Orthoplast; Vertex-Dental, Zeist, The Netherlands). A mounting device was used to ensure orientation along the long axis of the tooth. A root dentin block with a length of 4 mm was horizontally sectioned from each tooth at 1 mm below the CEJ by a 0.6-mm-thick precision diamond saw (Isomet; Buehler Ltd., Lake Bluff, IL) at 1000 rpm under water cooling. The root canals inside the blocks were enlarged with a complete pass of Gates Glidden burs (Dentsply Maillefer, Ballaigues, Switzerland), sizes 2, 3, 4, 5, and 6, at 300 rpm under water cooling to achieve a diameter of 1.5 mm. Each cylindrical dentin block was sectioned into 2 semicylindrical halves using the diamond saw. For specimen standardization, the cementum was removed from the outer surfaces of the semicylindrical halves using 600-grit fine-grain sandpaper (Buehler, Lake Bluff, IL). The size of each specimen was 4 × 4 × 2 mm (width × length × height). The smear layer was then removed by immersing the dentin sections in 5.25% NaOCl for 4 min and 17% ethylenediamine tetraacetic acid (EDTA) (Vista Dental Products, Racine, WI, USA) for 1 min in an ultrasonic bath. Sodium thiosulfate was used for 1 min to inactivate NaOCl. Finally, all sections were rinsed in sterile water for 1 min and sterilized using gamma irradiation with a dose of 25 kGy. Three dentin sections were then incubated in 5 mL of brain heart infusion (BHI) broth for 24 h at 37 °C to ensure that there was no bacterial contamination.
Dentin Infection with E. faecalis Biofilms
Enterococcus faecalis strain (American Type Culture Collection 47077) was used for biofilm formation. The bacterial strain was plated in BHI broth and incubated at 37 °C for 24 h. A single colony of E. faecalis from BHI agar plates was collected and suspended in 5 mL of sterile BHI broth at 37 °C. The cell suspension was standardized spectrophotometrically to achieve a turbidity equivalent to a 0.5 McFarland standard, which corresponded to an optical density of 0.08 to 0.1 based on the absorbance at 600 nm. The sterilized dentin specimens were placed in sterile centrifuge tubes containing 3 mL of E. faecalis suspension. The specimens were incubated at 37 °C for 3 weeks. The medium was replaced with fresh BHI broth every third day using a 24-h prepared culture to remove dead cells and to ensure bacterial viability.
Placement of sealer
The dentin specimens were removed from each tube aseptically and gently rinsed with sterile phosphate-buffered saline for 1 min to remove the culture medium and loosely attached planktonic bacteria. Three dentin specimens were randomly selected and examined by CLSM (Leica TCS SP2, Leica Microsystems, Heidelberg GmbH, UK) to verify the viability of E. faecalis biofilms. The sixty specimens were randomly divided into four groups (n = 15/group) according to the root canal sealer used, as follows: AH Plus Jet, BioRoot RCS, Totalfill BC sealer, and no sealer (control) (Fig. 1). AH Plus was mixed and placed inside the blocks using an automixing syringe. Totalfill BC sealer is a premixed ready-to-use sealer that was applied directly with its tip. BioRoot RCS was prepared according to the manufacturer’s instructions and placed on the root canal inside the blocks using a disposable 3-mL syringe. A scalpel was used to remove the excess material. All specimens were placed at 37 °C in 100% relative humidity for 1, 7, and 30 days.
CLSM examination
At each timepoint, five dentin sections from each group were stained with the fluorescent LIVE/DEAD BacLight Bacterial Viability stain (Molecular Probes, Eugene, OR) and examined by CLSM. The LIVE/DEAD BacLight Bacterial Viability kit contains two nucleic acid-binding dyes: SYTO 9, which labels all bacteria within a population regardless of membrane integrity, and propidium iodide (PI), which crosses compromised or damaged cell membranes. When both stains are present in cells with damaged membranes, SYTO 9 fluorescence is reduced due to displacement by the PI stain. Bacteria with intact cell membranes stain green, whereas those with damaged membranes stain red. The sealers were scraped off from dentin walls. The specimens were rinsed in sterile phosphate-buffered saline for 1 min. The fluorescent LIVE/DEAD BacLight Bacterial Viability stain kit was used following the manufacturer’s instructions. The mounted specimens were observed by CLSM through a 10/0.49 NA air immersion objective. For standardization, four corners of each dentin disc were scanned. The fluorescence signal of live bacteria was obtained by illuminating the samples with a 488 nm laser, and the emission was recorded through a 520–540 nm bandpass filter (green channel). Excitation by a 568 nm laser and emission through a 600–630 nm filter were used to detect the fluorescence of dead bacteria (red channel). The mean numbers of both live and dead bacteria were calculated using the ImageJ software (Wayne Rasband, NIH, USA) by automated calculation of any ovoid cell with 0.5–1 µm in diameter. The percentage of dead cells was calculated as follows:
Statistical analysis
The results of the Shapiro–Wilk normality test revealed that the data were normally distributed. The means of the differences in dead cell percentages after exposure to different sealers were compared by two-way analysis of variance (ANOVA), with type of sealer and time as independent variables and percentage of killed cells as dependent variables. Tukey’s post hoc test was used for multiple comparisons. Data were expressed as the mean ± the standard error of the mean. The level of significance was set at 0.05. Statistical analysis was performed using the SPSS statistical software for Windows (version 23; SPSS Inc., Chicago IL, USA).
Results
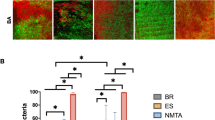
The CLSM images confirmed the presence of homogeneous E. faecalis biofilms on the dentin surfaces, with dense penetration into the dentinal tubules after a 3-week incubation period (Fig. 2). CLSM images verified the presence of E. faecalis biofilms, during the experimental period, in the control group (Fig. 3d, h, l).
The CLSM images of dentin exposed to AH Plus showed few dead bacteria attached to the live bacterial biofilm. These biofilms were affected more by the AH Plus sealer after 30 days of exposure (Fig. 3a, e, i). The majority of E. faecalis biofilms in discs exposed to BioRoot RCS at days 1 and 30 appeared as dead cells attached to the surface (Fig. 3b, j). However, these biofilms were not affected by BioRoot RCS after 7 days of exposure (Fig. 3f). The CLSM images of dentin exposed to Totalfill BC sealer showed the presence of dead bacteria attached to the live bacterial biofilm. Day 7 showed the highest number of dead bacteria compared to day 1 and day 30 (Fig. 3c, g, k).
Two-way ANOVA showed statistically significant differences in the percentage of killed cells among different groups over time (Fig. 4). Almost half of the bacteria were killed by BioRoot RCS (47.88%) on day 1 and by Totalfill BC (46.2%) on day 7, which was significantly higher than the values of the control group at the same timepoints (P = .009 and P = .013, respectively). All the three sealers killed significantly more bacteria than the control group at 30 days (P < .05). There was no significant difference among the AH Plus, BioRoot RCS, and Totalfill BC sealers on day 1. However, on day 7, the latter sealer showed a significantly higher percentage of dead bacteria than BioRoot RCS (P = .000). On day 30, the BioRoot RCS group had the highest percentage of killed cells (61.75%), which was significantly higher than those of the AH Plus (P = .000) and Totalfill BC (P = .04) groups.
Discussion
Calcium silicate-based root canal sealers have gained popularity in endodontics due to their excellent biological and physiochemical properties [9, 27]. An ideal endodontic sealer should have antimicrobial activity to help eliminate the microorganisms that survive inside the root canal system after chemomechanical shaping and cleaning and thereby improve the success rate of endodontic treatment. This study aimed to evaluate the antibacterial effectiveness of two bioceramic sealers. The epoxy-based AH Plus root canal sealer was used for comparison. Dentin discs were prepared from extracted human teeth to reflect the microanatomy of the root canal system. For sterilization, gamma radiation was selected, because it does not alter the dentin structure [28]. The antimicrobial properties were tested after different periods of incubation to evaluate the antibacterial properties over time.
Microorganisms are established in biofilms in infected root canal systems [29]. Bacteria living in biofilms are more resistant to antimicrobials than their planktonic counterparts [30]. Thus, it was crucial to evaluate the antibacterial effect of the sealers in conditions that most closely resemble those found in endodontic infections. The present study utilized a previous model by Ma et al. [21] with some modification. For bacterial inoculation and biofilm generation, the sterilized discs were placed in sterile centrifuge tubes containing E. faecalis suspension. The previous studies demonstrated different incubation periods for E. faecalis biofilm formation. However, bacteria in mature biofilms are more resistant than cells in young biofilms [31]. Thus, in this study, a 3-week incubation period was selected. The formation of dense E. faecalis biofilms on the dentin surface was observed in the present study with CLSM.
CLSM has the ability to control the field depth and eliminate the background signal away from the focal plane. The crucial key to the confocal approach is the use of an aperture in the conjugate focal plane of an objective lens in both the illuminating and imaging pathways of a microscope [32]. The area surrounding the aperture rejects stray photons returning from areas that are not in the focal plane of the lens. Thus, the sound dentin overwhelming autofluorescence signal is eliminated and the signal from the live and dead stained bacteria is enhanced. In addition, the use of the ImageJ software allowed the calculation of bacterial cells without the influence of the autofluorescence signal.
The results of the present study showed that AH Plus had weak activity against E. faecalis. Similar results were reported by the previous investigators who showed that only fresh AH Plus possessed antibacterial activity, whereas 24-h and 7-day-old samples did not show antibacterial effects against E. faecalis [33, 34]. The antimicrobial activity of fresh AH Plus might be related to the toxic effects of amines and epoxy resin present in its components [35]. In addition, this activity could be due to the minimum release of formaldehyde during the polymerization process [34, 36]. The results of this study showed that AH Plus killed significantly more bacteria than the no sealer group after 30 days. A similar finding was reported when infected human dentin discs were used for evaluation [18].
This study showed that both the BioRoot RCS and Totalfill BC sealers possessed antimicrobial effects. The antibacterial activity of sealers is based mainly on their ability to release hydroxyl ions and raise pH values [37]. During the hydration reaction, endodontic bioceramics form calcium hydroxide that dissociates into calcium and hydroxyl ions [38]. It has been reported that the BioRoot RCS and Totalfill BC sealers release calcium ions and raise the pH of the surrounding environment [11, 39, 40], which might explain their antimicrobial effect. The findings of this study concerning the effectiveness of Totalfill BC against E. faecalis are consistent with those from the previous studies [18, 34]. This study also showed that BC had consistent antimicrobial activity throughout the 30-day study, which is in accordance with the findings reported by Wang et al. [18]. In the present work, there was no significant difference in the ability to eliminate E. faecalis biofilm between the Totalfill BC and AH Plus sealers. However, these results are inconsistent with those reported by Candeiro et al., who reported a significantly greater ability of AH Plus to eliminate E. faecalis than the Totalfill BC sealer when assessed by the agar diffusion test [16]. These discrepant findings may be related to differences in experimental conditions. The agar diffusion test might produce false results because it depends on the diffusion capacity of the material in the agar medium. In the same study, when a DCT was used, both materials had significant antibacterial effectiveness [16]. When infected dentin and CLSM were used, it was shown that Totalfill BC had antibacterial effects similar to those of AH Plus [18].
The results of the present study revealed that BioRoot RCS exerted a strong antimicrobial effect on day 1, which was greatly diminished on day 7. Previous reports have shown that BioRoot RCS had time-dependent toxic effect [35, 41], which might explain the higher antibacterial effect on day 1. However, BioRoot RCS showed the strongest effect in 30-day specimens. The possible reason for this pattern is the difference in ion leaching from the sealer. BioRoot RCS showed an increase in calcium hydroxide and pH values after 28 days in Hank’s Balanced Salt Solution compared with the values obtained after 1 day [42]. Further investigations are required to evaluate the impact of biocompatibility and molecular leaching on BioRoot RCS antibacterial activity.
This study is the first to evaluate the long-term antimicrobial effectiveness of the BioRoot RCS sealer. Results in the present study showed that the antibacterial activity of BioRoot RCS was significantly higher than that of the other sealers after 30 days of exposure. In a previous study, BioRoot RCS was reported to have a significantly higher antimicrobial activity than AH Plus after 7 days of exposure [43]. This inconsistency might be explained by differences in experimental design, including the incubation time to establish a biofilm. Arias-Moliz and Camilleri established a 5-day-old biofilm in dentin for antimicrobial evaluation. Bacteria in young biofilms are more susceptible to antimicrobial medicaments due to their development and extracellular polymeric matrix formation [44]. Furthermore, in their study, the dentin discs were sterilized by autoclave, while gamma radiation was selected for the present study. It has been reported that less E. faecalis adheres to autoclaved dentin than to freshly split or gamma-irradiated dentin [45].
The in vitro method used in the current study evaluated the antimicrobial effectiveness against single species. Further studies are indicated to evaluate the antibacterial activity of bioceramic sealers against polymicrobial biofilms.
Conclusions
Calcium silicate-based root canal sealers possess antimicrobial effects against Enterococcus faecalis biofilms. The antibacterial activity of BioRoot RCS is significantly higher than that of the Totalfill BC and AH Plus sealers after 30 days of exposure.
References
Kakehashi S, Stanley HR, Fitzgerald RJ. The effects of surgical exposures of dental pulps in germ-free and conventional laboratory rats. Oral Surg Oral Med Oral Pathol. 1965;20:340–9.
Ørstavik D. Materials used for root canal obturation: technical, biological and clinical testing. Endod Topics. 2005;12:25–38.
Kayaoglu G, Erten H, Alacam T, Orstavik D. Short-term antibacterial activity of root canal sealers towards Enterococcus faecalis. Int Endod J. 2005;38:483–8.
Sousa-Neto MD, Silva Coelho FI, Marchesan MA, Alfredo E, Silva-Sousa YT. Ex vivo study of the adhesion of an epoxy-based sealer to human dentine submitted to irradiation with Er:YAG and Nd:YAG lasers. Int Endod J. 2005;38:866–70.
Miletic I, Devcic N, Anic I, Borcic J, Karlovic Z, Osmak M. The cytotoxicity of RoekoSeal and AH plus compared during different setting periods. J Endod. 2005;31:307–9.
Zhou HM, Du TF, Shen Y, Wang ZJ, Zheng YF, Haapasalo M. In vitro cytotoxicity of calcium silicate-containing endodontic sealers. J Endod. 2015;41:56–61.
Viapiana R, Guerreiro-Tanomaru JM, Hungaro-Duarte MA, Tanomaru-Filho M, Camilleri J. Chemical characterization and bioactivity of epoxy resin and Portland cement-based sealers with niobium and zirconium oxide radiopacifiers. Dent Mater. 2014;30:1005–20.
Candeiro GT, Correia FC, Duarte MA, Ribeiro-Siqueira DC, Gavini G. Evaluation of radiopacity, pH, release of calcium ions, and flow of a bioceramic root canal sealer. J Endod. 2012;38:842–5.
Camps J, Jeanneau C, El Ayachi I, Laurent P, About I. Bioactivity of a calcium silicate-based endodontic cement (BioRoot RCS): interactions with human periodontal ligament cells in vitro. J Endod. 2015;41:1469–73.
Eldeniz AU, Shehata M, Högg C, Reichl FX. DNA double-strand breaks caused by new and contemporary endodontic sealers. Int Endod J. 2016;49:1141–51.
Siboni F, Taddei P, Zamparini F, Prati C, Gandolfi MG. Properties of BioRoot RCS, a tricalcium silicate endodontic sealer modified with povidone and polycarboxylate. Int Endod J. 2017;50(Suppl 2):e120-e36.
Viapiana R, Moinzadeh AT, Camilleri L, Wesselink PR, Tanomaru Filho M, Camilleri J. Porosity and sealing ability of root fillings with gutta-percha and BioRoot RCS or AH Plus sealers. Evaluation by three ex vivo methods. Int Endod J. 2016;49:774–82.
Uzunoglu-Özyürek E, Erdoğan Ö, Aktemur Türker S. Effect of calcium hydroxide dressing on the dentinal tubule penetration of 2 different root canal sealers: a confocal laser scanning microscopic study. J Endod. 2018;44:1018–23.
Abdulkader A, Duguid R, Saunders EM. The antimicrobial activity of endodontic sealers to anaerobic bacteria. Int Endod J. 1996;29:280–3.
Siqueira JF Jr, Favieri A, Gahyva SM, Moraes SR, Lima KC, Lopes HP. Antimicrobial activity and flow rate of newer and established root canal sealers. J Endod. 2000;26:274–7.
Candeiro GT, Moura-Netto C, D’Almeida-Couto RS, Azambuja-Junior N, Marques MM, Cai S, Gavini G. Cytotoxicity, genotoxicity and antibacterial effectiveness of a bioceramic endodontic sealer. Int Endod J. 2016;49:858–64.
Weiss EI, Shalhav M, Fuss Z. Assessment of antibacterial activity of endodontic sealers by a direct contact test. Endod Dent Traumatol. 1996;12:179–84.
Wang Z, Shen Y, Haapasalo M. Dentin extends the antibacterial effect of endodontic sealers against Enterococcus faecalis biofilms. J Endod. 2014;40:505–8.
Haapasalo M, Qian W, Portenier I, Waltimo T. Effects of dentin on the antimicrobial properties of endodontic medicaments. J Endod. 2007;33:917–25.
Morgental RD, Singh A, Sappal H, Kopper PM, Vier-Pelisser FV, Peters OA. Dentin inhibits the antibacterial effect of new and conventional endodontic irrigants. J Endod. 2013;39:406–10.
Ma J, Wang Z, Shen Y, Haapasalo M. A new noninvasive model to study the effectiveness of dentin disinfection by using confocal laser scanning microscopy. J Endod. 2011;37:1380–5.
Stuart CH, Schwartz SA, Beeson TJ, Owatz CB. Enterococcus faecalis: its role in root canal treatment failure and current concepts in retreatment. J Endod. 2006;32:93–8.
Sundqvist G, Figdor D, Persson S, Sjogren U. Microbiologic analysis of teeth with failed endodontic treatment and the outcome of conservative re-treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:86–93.
George S, Kishen A, Song KP. The role of environmental changes on monospecies biofilm formation on root canal wall by Enterococcus faecalis. J Endod. 2005;31:867–72.
Schäfer E, Bössmann K. Antimicrobial efficacy of chlorhexidine and two calcium hydroxide formulations against Enterococcus faecalis. J Endod. 2005;31:53–6.
Lima RK, Guerreiro-Tanomaru JM, Faria-Junior NB, Tanomaru-Filho M. Effectiveness of calcium hydroxide-based intracanal medicaments against Enterococcus faecalis. Int Endod J. 2012;45:311–6.
Topcuoglu HS, Tuncay O, Karatas E, Arslan H, Yeter K. In vitro fracture resistance of roots obturated with epoxy resin-based, mineral trioxide aggregate-based, and bioceramic root canal sealers. J Endod. 2013;39:1630–3.
White JM, Goodis HE, Marshall SJ, Marshall GW. Sterilization of teeth by gamma radiation. J Dent Res. 1994;73:1560–7.
Ricucci D, Siqueira JF Jr. Biofilms and apical periodontitis: study of prevalence and association with clinical and histopathologic findings. J Endod. 2010;36:1277–88.
Upadya MH, Kishen A. Influence of bacterial growth modes on the susceptibility to light-activated disinfection. Int Endod J. 2010;43:978–87.
Wang Z, Shen Y, Haapasalo M. Effectiveness of endodontic disinfecting solutions against young and old Enterococcus faecalis biofilms in dentin canals. J Endod. 2012;38:1376–9.
Watson TF, Cook RJ, Festy F, Pilecki P, Sauro S. Optical imaging techniques for dental biomaterials interfaces. In: Curtis RV, Watson TF, editors. Dental biomaterials: imaging, testing and modelling. Cambridge: Woodhead; 2008.
Pizzo G, Giammanco GM, Cumbo E, Nicolosi G, Gallina G. In vitro antibacterial activity of endodontic sealers. J Dent. 2006;34:35–40.
Zhang H, Shen Y, Ruse ND, Haapasalo M. Antibacterial activity of endodontic sealers by modified direct contact test against Enterococcus faecalis. J Endod. 2009;35:1051–5.
Schweikl H, Schmalz G. The induction of micronuclei in V79 cells by the root canal filling material AH plus. Biomaterials. 2000;21:939–44.
Leonardo MR, Bezerra da Silva LA, Filho MT, Santana da Silva R. Release of formaldehyde by 4 endodontic sealers. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;88:221–5.
Al-Haddad A, Che Ab Aziz ZA. Bioceramic-based root canal sealers: a review. Int J Biomater. 2016;2016:9753210.
Trope M, Bunes A, Debelian G. Root filling materials and techniques: bioceramics a new hope? Endod Topics. 2015;32:86–96.
Zhou HM, Shen Y, Zheng W, Li L, Zheng YF, Haapasalo M. Physical properties of 5 root canal sealers. J Endod. 2013;39:1281–6.
Borges RP, Sousa-Neto MD, Versiani MA, Rached-Junior FA, De-Deus G, Miranda CE, Pecora JD. Changes in the surface of four calcium silicate-containing endodontic materials and an epoxy resin-based sealer after a solubility test. Int Endod J. 2012;45:419–28.
Alsubait SA, Al Ajlan R, Mitwalli H, Aburaisi N, Mahmood A, Muthurangan M, Almadhri R, Alfayez M, Anil S. Cytotoxicity of different concentrations of three root canal sealers on human mesenchymal stem cells. Biomolecules. 2018;1:8:E68.
Khalil I, Naaman A, Camilleri J. Properties of tricalcium silicate sealers. J Endod. 2016;42:1529–35.
Arias-Moliz MT, Camilleri J. The effect of the final irrigant on the antimicrobial activity of root canal sealers. J Dent. 2016;52:30–6.
Du T, Wang Z, Shen Y, Ma J, Cao Y, Haapasalo M. Effect of long-term exposure to endodontic disinfecting solutions on young and old Enterococcus faecalis biofilms in dentin canals. J Endod. 2014;40:509–14.
Chivatxaranukul P, Dashper SG, Messer HH. Dentinal tubule invasion and adherence by Enterococcus faecalis. Int Endod J. 2008;41:873–82.
Acknowledgements
This work was approved by the Institutional Review Board of King Saud University in Riyadh, Saudi Arabia (E-17-2687), and the College of Dentistry Research Center (IR0248) of King Saud University in Riyadh, Saudi Arabia. The project was carried out in the Molecular and Cell Biology Laboratory, a core research facility of the King Saud University, College of Dentistry in collaboration with the Prince Naif bin AbdulAziz Health Research Center.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Alsubait, S., Albader, S., Alajlan, N. et al. Comparison of the antibacterial activity of calcium silicate- and epoxy resin-based endodontic sealers against Enterococcus faecalis biofilms: a confocal laser-scanning microscopy analysis. Odontology 107, 513–520 (2019). https://doi.org/10.1007/s10266-019-00425-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10266-019-00425-7