Abstract
Pollination in Marantaceae is mediated by an explosive style movement. Before release, style tension is held by the hooded staminode. When a pollinator touches the trigger appendage of the hooded staminode the latter deforms and the style rapidly curls upwards. This movement has been interpreted as a turgor movement by some authors, but recent studies clearly indicate that setup, hold and release of tension are purely mechanical processes. However, in view of the high diversity of hooded staminodes, the question arises what keeps the tension in species with very thin staminodes. To test the holding mechanisms, we conducted mechanical and physico-chemical release experiments in four species with robust and four species with thin hooded staminodes in their natural tropical environment. We found almost the same response of all species to mechanical treatments, but species-specific reactions to different physico-chemical conditions. This indicates that style release follows the same mechanical principles in all species, but that the sensitivity of the explosive movement depends on material properties like tissue thickness and turgescence. As to the holding mechanisms, we found different degrees of floral synorganization. The hood of the hooded staminode formerly interpreted as an important holding structure does not play a noteworthy role. Instead, the basal plate of the hooded staminode antagonises the pressure of the style head against the holding point of the hooded staminode in species with robust hooded staminodes and well-developed basal plates. In some species with a thin hooded staminode, the latter is closely attached to the style and most likely stabilises tension by adhesive forces. In another species, a morphologically analogous structure adopts the function of the basal plate. We conclude that the holding mechanism of the style tension diversified during the evolution of Marantaceae whereas the release mechanism itself has been conserved throughout the family.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Marantaceae is an important element of tropical forest ecosystems. Nearly all species grow in the understory of tropical rainforests as perennial herbs and lianas. They account for a considerable biomass, provide food for pollinators and frugivores and shelter for small animals. Together with its small monogeneric sister family Cannaceae, Marantaceae forms the most advanced clade within the order Zingiberales. Most of the approx. 350 species (>80%) is distributed in the New World (Andersson 1981, 1998; Kennedy 2000). Except Halopegia and Thalia, each of the 29 genera is endemic to a single continent, i.e., Africa, America, and Asia (Govaerts and Kennedy 2016).
The family is characterized by a unique pollination mechanism combining extreme protandry and secondary pollen presentation with an explosive style movement (Claßen-Bockhoff and Heller 2008a; Eichler 1884; El Ottra et al. 2023; Kennedy 2000; Locatelli et al. 2004). Pollen is transferred in a split second (Claßen-Bockhoff 1991; Kunze 1984) and mediated by sophisticated staminode structures and pollen presenters which are precisely synorganized and synchronized with the pollinators’ behavior (Ley and Claßen-Bockhoff 2011, 2012).
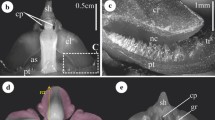
Style tension is set up in late bud stage mediated by the fleshy and hooded staminodes. In the adult flower (Fig. 1a, b), the fleshy staminode narrows the flower entrance thus forcing the pollinator to touch a trigger structure. The most important staminode with respect to the style movement is the hooded staminode (Fig. 1c, d: hs). It encloses the style head with a hood-like structure (ho) and usually forms a basal plate (bp) and trigger appendage (ta) at the side. In open flowers, the style (st) lies under tension in the hooded staminode. Style tension is set up before flowering, when own pollen is squeezed out of the anther by the elongating style (Claßen-Bockhoff and Heller 2008a; Pischtschan and Claßen-Bockhoff 2008). It is pressed onto the pollen plate (pp) at the back of the style head and protected by the hood of the hooded staminode. In the last hours before anthesis, the style elongates more strongly than the jacketing hooded staminode (Delpino 1869; Kennedy 1978). In many species, the resulting style tension is recognized by the arched form of the style (Fig. 1c).
Flower construction and pollination mechanism in Maranta leuconeura. a Front view of a flower. fs, fleshy staminode; os, outer staminode; pe, petal; th, theca of the half-fertile stamen. b Floral diagram. hs, hooded staminode; se, sepal; st, style. c,d Style movement. c In the open flower, the style lies overstretched in the hooded staminode. Own pollen is secondarily presented by the pollen plate (pp) at the back of the style head. When a pollinator loaded with foreign pollen (po) touches the trigger appendage of the hooded staminode (d: ta), style tension is released and the style curls inwards. d During the rapid style movement, pollen is exchanged. bp, basal plate; hp, holding point; gl, gland; ho, hood; sc, style canal; so, stigmatic orifice.
When searching for nectar, the pollinator touches the trigger appendage and releases the explosive style movement. The style springs upwards, and during this rapid movement pollen is exchanged. Given that the pollinator is loaded with foreign pollen on its proboscis or bill (Fig. 1c: po), this pollen is scraped off by the margin of the stigmatic orifice (so). Immediately thereafter, and facilitated by the curling movement, own pollen is pasted on precisely the same site of the pollinators’ mouth parts by a glue originating from the gland (gl) on the stylar head (Fig. 1c).
Whereas Delpino (1869), Eichler (1884), Schumann (1902) and Kennedy (1978) considered the style movement to be a purely mechanical process, Kunze (1984) postulated that it might be a turgor movement resembling the trap mechanism in the Venus flytrap Dionaea muscipula J. Ellis. He tested the release of the style tension in Maranta leuconeura E. Morren and Calathea undulata Regel by experiment and found that shifting the basal plate resulted in a rapid style movement, whereas cutting off large parts of the hood did not. He concluded that the hood was not able to hold the style tension mechanically and that the latter, therefore, is not set up by the counter pressure of the hooded staminode but by its internal tissue construction. Slow motion pictures confirmed his view as the whole style movement only needed 0.2 s which is too fast for a simple shift of water (Skotheim and Mahadevan 2005).
To test the hypothesis of a turgor movement, Claßen-Bockhoff and Heller (2008b) conducted release experiments in Costa Rica in four species, three with robust hooded staminodes (all Calathea clade) and Hylaeanthe hoffmannii (K. Schum.) A.M.E. Jonker & Jonker ex H. Kenn. (Maranta clade) with a very thin hooded staminode (Fig. 2a). They expected that in case of a turgor movement touching or piercing the style surface would cause membrane depolarization and, subsequently, style release. The authors could indeed directly trigger the style movement in Hylaeanthe hoffmannii, but not in the three remaining species. They concluded that two different mechanisms might have evolved in the family, a mechanical release in genera with strong hooded staminodes (like Calathea) and a turgor mechanism in more advanced genera with thin hooded staminodes (like Hylaeanthe). However, they proposed to repeat the treatments to clearly exclude any elastic deformations and wilting processes.
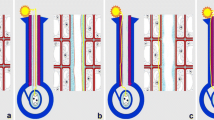
Hylaeanthe hoffmanii. a Side view of the hooded staminode and style. The hooded staminode is very thin and follows the curved (convex) shape of the style. Its trigger appendage (ta) is in a proximal position; basal plate and longitudinal swellings are lacking. At the holding point (hp), the style presses against the hooded staminode and keeps it stretched; at the fusion point (fp), style and hooded staminode separate from each other. b–f When the trigger appendage is bent backwards, the style is released from the hooded staminode and rapidly springs forward. Pictures taken from a video with 30 frames per second. The selected pictures correspond to frames 4, 8, 9, and 14 illustrating the extremely short time of the style bending (about 0.3 s). All figures in the same scale.
Subsequent histological investigations did not confirm the presence of a motor tissue (Jerominek et al. 2018; Pischtschan and Claßen-Bockhoff 2010). A detailed study dealing with the synorganization of floral structures in Marantaceae furthermore illustrated that the abaxial base of the style head pressed against the hood (holding point, Fig. 1c: hp) thereby stretching the tissue of the hooded staminode (Ley and Claßen-Bockhoff 2012). This finding explains that parts of the hood can be removed without releasing the style movement and that the hooded staminode bends backwards when the style curls upwards. Finally, electrophysiological measurements in Goeppetia bachemiana (Morren) Borchs. & Suárez with strong and Donax canniformis (G. Forst.) K. Schum. with weak staminode structures revealed that the release is purely mechanical in both species (Jerominek and Claßen-Bockhoff 2015). Based on quantitative histological investigations in Goeppertia bachemiana (E. Morren) Borchs. & S. Suárez, Jerominek et al. (2018) proved that the upper epidermis is under tensile stress in the unreleased style and that the shift of water stabilizing the final shape of the style is a consequence of and not the reason for the breakdown of tension. They concluded that the high speed of the style movement is based on elastically stored energy (Forterre et al. 2005).
All data at hand indicate that the setup, hold and release of tension are purely mechanical processes. However, it is still unclear how weak-hooded styles like those of Hylaeanthe hoffmannii are hold and released. In this species, the style is extremely arched (Fig. 2a), but the hooded staminode is not stretched in such a way that it moves backwards after the release of style tension (Fig. 2f). It rather has a convex shape and closely adheres to the style. When mimicking a pollinator by inserting a needle into the channel between style and trigger appendage (Fig. 2b), the connection is solved, the style springs upwards and the hooded staminode relaxes (Claßen-Bockhoff and Heller 2008a, b).
In the present study, we conduct style release experiments in eight species not previously investigated. We test the hypothesis that the style movement in Marantaceae is a purely mechanical process. For this purpose, we consider all data at hand and discuss the arguments apparently advocating a turgor movement, i.e., that the style itself is sensitive, that the hooded staminode is not able to hold the style tension and that the speed of the movement cannot be explained by a mechanical model.
Material and methods
Plant material
Eight taxa (four genera/seven species) were chosen for the experiments (Table 1; Fig. 3). The species represent different phylogenetic clades and are characterized by different flower morphologies. Weak and strong hooded staminodes were distinguished according to the hood types defined by Pischtschan et al. (2010).
Inflorescences and dissected flowers with hooded staminodes. a–d Species with strong hooded staminodes. Note the swellings in b–d. a Calathea crotalifera (lateral and top view). b Goeppertia ecuadoriana (top view). c G. zebrina var. zebrina (lateral and top view). d G. zebrina var. humilior (lateral and top view). e–h Species with weak-hooded staminodes. e Stromanthe tonkat (unreleased and released state). f S. sanguinea (unreleased and released state). g Phrynium pubinerve (whole flower in side view). h P. imbricatum (whole flower in side view). a–h Bar = 5 mm
To avoid undesired greenhouse effects, flowers were collected and investigated in their natural habitats, i.e., the Wilson Botanical Garden in the Field Station Las Cruces, Costa Rica (January to March 2010) and the Queen Sirikit Botanic Garden in Chiang Mai, Thailand (March to April 2010).
Picked flowers are highly sensitive to humidity. They either release spontaneously or lose their tension in the unreleased state. To avoid such desiccation effects, all flowers were picked in the morning and kept moist in a humid box for a maximum of 2 h till dissection. Stromanthe tonckat grew outside the Wilson Botanic Gardens (N 8.78604°, W 82.97257°) and was investigated in the field. Flowers were dissected under a dissecting microscope to uncover style and hooded staminode. Afterwards, the mechanical, chemical, and temperature manipulation tests were performed. Experiments were documented with a Canon Powershoot G9 mounted on a dissecting microscope.
Morphometric data
Morphological characters like length, width, and height of the style (Fig. 4c, e) were measured and tested for correlations between shape and size of floral structures and different style responses. Measurements were performed using photographs of the styles. All measurements were performed in the unreleased state.
-
Style length was recorded from the fusion point with the hooded staminode to the head of the style when still covered by the hooded staminode (Fig. 4c). Additionally, based on the lateral view, the area of the style and its proportion covered by the hooded staminode (Fig. 4d) was calculated using Photoshop CS4.
-
To investigate whether the position of the basal plate influenced style response its relative position to the style was determined as distance between basal plate and tip of the style (Fig. 4c: pb) divided by the length of the style.
-
The extension of the basal plate on the upper side of the style might be (1) minute, rarely reaching to the upper side of the style, (2) small, reaching up to one fourth of the style width, or (3) large, covering up to one half of the style width (Fig. 4a).
-
Morphological characters such as the dimensions of the frontal and adaxial lobes were classified for analyses as follows: (1) absent or small, (2) medium, and (3) large. The latter describes a frontal lobe covering even the stigmatic cavity (Fig. 4a: fl) or an adaxial lobe extended up to the basal plate (Fig. 4a: al).
-
The strength of the hooded staminode was coded as (1) thin, (2) thin with a swelling along the midrib, or (3) robust, with thickening not restricted to the midrib but affecting the whole staminode.
Mechanical tests and morphometric measurements. Example: Goepperta zebrina var. zebrina. a–c Top views. a Positions and structures for touching, stinging, and cutting (dotted line) experiments; al, adaxial lobe; bp, basal plate; fl, frontal lobe; ss, stamen side; ts, trigger side; us, upper side; x, position at basal plate. b Longitudinal (solid line) and transversal (dotted line) cutting positions; ba, bending area. c Morphometric measurements of length and width of the style and the asymmetric position of the basal plate. pb, position basal plate; x, position close to the basal plate. d,e Lateral views. d Directions of deflection: aw, away from style; to, towards style. hp, holding point. e Position for measuring the height of the style; arrows indicating directions for lifting (upwards) and moving (laterally) the basal plate
Style release experiments
In total, 42 different tests were performed to identify (1) which structure or tissue held the style and thus stored the elastic energy needed for the explosive style movement, and (2) which structures accounted for the perception of the release stimulus. Among these tests, 30 approaches manipulated the flowers mechanically, ten chemically, and two were based on the effect of temperature. Usually, three flowers per species were investigated for each approach. In case of doubtful results, sample size was increased to ten flowers (indicated in the respective tables). Sample size (total numbers of dissected flowers per species) slightly differed according to the availability of a suitable number of flowers.
To analyse and compare the data, an indicator for the style response (sensitivity) was calculated. It is defined as the frequency of style release after a certain treatment with the following five predefined ranges for style response: (1) 0% (never), (2) 1–25% (rare), (3) 26–75% (ambiguous), (4) 76–99% (frequent), and (5) 100% (always).
Mechanical tests
Mechanical stimuli were applied to test the importance of different morphological structures and whether they (1) have a holding function, (2) initiate the style movement, (3) are part of signal transmission, or (4) do not account for any of these functions.
The mechanical tests comprised five major approaches, i.e., stinging into the tissue of the style (Fig. 4a), cutting at various positions (Fig. 4b), deflecting the trigger appendage (Fig. 4d) by touching style and hooded staminode at different positions (like basal plate, frontal, and adaxial lobe), moving and lifting the basal plate (Fig. 4e), frontal, and adaxial lobes (Fig. 4a: fl, al).
Deflecting and touching manipulations were performed with a preparation needle, while a syringe was used for the stinging and a razor blade for the cutting experiments. The trigger appendage was deflected in four directions, two of them longitudinal to the style, i.e., distal (towards stigmatic cavity) and proximal (towards ovary) and two of them crosswise (deflected towards and away from the style; Fig. 4d, e). To identify the elastic tissue tension (stress distribution in the style), longitudinal and transversal cuts were performed (Fig. 4b). Potential holding structures were manipulated by cutting to test whether the corresponding treatment leads to the release of the style. Touching and stinging experiments were performed on the upper side of the style, on its flanks and at/close to the basal plate.
Chemical and physical treatments
Previous investigations have shown that styles of some species can be fixed in the unreleased state using ethanol, methanol, AFP, GFE, anaesthetics, hypertonic solutions, and/or by freezing (Claßen-Bockhoff and Heller 2008b; Pischtschan and Claßen-Bockhoff 2008, 2010). However, the different response to temperature and narcotizing substances has not been explained so far. The original assumption that the treatments could directly affect the membrane potential was rejected by Jerominek and Claßen-Bockhoff (2015), who excluded active signaling by applying chloroform.
The influence of temperature was tested by storing the dissected flowers either in a freezer (− 6° C) or in a heater for herbarium material (+ 60° C). After 30 min, the released or unreleased state of the styles was controlled. Sodium chloride (NaCl) was used as hypertonic solution with a 1 M concentration. Alcohols (ethanol and methanol) in concentrations ranging from 50 to 99% were applied to dissected flower allowing the chemical to fully cover style and hooded staminode. The influence of chloroform steam was tested using different concentrations, i.e., high (40 ml chloroform in 100 ml bottle, evaporating diameter: 5 cm), medium (1 ml chloroform in 80 ml tube, evaporating diameter: 17 mm), and low concentrations (300 µl chloroform in 80 ml tube, diameter of the evaporating surface area: 8 mm). Each time, the chemicals were applied for 20 min. The styles either released spontaneously during this time or were fixed in the unreleased state. To test for residual tension, the hood of the unreleased styles was removed. In single cases, a fixation period of 45 min was needed to irreversibly fix the style. For each chloroform approach the samples were only exposed to the steam but not wetted by the chemical.
Statistics
Several analyses were performed to identify (1) treatments with similar effects on the style of a certain taxon, (2) morphological parameters that account for the same reaction (style response) in different taxa, and (3) to test whether it is possible to distinguish the genera based on their style response. All parameters (ordinal and metric) were tested for correlation. Auto-correlated characters, e.g., morphological parameters vs. genera and hood type or those showing allometric relationships such as style width and height were not considered here. Pearson`s correlation coefficient (r) and significance (P) were calculated using SPSS 20.0.01 for both, the whole taxon set (8 taxa) and each of the two groups with weak (four taxa) and strong hooded staminodes (four taxa). Scatter plots were generated using SigmaPlot 10.0. For testing differences between weak- vs. strong-hooded species and between genera, a non-parametric U-Test was conducted using SPSS 20.0.01.
Results
Morphological diversity of the style-hooded staminode complex
The strong-hooded group includes the Calathea and Goeppetia species (Table 2). It is characterized by robust hooded staminodes with trigger appendages and basal plates (Fig. 5a–d). Calathea has stiff hooded staminodes throughout (Table 2) whereas Goeppertia has stiff tissue swellings only along the midrib of the hooded staminode (Fig. 3b–d). Frontal and adaxial lobes are present in all four species. Goeppertia zebrina var. humilor showed shorter adaxial lobes than the other three taxa (Fig. 5d). Generally, among the investigated species, the strong-hooded species have larger, broader, and wider styles and thicker hooded staminodes than the weak-hooded species (Fig. 5; Table 2). Appendages of the hooded staminode (basal plate and lobes) are also more prominent and larger in the strong-hooded species, and basal plates cover up to one half of the style width (Fig. 5a–c).
Morphology of the functional unit composed of hooded staminode (grey) and style (white). a–d Strong-hooded species with ‘thumb-like’ trigger appendages and staminode lobes. a Calathea crotalifera. b Goeppertia ecuadoriana. c G. zebrina var. zebrina. d G. zebrina var. humilior. e–f Weak-hooded species with reduced lobes. e Stromanthe tonckat. f S. sanguinea. g Phrynium imbricatum. h P. pubinerve. 1 Top views showing the dimension of the basal plates and the staminode appendages. 2 Lateral views illustrating the bending of the styles, their coverage by the hooded staminodes and the relative positions of the basal plates. al, adaxial lobe; bp, basal plate; hp, holding point; fl, frontal lobe; so, stigmatic orifice; ta, trigger appendage. All schemes are in the same scale
The four species with weak-hooded staminodes differ in their hooded staminodes. Frontal and adaxial lobes are reduced or even absent. Stromanthe has trigger appendages with very small basal plates (Fig. 5e, f). The relative position of the latter is more proximal (relative position: 0.3–0.37) than in strong-hooded taxa whose relative positions range from 0.22 to 0.28 (Table 2). In contrast, the two Phrynium species show folded trigger appendages (Fig. 5g, h) and form a slightly stiff basal plate. The relative position of the latter is proximal almost in the middle of the bending area (relative position: 0.4–0.45; Table 2). Morphologically, the two Phrynium species (Fig. 5g, h) clearly differed from all other taxa investigated by having a convex shape of the hooded staminode that is closely attached to the style.
The proportion of the style covered by the hooded staminode is highly variable within and among genera (Table 2). For weak-hooded species the coverage values range from 34.0 to 50.7% while this range is slightly smaller for strong-hooded taxa (44.4–58.1%).
Style response to mechanical stimuli
Despite the different morphology of the style-hooded staminode units, the mechanical tests revealed only little interspecific differences (Table 3). The values for style responses (sensitivity) to a certain treatment were quite distinctive with either a high frequency of style release (value: 5) or a negligible sensitivity (value: 1).
Trigger appendage
In all investigated taxa, the style was released when the trigger appendage was deflected longitudinally in proximal direction (Fig. 4d; Table 3). The same reaction was observed when deflecting the trigger appendage away from the style. In contrast, the two other directions (distal and towards the style) did not lead to the release of the style (Video V1). The only exception for this reaction was found in Stromanthe sanguinea, which showed an ambiguous style response (sensitivity) for the deflection of the trigger appendage towards the style (Table 3). In all experiments it was impossible to cut off the trigger without inducing a style release.
Basal plate
Mechanical manipulations affecting the basal plate showed opposite reactions. Whereas slightly touching the basal plate from above did not lead to the style release (except for Stromanthe tonckat), lifting, moving, or cutting the basal plate always resulted in style release. The basal plate slipped away before the style started to move (Video V2).
Staminode lobes
Lifting the frontal or adaxial lobes did never release the style. Longitudinal cuts into the distal hood showed no response (except when the manipulation was located close to holding point between style and hooded staminode, Video V3). The adaxial lobe could be removed by cutting without any style response, whereas cutting off the frontal lobe induced style release in the three strong hooded Goeppertia taxa (Table 3).
Style
Touching the style at different sites never stimulated a response (Table 3). Similarly, style tension was not released after stinging in the style with a preparation needle. Only Goeppertia zebrina (both var. zebrina and var. humilior) showed a rupture when the bending area was concerned (Table 3). In all other species and stinging positions, the style was never released after a single stinging but released after repeated stimulation and corresponding wounding effects (Video V4). The only example in which one single sting led to a style release was found in Stromanthe tockat. Here, the reaction was restricted to a manipulation close to the basal plate (Table 3). Transversal cuttings of the style always released its tension or was followed by a downward buckling (Table 3: *, Video V5). In some species such a rupture went along with the separation of the upper and lower cell-layer of the style. While the latter remained intact, the former splits and curls-up in the opposite direction (Video V6). Longitudinal cuts in the style led to a release of tension in three of the four weak-hooded staminodes (Table 3). The response in the remaining species was negligible (Video V7). After wounding, both varieties of Goeppertia zebrina showed a colour change of the style, which turned from white to black (Video V8).
Style response to chemical and physical tests
Compared to the mechanical treatments (Table 3), single chemical and physical tests revealed strong interspecific differences (Table 4). In Phrynium, the two species clearly differed in their response to low-concentrated chloroform steam and different temperatures (Table 4). In contrast, medium to high sodium chloride concentrations, alcohols, and NaCl are not suitable to distinguish between the two species. As to the two varieties of Goeppertia zebrina, the style response to alcohol can be used to discriminate between both taxa (Table 4; 70% alcohol).
Alcohol treatments
Treatments with highly concentrated ethanol and methanol (99%) always led to style release. The application of lower concentrations reduced the style response in all taxa (Table 4). Long exposure times in low concentrated alcohol generally led to a reduction or even complete loss of sensitivity that allows the fixation of styles even in species that show a high sensitivity (self-release). In the latter case, the style was fixed in its position and it was possible to induce any response even not by mechanical stimuli. For Goeppertia zebrina, each experiment with alcohol caused a colour change of the style, which turned from white to black after the chemical was applied (Video V9).
Chloroform steam
In all species, high concentrations of chloroform steam induced style release more often than lower concentrations (Table 4). However, species differed considerably in their response as shown in the two Goeppertia taxa. Similar to the alcohol tests, also low concentrated chloroform steam caused a fixation of the styles (Video V10) except for Phrynium pubinerve and Goeppertia zebrina var. humilor.
Sodium chloride
The application of NaCl rarely caused a style release (Table 4). It led to a shrinking of the style tissue (Video V11). In contrast to other treatments, NaCl did not induce a colour change in the style of Goeppertia zebrina (Video V8).
Temperature
The temperature experiments (Table 4) revealed quite variable results ranging from low (indicator value 1) to high sensitivity (indicator value 5). This tendency was observed for all species and within both, strong- and weak-hooded taxa to the same extend. Representatives of the same genus also showed a high variability in their style response (sensitivity), e.g., Phrynium pubinerve (5) and P. imbricatum (1). Both varieties of Goeppertia zebrina showed a colour change of the style tissue when samples were exposed to room temperature after freezing (Video V12).
Statistics
Style responses to different treatments among all species revealed three significant correlations (Table 5), i.e., ethanol/methanol (Fig. 6a), NaCl/methanol (Fig. 6b), and high temperature/chloroform steam (Fig. 6d). As to morphological parameters, no significant correlations were found in the investigated taxa (Table 5).
Strong hooded species
Within the strong-hooded group, the following five correlations were significant (Table 5): methanol/ethanol (Fig. 6a), heat/chloroform (Fig. 6d), chloroform/relative position of the basal plate (Fig. 6g), high temperature/relative position of the basal plate (Fig. 6j), and low temperature/height of the style (Fig. 6l).
Weak-hooded species
The following correlations were identified within the weak-hooded group (Table 5): NaCl/chloroform (Fig. 6c), low/high temperature (Fig. 6e), NaCl/dimensions of the basal plate (Fig. 6f), chloroform/dimensions of the basal plate (Fig. 6h), high and low temperature/style proportion covered by the hooded staminode (Fig. 6i, k).
With respect to the two hood types, none of the mechanical, chemical and physical tests showed any significant difference in style responses (U-test,).
Discussion
The high speed of the style movement (Claßen-Bockhoff 1991; Kunze 1984) and the controverse style responses after mechanical and physico-chemical treatments (Claßen-Bockhoff and Heller 2008b) gave reason to interpret the style movement, at least in some species, as a turgor movement. However, in contrast to the model of the Venus fly trap, style movement in Marantaceae is irreversible and tension is not set up within a single organ. It rather arises by the counter pressure of two synorganized organs, i.e., the style and hooded staminode. This construction does not contradict the assumption of an underlying turgor movement, but spotlights the possibility of a completely mechanical movement.
The most important findings of the present experiments are, first, that species with strong and weak-hooded staminodes respond equally to mechanical stimulation indicating that they share the same underlying release mechanism. We found, second, that all species differ in their response to chemical and temperature stimuli illustrating the influence of species-specific material properties on style sensitivity.
Influence of species-specific properties on style sensitivity
Alcohol application
The two alcohols had similar effects, i.e., they either released or irreversibly fixed the style. The releasing effect of high concentrated alcohols (99%) is most likely based on the low surface tension of alcohol (ethanol 22.39 mN/m, methanol 22.45 mN/m) in comparison to water (72.80 mN/m) at 20 °C (Dean 1999; Harkins and McLaughlin 1925). In contrast, lower concentrations could also release the style by changing the mechanical properties, volume, and density of plant tissues (see Video V09: although the style did not release in this case, the video shows clear changes in the tissue).
Jerominek and Claßen-Bockhoff (2015) showed by experiment that liquids with a low surface tension released the style by infiltration into the gap between style and hooded staminode. This means that the style was not released physiologically by membrane depolarisation, but purely mechanically by disconnecting both organs. Consequently, the species-specific style responses (sensitivities) to alcohols were obviously related to properties that facilitated fluid infiltration. The highest sensitivity appeared in Goeppertia ecuadoriana (Table 4), a species with a loose hooded staminode that was characterised by a comparatively large, undulate adaxial fold (Figs. 3b, 5b). In contrast, the lowest sensitivity (Stromanthe sanguinea) was associated with a very tight hooded staminode probably hampering infiltration (Figs. 3f, 5f). The high sensibility of the Phrynium species may be caused by the tight connection of the hooded staminode and style. The thin gap between these structures most likely increased the capillary effect and the infiltration of alcohols in the experiment. In Goeppertia zebrina, alcohol application had a destructive effect on cell level. The style became black indicating an oxidation of phenols. These are stored in the vacuole and can only react with polyphenol oxidases located in the plastids when the enzymes are released through an injury or damage of the cells (Steffens et al. 1994).
Decreasing the concentration of alcohols decreased the sensitivity of the style response in all species and even led to a complete loss of sensitivity (indicator for style release <5). Whereas Claßen-Bockhoff and Heller (2008b) and Pischtschan and Claßen-Bockhoff (2008, 2010) interpreted this response as a proof for an underlying turgor movement, we here explain it with the hygroscopic capacity of alcohol. Water is extracted from the plant’s tissue; the style hardens and becomes irreversibly fixed. Consequently, the effect of alcohols to release or to fix the style is a function of concentration and exposure time and not necessarily a proof for a turgor movement.
Interestingly, there is no correlation between single morphological characters and style sensitivity to alcohols. This indicates that the effect of alcohols may depend on a morphological character syndrome including relative proportions, properties, and configurations of the style, hooded staminode and their parts.
Sodium chloride
Application of NaCl solutions did not considerably affect the style. The same result was found in Thalia geniculata and interpreted as proof for an underlying seismonastic turgor movement (Claßen-Bockhoff 1991). However, our time-lapse recordings showed that the style tissue was shrinking by plasmolysis after NaCl application. We conclude that the decrease of turgor pressure in the cells causes the irreversible loss of function and that no change in membrane tension has to be considered. As to style release, we assume a similar mechanism as to methanol (Table 5; Fig. 6b). Most likely, the NaCl-solution infiltrated into the gap between style and hooded staminode and resulted mechanical disconnection.
Chloroform
Exposed to chloroform steam, the tissues of the style and hooded staminode became soft and lost water resembling the shrinking effect observed after NaCl application. In consequence, high concentrated chloroform steam generally induced style movement while decreasing concentration lowered the probability of release (Table 4).
In strong hooded species, sensitivity increased with a more proximal position of the basal plate (Fig. 6d) and in weak-hooded species with the increasing dimension of the basal plate (Fig. 6h). Both findings indicate that the turgescence of the style-hooded staminode complex is essential for holding the style’s tension. The high sensibility in Phrynium species (and Thalia geniculata, Pischtschan and Claßen-Bockhoff 2010) might be due to the thin texture of the hooded staminode as tissue shrinkage directly dissolves the holding point between style and staminode. Goeppertia zebrina var. humilior, has a stiffened hooded staminode, but a comparable small basal plate (medium size). We assume that the latter easily slips off the style when the tissue is softening, thus explaining its high sensitivity.
Using low concentrations of chloroform steam, it was possible to fix the style as far as the exposure time was long enough. In our experiments, this happened approximately 2–5 min after exposing the styles to the steam; in the extremely excitable Thalia geniculata a 60-min narcosis with chloroform was needed to suppress style release (Pischtschan and Claßen-Bockhoff 2010).
Temperature
As heat leads to a loss of water and freezing to cell damage it was expected that weak-hooded staminodes would be more affected than strong ones. However, no clear pattern was found. High and low temperatures resulted in all kinds of response from rapid style release to a complete loss of mobility.
Correlation analyses indicated that chloroform and heat had a similar effect on tissue softening (Fig. 6d) and that the response to temperature was linked to the degree of coverage in weak-hooded species, and the relative position of the basal plate and height of the style in strong-hooded species. We assume that the ease of the basal plate to slip off the style and the relative tissue thickness of style and hooded staminode may explain the results.
The black colouration of frozen Goeppertia zebrina styles being exposed to room temperature again indicated a disruptive effect. The extreme damage confirms previous histological investigations (Pischtschan and Claßen-Bockhoff 2010).
No evidence for style sensitivity
Touching the style at different positions revealed low sensitivities among all species which is in contrast to previous studies of Claßen-Bockhoff and Heller (2008b). The authors observed a high sensitivity in the strong-hooded Pleiostachya pruinosa (Regel) K. Schum. and the weak-hooded Hylaeanthe hoffmannii in Costa Rica. They explained this finding as a strong indication for the assumed turgor movement. However, based on present knowledge, both reactions can be explained mechanically. In Pleiostachya pruinosa, the frontal lobe only loosely covers the stigmatic orifice and the small basal plate easily glides off the style. The overall weak mechanical hold is associated with self-release and self-pollination (Ley and Claßen-Bockhoff 2012). In Hylaeanthe hoffmannii, the style could be released when touching the lower side of the style and the area below the stigmatic orifice. These treatments directly affected the holding point and, therefore, resulted in style release.
Stinging into the style tissue likewise indicated no obvious style sensitivity. Goeppertia zebrina immediately responded with a rupture of the upper style epidermis (Table 3) indicating that this tissue layer is under tensile stress (Jerominek et al. 2018). Stromanthe tonckat responded with a style movement when the style was stinged close to the basal plate. We interpret this finding as a possible artefact caused by the small size of the flower. Preparation was extremely difficult and often led to an undesired lift of the basal plate (indirect initiation).
Transverse cuttings resulted in a separation of the tissue of the upper and lower side of the style. This finding disproves the view that style release is based on a turgor movement as compressive stress, i.e., a high turgor in the upper side pressing against the lower side (Pischtschan and Claßen-Bockhoff 2010), has not been confirmed by the current experiments.
Longitudinal cuttings revealed low sensitivities in the strong-hooded species and ambivalent results in the weak-hooded species (Table 3). The absence of a reaction is probably based on the orientation of the tissue tension. Tensile and compressive stress can only be relieved by a rupture in the direction opposite to the stress, i.e., by transverse cuttings. Style release was only observed when the cuttings affected the holding point. We assume that in the weak-hooded species, the holding point was indirectly affected by the manipulations which is in accordance with our observations for the longitudinal cuttings in the distal hood.
Diverse holding mechanisms
In the past, the hood of the hooded staminode was regarded to hold the style tension (Kunze 1984). However, the results of the present study combined with already available data confirm that the holding point is the most important site setting up and holding style tension (Ley and Claßen-Bockhoff 2012). The style head presses against the hooded staminode thereby stretching it in longitudinal direction. The movement of the trigger appendage deforms the hooded staminode and separates the style tip from the hood (Jerominek and Claßen-Bockhoff 2015; Ley and Claßen-Bockhoff 2012). The style curls upwards whereas the hooded staminode bends backwards. The release of mechanical tension causes a turgor change mediating the enormous cell expansion on the lower side of the style (Jerominek et al. 2018). Contrary to the expectation, the hood has no holding function. It most likely protects pollen against pollen collecting bees and/or facilitates secondary pollen presentation (Pischtschan et al. 2010).
Our experiments with wilted or narcotized flowers support the holding function of the holding point. In the corresponding treatments the basal plate could be lifted, moved, or even removed without any reaction although the style was still under tension and could be released by manipulating the holding point. Wilting and narcotizing obviously caused a loss of turgor which was indicated by a decreasing style length. We conclude that the tension between hooded staminode and style was reduced and the latter only held by the holding point.
Additional hold is usually provided by the basal plate acting as counterforce to the holding point. However, we add two further holding mechanisms disregarded in the past: the swelling of the frontal lobe (Hylaeanthe hoffmannii) and adhesive forces (thin hooded species).
Trigger appendage and basal plate
The basal plate forms a functional unit with the trigger appendage in the process of pollen transfer. The trigger appendage makes the connection between pollinator and basal plate. Its diverse shape does not directly contribute to the style release but has evolved in concert with the diversification of the flower construction (Ley and Claßen-Bockhoff 2011, 2012; Pischtschan et al. 2010). Our experiments indicated that the function of the trigger appendage was to lift the basal plate which only happened when the trigger appendage was deflected away from the style or in proximal direction.
Moving, lifting, and cutting the basal plate always led to a release of style tension which is in accordance with previous studies (Claßen-Bockhoff and Heller 2008a, b). In Stromanthe tonckat, with a very small and laterally arranged basal plate, touching the basal plate resulted in its gliding off the style which likewise released tension. Altogether, it is obvious that the basal plate is involved in holding style tension. It may either induce a deformation of the hooded staminode (relieving the holding point) as assumed by Claßen-Bockhoff and Heller (2008b) or solely relieve the tension at the upper side of the style (lifting basal plate).
Despite its functional importance, the basal plate is not essential. This is indicated by species like Hylaeanthe hoffmannii lacking the basal plate. Moreover, our experiments indicate that the basal plate might only have a holding function in fully-turgescent styles.
Staminode lobes
In most species investigated so far, lobes are not directly involved in holding style tension. They rather protect the stigmatic orifice and contribute to the reduction or even avoidance of self-pollination (Ley and Claßen-Bockhoff 2012).
Cutting experiments rarely led to a style release in the Goeppertia species. We interpret this finding as a false positive result caused by the pressure of the razor blade against the hooded staminode. As the latter is only thickened along the midrib, deformation of the hooded staminode could not be avoided when cutting its appendages. This view is indirectly supported by Calathea crotalifera, which has a completely stiff hooded staminode able to buffer the pressure (mechanical force) while cutting the appendages.
A species in which lobes directly contribute to the hold of style tension is Hylaenathe hoffmannii (Claßen-Bockhoff and Heller 2008a, b). First, the petaloid appendage of the monothecate anther envelops the style-hooded staminode complex and stabilises the functional unit mechanically. Second, the frontal lobe covering a large part of the style head has a distinct dint below the stigmatic orifice that gains stability by its swollen margin. This structure has been disregarded in the past but gains importance under the mechanical release hypothesis. It has the same position as the basal plate in other species and compensates the loss of this holding structure. Being morphologically analogous, it has the same function and acts as a basal plate equivalent.
Adhesive forces
Some species with thin hooded staminodes appeared to be less dependent on the basal plate. The two Phrynium species had medium-sized basal plates and overarched styles to which the hooded staminodes were closely attached. The latter were not stretched in longitudinal direction but along the style curvature. After release of tension, they did not bend backwards but relaxed in straight orientation.
This configuration of the style-hooded staminode complex resembles that of Hylaeanthe hoffmannii (Fig. 2a). Jerominek (2015) postulated that the closely spaced organs are hold together by adhesive forces which increase the holding function. Given the dense arrangement of the floral structures in bud stage (Claßen-Bockhoff and Heller 2008a), this arrangement could represent a persisting juvenile stage, in which the organs do not separate from each other.
As to style release, previously conducted chemical experiments (Jerominek and Claßen-Bockhoff 2015) illustrated, that large droplets of chloroform and 70% alcohol flew under the basal plate into the hood of the staminode and caused a mechanical release by separating the holding point between head and hood. Jerominek (2015), thus, concluded, that the release mechanism was based on an air ingress that broke the connection of the two organs when the trigger appendage was deflected.
Such an adhesive mechanism is also assumed for the Phrynium species and further Marantaceae with thin hooded staminodes. It goes along with the spatial independence of the trigger appendage from the basal plate and its shifting to a more proximal position. The alternative flower construction directly influences pollination as only visitors able to reach the trigger can act as pollinators (Ley and Claßen-Bockhoff 2012).
Conclusions
The present paper is the last of a series dealing with the evolution and functional morphology of the explosive style movement in Marantaceae (Claßen-Bockhoff 1991; Claßen-Bockhoff and Heller 2008a, b; Jerominek and Claßen-Bockhoff 2015; Jerominek et al. 2018; Ley and Claßen-Bockhoff 2011, 2012; Pischtschan et al. 2010; Pischtschan and Claßen-Bockhoff 2008, 2010). It confirms that the style movement can be explained as a completely mechanical process. Style tension is primarily held by the counterpressure of style and hooded staminode at the holding point. The basal plate functions as additional holding structure particularly in species with strong hooded staminodes. In some species with thin hooded staminodes, adhesive forces between style and hooded staminode contribute to the hold of tension. Together with the basal plate equivalent in Hylaeanthe hoffmannii, we, thus, identified three different holding mechanisms. This is a surprising result as previous interpretations considered the hood as most important holding structure (Kunze 1984) and favoured a turgor movement in at least some species with thin hooded staminodes (Claßen-Bockhoff and Heller 2008b). These assumptions are rejected here.
Within the Marantaceae, flowers with strong and thin hooded staminodes evolved each requiring specific holding structures to assure pollen transfer. Strong hooded staminodes with the basal plate as counterforce are predominantly found in basal clades of Marantaceae like the African Sarcophyrynium or American Calathea clade, whereas thin hooded staminodes with adhesive forces holding the tension appear in different derived clades (Ley and Claßen-Bockhoff 2012; Pischtschan et al 2010). As to the present knowledge, this holding mode has been confirmed or assumed for the predominantly American Maranta clade (Hylaenanthe, Maranta) and predominantly Asian Donax clade (Donax, Phrynium, Thalia) indicating that this construction may have evolved several times in parallel.
Summarising, the rapid style movement in Marantaceae is a further example of purely mechanically released explosive movements in flowers. It joins the well-known examples of explosive keel blossoms in Fabaceae (Raju and Rao 2006; Westerkamp 1993), Hyptis (Lamiaceae, Brantjes and Vos 1981) and Schizanthus (Cocucci 1989), and the catapult movements of pollinaria and pollen release in orchids (Dodson 1962) and Cornus (Edwards et al. 2005).
The explosive style movement in Marantaceae is a family-wide character (Andersson 1981, 1998). As the sister group Cannaceae has only few species, the specific pollination mechanism has been considered a key innovation triggering speciation in the family (Kennedy 2000). However, neither mechanical isolation (Grant 1994) through differential pollen deposition onto the same pollinator nor through specific adaptations of the style-hooded staminode complex to certain pollinators could be confirmed (Ley and Claßen-Bockhoff 2010). The family rather diversified through geographical isolation (Ley and Claßen-Bockhoff 2011), thereby modifying the shape of floral structures without changing the pollination mechanism.
The latter is an inherited character which is most likely based on ontogenetic abbreviation. Considering the sister family Cannaceae, both families share extreme protandry and secondary pollen transfer to the style already in bud stage (Glinos and Cocucci 2011). However, whereas staminodes and style separate in Cannaceae, the style is not freed from the hooded staminode in Marantaceae. The straightjacket condition results in style tension and may have triggered the evolution of the explosive pollen transfer.
Data availabitily
All row data are available from the authors.
References
Andersson L (1981) The neotropical genera of Marantaceae: circumscription and relationships. Nordic J Bot 1:218–245. https://doi.org/10.1111/j.1756-1051.1981.tb00692.x
Andersson L (1998) Marantaceae. In: Kubitzky K (ed) The families and genera of vascular plants. Vol. IV. Flowering plants, Monocotyledons, Alismatanae and Commelinanae (except Gramineae). Springer, Berlin, pp 278–293
Brantjes NBM, de Vos OC (1981) The explosive release of pollen in flowers of Hyptis (Lamiaceae). New Phytol 87:425–430. https://doi.org/10.1111/j.1469-8137.1981.tb03213.x
Claßen-Bockhoff R (1991) Untersuchungen zur Konstruktion des Bestäubungsapparates von Thalia geniculata (Marantaceen). Bot Acta 104:183–193
Claßen-Bockhoff R, Heller A (2008a) Floral synorganization and secondary pollen presentation in four Marantaceae from Costa Rica. Int J Plant Sci 169:745–760. https://doi.org/10.1086/588069
Claßen-Bockhoff R, Heller A (2008b) Style release experiments in four species of Marantaceae from the Golfo Dulce area, Costa Rica. Stapfia 88:557–571
Cocucci A (1989) El mecanismo floral de Schizanthus (Solanaceae). Kurtziana 20:113–132
Dean JA (1999) Lange’s handbook of chemistry. McGraw-Hill, New York
Delpino F (1869) Breve cenno sulle relazioni biologiche e geneologiche delle Matantaceae. Nuovo Giorn Bot Ital 1:293–306
Dodson CH (1962) Pollination and variation in the subtribe Catasetinae (Orchidaceae). Ann Missouri Bot Garden 49:35–56. https://doi.org/10.2307/2394740
Edwards J, Whitaker D, Klionsky S, Laskowski MJ (2005) A record-breaking pollen catapult. Nature 435:164. https://doi.org/10.1038/435164a
Eichler AW (1884) Beiträge zur Morphologie und Systematik der Marantaceen. Kgl. Akad. Wiss., Berlin
Forterre Y, Skotheim JM, Dumais J, Mahadevan L (2005) How the Venus flytrap snaps. Nature 433:421–425
Glinos E, Cocucci AA (2011) Pollination biology of Canna indica (Cannaceae) with particular reference to the functional morphology of the style. Plant Syst Evol 291:49–58. https://doi.org/10.1007/s00606-010-0379-x
Govaerts R, Kennedy H (2016) World checklist of Marantaceae. Facilitated by the royal botanic gardens, Kew. https://apps.kew.org/wcsp/. Retrieved 23 Jan 2016
Grant V (1994) Modes and origins of mechanical and ethological isolation in angiosperms. Proc Natl Acad Sci USA 91:3–10. https://doi.org/10.1073/pnas.91.1.3
Harkins WD, McLaughlin HM (1925) The structure of films of water on salt solutions I. Surface tension and adsorption for aqueous solutions of sodium chloride. J Am Chem Soc 47:2083–2089
Jerominek M, Claßen-Bockhoff R (2015) Electrical signals in prayer plants (Marantaceae)? Insights into the trigger mechanism of the explosive style movement. PLoS ONE 10:e0126411. https://doi.org/10.1371/journal.pone.0126411
Jerominek M, Will M, Claßen-Bockhoff R (2018) Insights into the inside—a quantitative histological study of the explosively moving style in Marantaceae. Front Plant Sci 9:1695. https://doi.org/10.3389/fpls.2018.01695
Jerominek M (2015) Experimental investigations on the explosive pollination mechanism in Marantaceae. Dissertation, Johannes Gutenberg-University Mainz, Germany
Kennedy K (1978) Systematics and pollination of the “cloed flowered” species of Calathea (Marantaceae). Univ Calif Publ Bot 71:1–90
Kennedy H (2000) Diversification in pollination mechanisms in the Marantaceae. In: Wilson KL, Morrison DA (eds) Monocots: systematics and evolution. CSIRO, Melbourne, pp 335–343
Kunze H (1984) Vergleichende Studien an Cannaceen- und Marantaceenblüten. Flora 175:301–318. https://doi.org/10.1016/S0367-2530(17)31453-6
Ley AC, Claßen-Bockhoff R (2010) Parallel evolution in plant-pollinator interaction in African Marantaceae. In: van der Burgt X, van der Maesen J, Onana JM (eds) Systematics and conservation of African plants. Royal Botanic Gardens, Kew, pp 847–854
Ley AC, Claßen-Bockhoff R (2011) Ontogenetic and phylogenetic diversification in Marantaceae. In: Wanntorp L, Ronse de Craene L (eds) Flowers on the tree of life. Cambridge University Press, Cambridge, pp 239–255
Ley AC, Claßen-Bockhoff R (2012) Floral synorganization and its influence on mechanical isolation and autogamy in Marantaceae. Bot J Linn Soc 168:300–322. https://doi.org/10.1111/j.1095-8339.2011.01202.x
Locatelli E, Machado IC, Medeiros P (2004) Saranthe klotzschiana (KOER.) EICHL. (Marantaceae) e seu mecanismo explosive de polinização. Rev Bras Bot 27:757–765
El Ottra JHL, Toni JFG, Thaowetsuwan P, Dos Santos P, Jeiter J, Ronse De Craene L, Bull-Hereñu K, Claβen-Bockhoff R (2023) Pollen transfer within flowers: how pollen is secondarily presented. Int J Plant Sci 185:15–31
Pischtschan E, Claßen-Bockhoff R (2008) Setting-up tension in the style of Marantaceae. Plant Biol 10:441–450. https://doi.org/10.1111/j.1438-8677.2008.00051.x
Pischtschan E, Claßen-Bockhoff R (2010) Anatomic insights into the thigmonastic style tissue in Marantaceae. Plant Syst Evol 286:91–102. https://doi.org/10.1007/s00606-010-0282-5
Pischtschan E, Ley AC, Classen-Bockhoff R (2010) Ontogenetic and phylogenetic diversification of the hooded staminode in Marantaceae. Taxon 59:1111–1125. https://doi.org/10.1002/tax.594011
Prince LM, Kress J (2006) Phylogenetic relationsships and classification in Marantaceae: insights from plastid DNA sequence data. Taxon 55:281–296. https://doi.org/10.2307/25065578
Raju AJS, Rao SP (2006) Explosive pollen release and pollination as a function of nectar-feeding activity of certain bees in the biodiesel plant, Pongamia pinnata (L.) Pierre (Fabaceae). Curr Sci 90:960–967
Schumann K (1902) Marantaceae. In: Engler A (ed) Das Pflanzenreich, vol IV. W. Engelmann, Leipzig, pp 1–184
Skotheim JM, Mahadevan L (2005) Physical limits and design principles for plant and fungal movements. Science 308:1308–1310. https://doi.org/10.1126/science.1107976
Steffens J, Harel E, Hunt M (1994) Polyphenol Oxidase. In: Ellis B, Kuroki G, Stafford H (eds) Genetic engineering o plant secondary metabolism, vol 28. Springer, Berlin, pp 275–312
Westerkamp C (1993) The co-operation between the asymmetric flower of Lathyrus latifolius (Fabaceae-Vicieae) and its visitors. Phyton 33:121–137
Acknowledgements
We thank Dr. Piyakaset Suksathan and Dr. Suyanee Vessabutr for local assistance and arrangements in the Queen Sirikit Botanic Garden (Thailand), and Dr. Zak Zahawi in the Wilson Botanical Garden (Costa Rica). We are grateful to Rodolfo Quirós and Pattarin Nunthamontree for their technical assistance. Thanks are also given to the editor of this special issue, Akitoshi Iwamoto, and to two unknown reviewers for their helpful comments.
Funding
Travel and accommodation expenses were funded by the German Academic Exchange Service (DAAD; D/09/49132).
Author information
Authors and Affiliations
Contributions
The project was initiated by RC-B. Both authors contributed to the study conception and design. Material preparation and data collection were performed by MJ. Data analysis and interpretation were conducted by MJ and RC-B. The first draft of the manuscript was written by MJ, the final version by RC-B. Both authors approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
There are no competing interests.
Ethics approval
There are no humans or animals involved in the research.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
V1: Trigger deflection (Goeppertia ecuadoriana) (MP4 9861 KB)
V2: Basal plate slipped away (Goeppertia zebrina var. humilior) (MP4 1174 KB)
V3: Longitudinal cut in distal hood (Goeppertia zebrina var. humilior) (MP4 3020 KB)
V4: Stinging style (Calathea crotalifera) (MP4 10215 KB)
V5: Transverse cut and downward buckling of the style (Goeppertia zebrina var. humilior) (MP4 2206 KB)
V6: Transverse cut and rupture of the style (Goeppertia zebrina var. zebrina) (MP4 5270 KB)
V7: Longitudinal cut at lower side of the style (Calathea crotalifera) (MP4 5383 KB)
V8: Time-lapse (20 min) black colouration after wounding (Goeppertia zebrina var. humilior) (MP4 5546 KB)
V9: Time-lapse (20 min) black colouration in 70% ethanol (Goeppertia zebrina var. zebrina) (MP4 5190 KB)
V10: Time-lapse (10 min) fixation chloroform steam (Stromanthe sanguinea) (MP4 5149 KB)
V11: Time-lapse (30 min) fixation in NaCl (Goeppertia zebrina var. zebrina) (MP4 7539 KB)
V12: Time-lapse (20 min) black colouration after freezing (Goeppertia zebrina var. humilior) (MP4 3036 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jerominek, M., Claßen-Bockhoff, R. What keeps the style under tension? Experimental tests to understand the biomechanics of the explosive style movement in Marantaceae. J Plant Res 137, 745–762 (2024). https://doi.org/10.1007/s10265-024-01535-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10265-024-01535-2