Abstract
Plants maintain populations of stem cells to generate new organs throughout the course of their lives. The pathways that regulate plant stem cell maintenance have garnered great interest over the past decades, as variation in these pathways contributes plant morphological diversity and can be harnessed for crop improvement. In order to facilitate cross-species comparisons of gene function and better understand how these stem cell regulatory pathways evolved, we undertook a functionally informed phylogenetic analysis of leucine-rich receptor-like kinases (LRR-RLK) and related proteins across diverse land plant model systems. Based on our phylogenetic analysis and on functional data, we propose a naming scheme for these stem cell signaling genes. We discovered evidence for frequent loss of protein domains in angiosperms but not in bryophytes. In addition, several clades of stem cell signaling genes are closely related to genes that function in immunity, although these distinct developmental and immune functions likely separated or after the divergence of lycophytes and angiosperms. Overall, the phylogenetic framework and evolutionary hypotheses we provide here will empower future research on cross-species comparisons of stem cell signaling pathways.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
New plant tissues develop from reserves of stem cells called meristems that are found at the tips of roots and shoots and at the sites of vasculature formation. Maintenance of stable stem cell populations poses a challenge during development: if the stem cell population grows too large then development becomes disorganized, whereas under-proliferation of the stem cell pool can lead to meristem consumption and the termination of development. Signaling pathways dedicated to meristem maintenance are thus critical for maintaining indeterminate growth, a hallmark of plant development and a strategic source of morphological diversity.
Decades of research have revealed widespread functions for a suite of leucine-rich receptor-like kinases (LRR-RLK), receptor-like proteins (LRR-RLP), and pseudokinases in the regulation of plant meristem maintenance. These include the LRR-RLKs CLAVATA1 (CLV1) (Clark et al. 1997), PHLOEM INTERCALATED WITH XYLEM (PXY) (Fisher and Turner 2007), and RECEPTOR-LIKE PROTEIN KINASE 2/TOADSTOOL 2 (RPK2) (Kinoshita et al. 2010), the LRR-RLPs CLAVATA2 (CLV2) (Kayes and Clark 1998) and FASCIATED EAR 3 (FEA3) (Wu et al. 2016), the pseudokinase CORYNE (CRN) (Miwa et al. 2008), and the CLAVATA INSENSITIVE KINASE (CIK) co-receptors (Hu et al. 2018). Each of these cell-surface proteins are thought to act in signal perception and transduction that is elicited by mobile CLAVATA3/EMBRYO SURROUNDING REGION (CLE) peptide ligands.
LRR RLKs and related proteins have been studied predominately in the model plant Arabidopsis thaliana. While much progress has been made, we are still far from understanding the downstream signaling pathways or how these signaling components are employed in different developmental contexts. More recently, research on crop and bryophyte species has revealed that many of these signaling components have conserved functions, and that these pathways can be altered for agronomic benefit (Bommert et al. 2013; Je et al. 2018; Rodríguez-Leal et al. 2017; Whitewoods et al. 2018). These results demonstrate the insights gleaned from and the benefits of studying stem cell maintenance in diverse model species.
This work promotes a strategy of using a framework of combined phylogenetic and functional data to facilitate future analyses of meristem regulatory LRR RLKs from diverse species. We assess how stem cell regulating LRR-RLKs and related proteins have evolved across several plant model organisms, namely Arabidopsis, Tomato, Maize, Rice, the moss Physcomitrella patens (Hedw.) Bruch & Schimp., and the liverwort Marchantia polymorpha L. We extend our analysis to include the lycophyte Selaginella moellendorfii Hieron. and the moss Sphagnum fallax H. Klinggr. in order to shed light on gene duplications associated with the evolution of vasculature, and to gain a broader understanding of bryophyte stem cell signaling. We also propose a cogent, functionally and phylogenetically based nomenclature for heretofore unannotated orthologs of these meristem signaling components (Table S1). Finally, we use our phylogenetic analysis to highlight trends and propose testable hypotheses about the evolution of stem cell signaling in land plants.
Materials and methods
Starting with Arabidopsis thaliana gene of interest (i.e. CLV1), except in the case of FEA3 where the maize ortholog was used, we performed pBLAST in Phytozome 11 against: Arabidopsis thaliana TAIR10, Solanum lycopersicum iTAG2.4, Zea mays Ensembl-18, Oryza sativa v7_JGI, Selaginella moellendorfii v1.0, Physcomitrella Patens v3.3, Marchantia polymorpha v3.1, and Sphagnum fallax v 0.5 proteomes. Peptide sequences for the top 250 blast hits were selected and then filtered so that only peptides encoded by primary transcripts remained.
We used the CIPRES portal to run mafft set to the slowest but most accurate mode (linsi) (Katoh 2005). We then trimmed multiple sequence alignments of positions high in gaps using trimal (Capella-Gutiérrez et al. 2009), removing any position comprised of over 50% gaps. Using these trimmed multiple sequence alignments, we then constructed phylogenetic trees using RaXML (Stamatakis 2014) set to the PROTCATDAYHOFF model with 1000 rapid bootstrap via the CIPRES server (Miller et al. 2010). We viewed trees with the highest likelihood score with bootstrap values represented on bipartitions using MEGAX. From these larger trees, we found the most basal bipartition with a support value over 90% that contained our gene family of interest and selected that subtree for representation here.
Trees were visualized and annotated in MEGAX or using ete3 (Huerta-Cepas et al. 2016). Alignments juxtaposed to trees were alignments only of the sequences referenced on that tree (not the whole set of 200) aligned using Muscle (Edgar 2004) visualized in Aliview.
Results
CLV1 and BAM: dynamic gene gain and loss
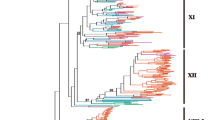
CLV1 encodes an LRR/RLK that regulates stem cell identity in the SAM by acting in a negative feedback loop wherein the homeobox transcription factor WUSCHEL (WUS), expressed in the middle of the shoot apical meristem (SAM), diffuses to overlying cells to activate the expression of the CLE peptide-encoding gene CLAVATA3 (CLV3) (Schoof et al. 2000). CLV3 is secreted and diffuses back down to the middle of the meristem, where it acts through the CLV1 receptor to repress WUS expression, completing the negative feedback loop. Angiosperm genomes contain a suite of paralogs of CLV1 called BARELY ANY MERISTEM (BAM). Our analysis of the CLV1/BAM clade of LRR-RLK genes suggests that Arabidopsis BAM1 and BAM2 were generated following a recent gene duplication (Fig. 1). We also detected a clade of BAM genes absent from the Arabidopsis genome that includes the recently characterized tomato gene SlBAM4 (Rodriguez-Leal et al. 2019). Meanwhile, maize lacks a member of the BAM3 clade, but contains two copies of BAM4. Overall, there is evidence for frequent gain and loss of BAM genes, while CLV1 was maintained as a single copy in each angiosperm sampled.
Maximum Likelihood tree of CLV1/BAM LRR-RLKs based on full length peptide sequences. Subtree shown here is taken from a larger maximum likelihood tree; bootstrap support at the base of this tree was 100%. CLAVATA1 and BAM genes are paralogs that evolved after the split between lycophytes and angiosperms, while Selaginella and bryophyte orthologs underwent lineage-specific duplications
Our analysis also shows that CLV1 and BAM genes diverged after the separation of the lycophyte and flowering plant lineages (Fig. 1). Thus, lycophyte and bryophyte genes presented here are co-orthologous to the CLV1 and BAM clades. Recent work demonstrated a conserved role for the moss genes PpCLV1a and PpCLV1b in inhibiting meristem identity and uncovered a previously undescribed function in the regulation of cell division plane orientation (Whitewoods et al. 2018). Intriguingly, a role for CLV1/BAM in the control of cell division plane orientation was also found to be conserved in Arabidopsis, wherein clv1, bam1, bam2, bam3 quadruple mutations resulted in cell division plane defects in the root (Whitewoods et al. 2018). It is likely that historical challenges in generating higher order mutants had obscured the role of CLV1/BAM during cell division plane orientation in Arabidopsis. Moreover, these reverse genetic challenges had previously rendered cross-species comparisons of ‘loss of clade’ rather than ‘loss of gene’ function untenable. However, with the advent of facile genome editing technologies and a wealth of genomic information, we can, informed by phylogenies, test and gain a general understanding of gene family function.
Many developmental functions for BAM1 and BAM2 have been demonstrated, including CLE perception and regulation of cell fate and periclinal divisions in root vasculature and in anther development, and buffering of CLE signaling in the SAM (Cui et al. 2018; DeYoung and Clark 2008; Hord et al. 2006; Qian et al. 2018; Shimizu et al. 2015). Despite these distinct roles for CLV1 and BAM1/BAM2, BAM1 also compensates for clv1 loss of function in shoot meristems (Nimchuk et al. 2015). These data suggest that BAM1/BAM2 can perform the same biochemical function as CLV1, and that differences in mutant phenotypes between these related LRR-RLKs are due to differences in gene expression.
PXY: an ancient LRR-RLK recruited to vascular development
Within the broader LRR-RLK phylogeny, the clade containing PHLOEM INTERCELATED WITH XYLEM (PXY) is sister to the CLV1 and BAM clade of receptor kinases (Liu et al. 2017). Like CLV1, PXY encodes a CLE receptor and regulates the activity of a WUSCHEL-like HOMEOBOX (WOX) gene, here WOX4 in the stem cell niche comprising the vascular procambium (Etchells et al. 2013; Hirakawa et al. 2010). The PXY ligand is TDIF/CLE41, a different class of CLE from CLV3 (Goad et al. 2017). Whereas PXY is conserved across flowering plants and Selaginella, the moss genomes sampled here lack both PXY (Fig. 2) and TDIF orthologs (Whitewoods et al. 2018). However, the genome of the liverwort Marchantia polymorpha harbors a PXY ortholog, as well as TDIF peptide encoding gene, which together reduce cell proliferation near the apical notch of the thallus (Hirakawa et al. 2019). This topology and the functional characterization of TDIF signaling in Marchantia suggests that PXY function predates the evolution of vasculature, and that a function during vascular formation was co-opted later in land plant evolution.
Maximum Likelihood tree of PXY LRR-RLKs based on full length peptide sequences. Subtree shown here is taken from a larger maximum likelihood tree that also included the CLV1/BAM clade; bootstrap support at the base of this subtree was 100%. PXY is typically associated with vascular development, but the non-vascular liverwort Marchantia polymorpha possesses one PXY ortholog
CLAVATA2 and CORYNE: pieces of a whole
Conclusive evidence for protein-protein interactions among LRR-RLKs is scarce, owing to the inherent difficulties in studying low-abundance membrane-associated proteins. However, data supporting the formation of a CLV2:CRN complex is compelling (Bleckmann et al. 2010; Guo et al. 2011; Somssich et al. 2015). CLV2 possesses an LRR-ectodomain while CRN possesses a cytoplasmic domain but no ectodomain; it is attractive to think that together these two proteins constitute a complete LRR-RLK. However, the CRN cytoplasmic domain possesses a pseudokinase that is important for its function, although the mechanism is unclear. Like other LRR-RLK complexes that maintain stem cell populations in the SAM, CLV2 and CRN have roles in diverse developmental processes including phloem development (Hazak et al. 2017). The function of CLV2 and CRN appear to be conserved in grasses, as mutants of the maize CLV2 ortholog FASCIATED EAR 2 (FEA2) also develop enlarged and fasciated inflorescence meristems (Taguchi-Shiobara et al. 2001). In both models, the effects of clv1 and clv2/crn loss of function are additive, suggesting that CLV1 and CV2/CRN comprise distinct CLE signaling pathways (Müller et al. 2008).
In our phylogenetic analysis, we find that CLV2 exists as a single-copy gene in the four angiosperm genomes sampled, and we did not detect CLV2 orthologs in Selaginella moellendorffii or bryophytes (Fig. S1). Further analysis, however, is limited by very low support values for relationships along the backbone of the phylogenetic tree, hindering our ability to draw further conclusions about the evolution of CLV2 within land plants.
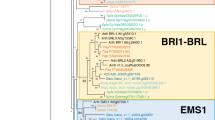
Similar to CLV2, each angiosperm genome assayed here possesses one ortholog of CRN (Fig. 3). In the case of CRN however, we were able to identify a well-supported sister clade containing the Arabidopsis receptor kinase gene SUPPRESSOR OF BIR1 (SOBIR1). Unlike CRN, SOBIR1 possesses an extracellular domain with LRRs and has been described to function in immunity-induced and developmentally programmed cell death (Gao et al. 2009; Leslie et al. 2010). We identified orthologs of SOBIR1 in Marchantia, Physcomitrella, and Selaginella; the Marchantia and Physcomitrella genes are predicted to encode proteins containing the longest extracellular domains of any in the clade (Fig. 3). Interestingly, the maize and rice orthologs of SOBIR1 have short extracellular domains, similar to CRN. These disparities raise questions as to whether these grass SOBIR1 orthologs functionally resemble SOBIR1 or CRN, or whether they possess separate functions entirely. These phylogenetic data support a model wherein CRN is evolutionarily derived from a full-length LRR-RLK, raising the appealing hypothesis that CLV2 is similarly derived from a gene encoding a full length LRR-RLK but lost its cytoplasmic domain. However, whereas conservation of the kinase domain enables phylogenetic analyses of CRN, discerning the evolution of CLV2 will prove much more difficult given that the sequence is largely composed of repeat domains.
Maximum Likelihood tree of the CRN pseudokinase and the related LRR-RLK SOBIR1 based on full length peptide sequences. Appended to the right is a realignment of the full-length peptide sequences from the genes represented in the tree. Subtree shown here is taken from a larger maximum likelihood tree; bootstrap support at the base of this subtree was 100%. CRN and SOBIR1 family members have distinct functions, but repeated domain loss has led to the convergent evolution of similar protein structures between the clades with truncated ectodomains
RPK1/RPK2: Structural changes and possible neofunctionalization
RPK2 acts downstream of CLE signaling in multiple developmental contexts. In the SAM, RPK2 performs a similar function as CLV1 and CLV2, but via a separate pathway (Kinoshita et al. 2010). During anther development, RPK2 acts with BAM1 and BAM2 to coordinate cell division plane orientation and cell identity (Cui et al. 2018; Mizuno et al. 2007). As a final example of overlapping function with CLV1/BAM type LRR-RLKs, RPK2 and BAM1 interact to inhibit cell proliferation in the root (Shimizu et al. 2015). Interestingly, and despite the repeated discovery of overlap between RPK2 and CLV1/BAM genes, the Arabidopsis RPK2 paralog RPK1 appears to function in a completely distinct pathway. RPK1 is required for ABA response (Osakabe et al. 2005) and is essential for shoot regeneration (Motte et al. 2014). However, there is an exception to this separation of RPK2 and RPK1 pathways, as these genes appear to have redundant functions in the embryo where they are implicated in organizing auxin efflux carriers during embryonic patterning (Nodine et al. 2007). While this impact on auxin efflux is the most mechanistic description of RPK1 or RPK2 function, whether such changes in auxin transport could account for other rpk1 or rpk2 mutant phenotypes has not been explored.
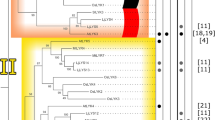
As RPK1 and RPK2 have distinct functions in most developmental contexts, we used our phylogenetic analyses to determine whether RPK2 or RPK1 is more likely to carry out the ancestral RPK1/RPK2 function, and whether one gene’s activity is likely the result of neofunctionalization. Given that Selaginella, Physcomitrella, and Marchantia each have a single RPK1/RPK2 homolog, tree topology alone provides little useful information toward answering this question (Fig. 4). Comparing the structures of the bryophyte and angiosperm RPK1/RPK2 homologs (Fig. 4) reveals that RPK1 and several angiosperm orthologs are truncated, with shorter extracellular domains, whereas RPK2 resembles the ancestral, full-length form. Together with recent functional data from Physcomitrella showing that PpRPK2 is a regulator of stem cell number and cell division plane similar to RPK2 (Whitewoods et al. 2018), this evidence suggests that RPK1’s role in ABA signaling might represent neofunctionalization of the ancestral RPK1/RPK2 gene, concurrent with a loss of LRR domains.
Maximum Likelihood tree of the RPK1 and RPK2 LRR-RLKs based on full length peptide sequences. Subtree shown here is taken from a larger maximum likelihood tree; bootstrap support at the base of this subtree was 100%. Adjacent to the phylogeny are the realigned, full-length peptide sequences encoded by the genes depicted on this subtree. AtRPK2 more closely resembles the bryophyte ortholog of RPK1/RPK2. Truncations of genes in this clade appear to be common derived features
Like RPK1, several other genes in this clade encode proteins that are truncated or appear to be missing internal LRR domains (Fig. 4). While one of these short-ectodomain variants includes the tomato gene most closely related to RPK1, in several cases the number of LRR domains is unrelated to the position of the gene on the tree. This suggests, as others have shown (Liu et al. 2017), that LRR domain number is highly dynamic. In the case of RPK1/RPK2, it would be interesting to see whether, as appears to be the case for moss and Arabidopsis RPK2, functional conservation can be predicted based on conserved LRR domain structure more than by relatedness as depicted by the gene tree (determined by sequence). In accordance with this hypothesis, we named short-ectodomain homologs of RPK1/RPK2 as RPK1 LIKE and long-ectodomain homologs RPK2 LIKE (Table S1).
FEA3 and TMM: close relatives with distinctive ligands
The RLP-encoding gene FEA3 was discovered in maize as a single copy gene regulating meristem size, akin to CLV1 (Wu et al. 2016). FEA3 is hypothesized to binds to and transduce signals from the CLE peptide FON2-LIKE CLE PROTEIN 1 (FCP1). The discovery of FEA3 led to the hypothesis that different LRR-RLKs contribute to the regulation of meristem size by controlling the expression of WUS in different meristematic domains. In this model, FEA3 represses ZmWUS1 in the center of the SAM toward the stem in response to FCP1, whereas in Arabidopsis CLV1 responds to CLV3 to repress WUS nearer the apex of the shoot meristem. While FEA3 was originally hypothesized to regulate SAM size based on leaf-derived FCP1 (Wu et al. 2016), more recent data conflicts with the originally reported expression domains for FCP1, indicating that the model of FCP1-FEA3 activity should be revisited (Knauer et al. 2019).
Unexpectedly, our phylogenetic analysis revealed that the clade sister to FEA3 contains the Arabidopsis gene AT4G28560, which is annotated as ROP-INTERACTIVE CRIB MOTIF-CONTAINING PROTEIN 7 (RIC7) that contains a CRIB (Cdc42/Rac-interactive binding) domain (Fig. 5). This annotation led us to question the close position of AT4G28560 to FEA3 in our maximum likelihood analysis. After subjecting AT4G28560 to a conserved protein domain search, we found that this gene encodes a protein predicted to contain 9 LRR domains and no CRIB domain (Fig. S2). Additionally, the highest scoring pBLAST hits against the Arabidopsis thaliana genome are the Arabidopsis orthologs of FEA3, but not other RIC gene family members (pBLAST data not shown). Finally, another gene model, AT4G28556, is annotated as RIC7 in the paper where RIC7 function was originally characterized (Jeon et al. 2008).Altogether, these results suggest that AT4G28560 is currently misannotated as RIC7. Furthermore, the clade containing AT4G28560 also contains one rice and one tomato gene, but no maize genes, suggesting that the maize ortholog may have been lost and that the current closest maize ortholog is FEA3.
Maximum Likelihood tree of the FEA3 and TMM LRR-RLPs based on full length peptide sequences. Subtree shown here is taken from a larger maximum likelihood tree; bootstrap support at the base of this subtree was 94%. Whereas FEA3 is a putative CLE receptor, TMM binds a distinct class of ligands. Support for placement of bryophyte orthologs of FEA3 is low
We next tried to determine whether FEA3 is conserved in bryophytes and Selaginella. Although the tree topology with the highest likelihood places a set of Selaginella and bryophyte genes sister to the clade containing FEA3, bootstrap support for these relationships are low (Fig. 5). It is thus difficult to tell conclusively whether the lycophyte and bryophyte clades are more closely related to FEA3, or to the gene family sister to FEA3 containing the ERECTA (ER) co-receptor TOO MANY MOUTHS (TMM) (Lee et al. 2012). Given that TMM has a well-supported moss ortholog (Pp3c3 3780V3.1) separate from the putative bryophyte FEA3 orthologs, we propose that the bryophyte clade sister to the FEA3 clade likely comprises true orthologs of FEA3. Interestingly, while Physcomitrella patens contains a high-confidence ortholog of TMM with a demonstrated conserved function (Caine et al. 2016), the peat moss Sphagnum fallax and the liverwort Marchantia polymorpha do not. Thus, as neither Sphagnum nor Marchantia possess stomata and Physcomitrella does, the presence/absence of TMM tracks well with the evolution of stomata. These analyses suggest that TMM functions specifically in stomatagenesis as far back as the earliest land plants.
Unlike the CRN and RPK1/RPK2 gene families, the RLPs comprising the FEA3 and TMM clades vary little in their protein length and overall domain structure, at least in the taxa sampled (Fig. S3). These data suggest that while dynamic LRR-domain gain and loss is common in many LRR-RLK gene families, it is not the case universally.
CIK: co-receptors at the crossroads of immune and stem cell signaling
CLAVATA insensitive kinases (CIKs) are recently discovered LRR-RLKs that act as co-receptors within diverse developmental contexts. CIKs form co-receptor complexes with many of the signaling proteins discussed above, including CLV1, BAM1/2, RPK2, CLV2, and CRN (Anne et al. 2018; Cui et al. 2018; Hu et al. 2018). CIK receptors are closely related to the NSP-INTERACTING KINASE (NIK) LRR-RLKs that function in plant immunity (Fontes et al. 2004; Zorzatto et al. 2015). Expansion and diversification of CIK and NIK receptors occurred following the speciation events that separated bryophytes and lycophytes from vascular plants (Fig. 6). We thus resolve a well-supported clade of bryophyte genes co-orthologous to all angiosperm CIK and NIK LRR-RLKs.
The CIK/NIK clade thus presents us with a family of receptors that function in both immunity and development. Expression of NIK genes in Arabidopsis under CIK promoters can complement cik mutant phenotype (Anne et al. 2018), indicating that while the function of these genes has diverged, the biochemical operations they can conduct have not. Immune and developmental pathways consistently exhibit substantial crosstalk, and how similar signaling pathways are parsed differently during development and immune response is an open question in plant biology. Given that CIK1/2 and NIK1/2 are such similar proteins with quite distinct functions, we propose that comparison of all CIK/NIK genes to their bryophyte orthologs will prove a fertile ground for experiments seeking to understand how subfunctionalization of LRR-RLKs occurs, how different receptor protein complexes evolve, and the crosstalk between immune and developmental signaling.
Discussion
Many of the LRR-RLKs discussed herein are have distinct functions across many tissues but are unified in their regulation of stem cell specification. Within a clade, the ability of various homologs to complement one another is widespread, despite differences in mutant phenotypes among these homologs. Often times these differences in loss-of-function phenotype are ascribed to variations in expression domain. However, given that a protein accumulates within a new domain, two distinct outcomes are possible. First, the protein can perform the same biochemical operation it did in its original domain (i.e. subfunctionalization); which can lead to unexpected mutant phenotypes for the subfunctionalized paralog. For example, a mutation that reduces proliferation in leaf initial cells will have quite distinct developmental consequences from a mutation of a paralogous gene functioning in SAM stem cell proliferation even though both regulate the same process, i.e. proliferation. Second, the protein may evolve new biochemical functions in its new domain (i.e. neofunctionalization), which might involve new binding new partners, phosphorylating novel downstream targets, or binding to different ligands. Such neofunctionalization may stem from structural changes to the protein itself that arise after gene duplication or might be entirely dependent on novel interactions in the new context. Uncovering which of the above scenarios are operating during the diversification of the LRR-RLKs and related proteins will lead to a better understanding of stem cell maintenance pathways in land plants. Working within a functionally informed phylogenetic framework like the one provided here will expedite such studies.
Given the degree of redundancy, apparent promiscuity in complex formation, and diversity of downstream responses possible even from the same receptors (Je et al. 2018), understanding LRR-RLK function will require experiments with high spatial and temporal resolution. This pursuit will be aided by the advent of single-cell technologies; high-throughput experiments providing a set of plausible protein-protein interactions (Smakowska-Luzan et al. 2018) can be combined with single-cell RNAseq data to generate hypotheses about which receptors might be forming a complex in a given cell type during development.
Here we presented an evolutionary framework for analyses of signaling genes involved in stem cell maintenance. We identified orthologs of these signaling components in diverse plant model systems and propose a nomenclature for unannotated genes based on functional and phylogenetic (Table S1). We see, as has been previously reported (Liu et al. 2017), that LRR domain number is highly dynamic within some (RPK1/RPK2) but not all (FEA3) gene families. We identify bryophyte orthologs for most LRR-RLK-like genes examined, reaffirming that LRR-RLK gene families diversified early in land plant evolution. Interestingly, even in clades with frequent truncations in extracellular protein domains such as CRN/SOBIR1 or RPK1/RPK2, bryophyte extracellular domains were always the longest and exhibited no evidence of domain loss. It will be interesting to see whether this is a general trend that extends beyond the taxa and gene families sampled here, and will require the assembly of a greater number of bryophyte genomes.
Within clades, we find evidence that the pseudokinase CRN evolved from a full-length LRR-RLK that is the likely ancestor of CRN and SOBIR1. In the case of CRN and SOBIR1 as well for CIK and NIK genes, we find that many of these regulators of stem cell signaling are closely related to genes that function in plant immunity. Intriguingly, cik mutations can be complemented by NIK genes, which suggests that the context within which a protein functions is determined not only by cell type, but also by that the biotic and abiotic stimuli perceived by that cell. Moreover, a gene closely related to FEA3 was identified to be mis-annotated, and we found that FEA3 is closely related to the ER co-receptor encoding gene TMM. This relationship is interesting, as the TMM/ER complex binds a distinct class of ligands from FEA3, adding another level of promiscuity to these LRR-RLK gene families that will need to be untangled.
In this work we sought to provide a useful reference to facilitate research on stem cell signaling pathways in diverse model and crop species. As plant transformation and genome editing technologies improve, the number of systems available for functional genetic studies will expand, and analyses like the one conducted here will need to be replicated. Altogether, increasing the number of model systems and performing clade to clade rather than gene to gene comparisons will provide us with a deeper and more general understanding of plant stem cell signaling.
References
Anne P, Amiguet-Vercher A, Brandt B et al (2018) CLERK is a novel receptor kinase required for sensing of root-active CLE peptides in Arabidopsis. Development 145:dev162354. https://doi.org/10.1242/dev.162354
Bleckmann A, Weidtkamp-Peters S, Seidel CAM, Simon R (2010) Stem cell signaling in Arabidopsis requires CRN to localize CLV2 to the plasma membrane. Plant Physiol 152:166–176. https://doi.org/10.1104/pp.109.149930
Bommert P, Nagasawa NS, Jackson D (2013) Quantitative variation in maize kernel row number is controlled by the FASCIATED EAR2 locus. Nat Genet 45:334–337. https://doi.org/10.1038/ng.2534
Caine RS, Chater CC, Kamisugi Y et al (2016) An ancestral stomatal patterning module revealed in the non-vascular land plant Physcomitrella patens. Development 143:3306–3314. https://doi.org/10.1242/dev.135038
Capella-Gutiérrez S, Silla-Martínez JM, Gabaldón T (2009) trimAl: A tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 25:1972–1973. https://doi.org/10.1093/bioinformatics/btp348
Clark SE, Williams RW, Meyerowitz EM (1997) The CLAVATA1 gene encodes a putative receptor kinase that controls shoot and floral meristem size in Arabidopsis. Cell 89:575–585. https://doi.org/10.1016/S0092-8674(00)80239-1
Cui Y, Hu C, Zhu Y et al (2018) CIK receptor kinases determine cell fate specification during early anther development in Arabidopsis. Plant Cell 30:2383–2401. https://doi.org/10.1105/TPC.17.00586
DeYoung BJ, Clark SE (2008) BAM receptors regulate stem cell specification and organ development through complex interactions with CLAVATA signaling. Genetics 180:895–904. https://doi.org/10.1534/genetics.108.091108
Edgar RC (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32:1792–1797. https://doi.org/10.1093/nar/gkh340
Etchells JP, Provost CM, Mishra L, Turner SR (2013) WOX4 and WOX14 act downstream of the PXY receptor kinase to regulate plant vascular proliferation independently of any role in vascular organisation. Development 140:2224–2234. https://doi.org/10.1242/dev.091314
Fisher K, Turner SR (2007) PXY, a receptor-like kinase essential for maintaining polarity during plant vascular-tissue development. Curr Biol 17:1061–1066. https://doi.org/10.1016/j.cub.2007.05.049
Fontes EPB, Santos AA, Luz DF et al (2004) The geminivirus nuclear shuttle protein is a virulence factor that suppresses transmembrane receptor kinase activity. Genes Dev 18:2545–2556. https://doi.org/10.1101/gad.1245904
Gao M, Wang X, Wang D et al (2009) Regulation of cell death and innate immunity by two receptor-like kinases in Arabidopsis. Cell Host Microbe 6:34–44. https://doi.org/10.1016/j.chom.2009.05.019
Goad DM, Zhu C, Kellogg EA (2017) Comprehensive identification and clustering of CLV3/ESR-related (CLE) genes in plants finds groups with potentially shared function. New Phytol 216:605–616. https://doi.org/10.1111/nph.14348
Guo Y, Han L, Hymes M et al (2011) CLAVATA2 forms a distinct CLE-binding receptor complex regulating Arabidopsis stem cell specification. Plant J 63:734–747. https://doi.org/10.1111/j.1365-313X.2010.04295.x.CLAVATA2
Hazak O, Brandt B, Cattaneo P et al (2017) Perception of root-active CLE peptides requires CORYNE function in the phloem vasculature. EMBO Rep 18:1367–1381. https://doi.org/10.15252/EMBR.201643535
Hirakawa Y, Kondo Y, Fukuda H (2010) TDIF peptide signaling regulates vascular stem cell proliferation via the WOX4 homeobox gene in Arabidopsis. Plant Cell 22:2618–2629. https://doi.org/10.1105/tpc.110.076083
Hirakawa Y, Uchida N, Yamaguchi YL et al (2019) Control of proliferation in the haploid meristem by CLE peptide signaling in Marchantia polymorpha. PLOS Genet 15:1–20. https://doi.org/10.1371/journal.pgen.1007997
Hord CLH, Chen C, DeYoung BJ et al (2006) The BAM1/BAM2 receptor-like kinases are important regulators of Arabidopsis early anther development. Plant Cell 18:1667–1680. https://doi.org/10.1105/tpc.105.036871
Hu C, Zhu Y, Cui Y et al (2018) A group of receptor kinases are essential for CLAVATA signalling to maintain stem cell homeostasis. Nat Plants 4:205–211. https://doi.org/10.1038/s41477-018-0123-z
Huerta-Cepas J, Serra F, Bork P (2016) ETE 3: reconstruction, analysis, and visualization of phylogenomic data. Mol Biol Evol 33:1635–1638. https://doi.org/10.1093/molbev/msw046
Je B, Il XuF, Wu Q et al (2018) The CLAVATA receptor FASCIATED EAR2 responds to distinct CLE peptides by signaling through two downstream effectors. Elife. https://doi.org/10.7554/eLife.35673
Jeon BW, Hwang J-U, Hwang Y et al (2008) The Arabidopsis small G protein ROP2 is activated by light in guard cells and inhibits light-induced stomatal opening. Plant Cell 20:75–87. https://doi.org/10.1105/tpc.107.054544
Katoh K (2005) MAFFT version 5: improvement in accuracy of multiple sequence alignment. Nucleic Acids Res 33:511–518. https://doi.org/10.1093/nar/gki198
Kayes JM, Clark SE (1998) CLAVATA2, a regulator of meristem and organ development in Arabidopsis. Development 125:3843–3851
Kinoshita A, Betsuyaku S, Osakabe Y et al (2010) RPK2 is an essential receptor-like kinase that transmits the CLV3 signal in Arabidopsis. Development 137:3911–3920. https://doi.org/10.1242/dev.061747
Knauer S, Javelle M, Li L et al (2019) A high-resolution gene expression atlas links dedicated meristem genes to key architectural traits. Genome Res 29:1962–1973. https://doi.org/10.1101/gr.250878.119
Lee JS, Kuroha T, Hnilova M et al (2012) Direct interaction of ligand-receptor pairs specifying stomatal patterning. Genes Dev 26:126–136. https://doi.org/10.1101/gad.179895.111
Leslie ME, Lewis MW, Youn JY et al (2010) The EVERSHED receptor-like kinase modulates floral organ shedding in Arabidopsis. Development 137:467–476. https://doi.org/10.1242/dev.041335
Liu P-L, Du L, Huang Y et al (2017) Origin and diversification of leucine-rich repeat receptor-like protein kinase (LRR-RLK) genes in plants. BMC Evol Biol 17:47. https://doi.org/10.1186/s12862-017-0891-5
Miller MA, Pfeiffer W, Schwartz T (2010) Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In: 2010 Gateway Computing Environments Workshop, GCE 2010
Miwa H, Betsuyaku S, Iwamoto K et al (2008) The receptor-like kinase SOL2 mediates CLE signaling in Arabidopsis. Plant Cell Physiol 49:1752–1757. https://doi.org/10.1093/pcp/pcn148
Mizuno S, Osakabe Y, Maruyama K et al (2007) Receptor-like protein kinase 2 (RPK 2) is a novel factor controlling anther development in Arabidopsis thaliana. Plant J 50:751–766. https://doi.org/10.1111/j.1365-313X.2007.03083.x
Motte H, Vercauteren A, Depuydt S et al (2014) Combining linkage and association mapping identifies RECEPTOR-LIKE PROTEIN KINASE1 as an essential Arabidopsis shoot regeneration gene. Proc Natl Acad Sci USA 111:8305–8310. https://doi.org/10.1073/pnas.1404978111
Müller R, Bleckmann A, Simon R (2008) The receptor kinase CORYNE of Arabidopsis transmits the stem cell-limiting signal CLAVATA3 independently of CLAVATA1. Plant Cell 20:934–946. https://doi.org/10.1105/tpc.107.057547
Nimchuk ZL, Zhou Y, Tarr PT et al (2015) Plant stem cell maintenance by transcriptional cross-regulation of related receptor kinases. Development 142:1043–1049. https://doi.org/10.1242/dev.119677
Nodine MD, Yadegari R, Tax FE (2007) RPK1 and TOAD2 are two receptor-like kinases redundantly required for Arabidopsis embryonic pattern formation. Dev Cell 12:943–956. https://doi.org/10.1016/j.devcel.2007.04.003
Osakabe Y, Maruyama K, Seki M et al (2005) Leucine-rich repeat receptor-like kinase1 is a key membrane-bound regulator of abscisic acid early signaling in Arabidopsis. Plant Cell 17:1105–1119. https://doi.org/10.1105/tpc.104.027474
Qian P, Song W, Yokoo T et al (2018) The CLE9/10 secretory peptide regulates stomatal and vascular development through distinct receptors. Nat Plants 4:1071–1081. https://doi.org/10.1038/s41477-018-0317-4
Rodríguez-Leal D, Lemmon ZH, Man J et al (2017) Engineering quantitative trait variation for crop improvement by genome editing. Cell. https://doi.org/10.1016/j.cell.2017.08.030
Rodriguez-Leal D, Xu C, Kwon CT et al (2019) Evolution of buffering in a genetic circuit controlling plant stem cell proliferation. Nat Genet 51:786–792
Schoof H, Lenhard M, Haecker a et al (2000) The stem cell population of Arabidopsis shoot meristems in maintained by a regulatory loop between the CLAVATA and WUSCHEL genes. Cell 100:635–644. https://doi.org/10.1016/S0092-8674(00)80700-X
Shimizu N, Ishida T, Yamada M et al (2015) BAM 1 and RECEPTOR-LIKE PROTEIN KINASE 2 constitute a signaling pathway and modulate CLE peptide-triggered growth inhibition in Arabidopsis root. New Phytol 208:1104–1113. https://doi.org/10.1111/nph.13520
Smakowska-Luzan E, Mott GA, Parys K et al (2018) An extracellular network of Arabidopsis leucine-rich repeat receptor kinases. Nature 553:342–346. https://doi.org/10.1038/nature25184
Somssich M, Ma Q, Weidtkamp-Peters S et al (2015) Real-time dynamics of peptide ligand-dependent receptor complex formation in planta. Sci Signal 8:ra76. https://doi.org/10.1126/scisignal.aab0598
Stamatakis A (2014) RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30:1312–1313. https://doi.org/10.1093/bioinformatics/btu033
Taguchi-Shiobara F, Yuan Z, Hake S, Jackson D (2001) The fasciated ear2 gene encodes a leucine-rich repeat receptor-like protein that regulates shoot meristem proliferation in maize. Genes Dev 15:2755–2766. https://doi.org/10.1101/gad.208501
Whitewoods CD, Cammarata J, Nemec Venza Z et al (2018) CLAVATA was a genetic novelty for the morphological innovation of 3D growth in land plants. Curr Biol. https://doi.org/10.1016/j.cub.2018.05.068
Wu Q, Gruel J, Lee YK, Bommert P (2016) Signaling from maize organ primordial via FASCIATED EAR3 regulates stem cell proliferation and yield traits. Nat Genet. http://www.nature.com/ng/journal/vaop/ncurrent/full/ng.3567.html. Accessed 22 Apr 2020
Zorzatto C, MacHado JPB, Lopes KVG et al (2015) NIK1-mediated translation suppression functions as a plant antiviral immunity mechanism. Nature 520:679–682. https://doi.org/10.1038/nature14171
Acknowledgements
The authors would like to acknowledge Jesus Martínez-Gómez for stimulating conversation on the topics discussed here. Joseph Cammarata would also like to thank the Provost Diversity Fellowship for Advanced Doctoral Students for funding.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliation.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cammarata, J., Scanlon, M.J. A functionally informed evolutionary framework for the study of LRR-RLKs during stem cell maintenance. J Plant Res 133, 331–342 (2020). https://doi.org/10.1007/s10265-020-01197-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10265-020-01197-w