Abstract
Field experiments in a contaminated farmland in Nihonmatsu city, Fukushima were conducted to assess the effectiveness of the plant–microbe interaction on removal of radiocesium. Before plowing, 93.3 % of radiocesium was found in the top 5 cm layer (5,718 Bq kg DW−1). After plowing, Cs radioactivity in the 0–15 cm layer ranged from 2,037 to 3,277 Bq kg DW−1. Based on sequential extraction, the percentage of available radiocesium (water soluble + exchangeable) was fewer than 10 % of the total radioactive Cs. The transfer of 137Cs was investigated in three agricultural crops; komatsuna (four cultivars), Indian mustard and buckwheat, inoculated with a Bacillus or an Azospirillum strains. Except for komatsuna Nikko and Indian mustard, inoculation with both strains resulted in an increase of biomass production by the tested plants. The highest 137Cs radioactivity concentration in above-ground parts was found in Bacillus-inoculated komatsuna Nikko (121 Bq kg DW−1), accompanied with the highest 137Cs TF (0.092). Furthermore, komatsuna Nikko-Bacillus and Indian mustard-Azospirillum associations gave the highest 137Cs removal, 131.5 and 113.8 Bq m−2, respectively. Despite the beneficial effect of inoculation, concentrations of 137Cs and its transfer to the tested plants were not very high; consequently, removal of 137Cs from soil would be very slow.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Accidents resulting from power generation, for example those in Chernobyl 1986 and Fukushima 2011 are the main origins of environmental contamination with radioactive Cs and other radionuclides. The Fukushima nuclear power plant (FNPP) accident caused large amount of radioactive materials, mainly 134Cs and 137Cs, to be deposited on areas in Japan causing widespread soil contamination, remarkably in Fukushima prefecture. Total atmospheric release due to this accident was estimated at about 18 petabecquerel (PBq) for 134Cs (Hamada and Ogino 2012) and 12 PBq for 137Cs (Chino et al. 2011). Among these two anthropogenic radioisotopes, 137Cs is of serious environmental concern, owing to its rapid incorporation into biological systems, long half-life of 30.2 years and emission of radiation during the decay process. Estimations showed that 22 % of the released 137Cs were deposited onto land in Japan and 60–70 % of this deposition was in Fukushima prefecture (Morino et al. 2011). The vertical distribution of 137Cs in soil is a key factor that is in relation with risk evaluation in both external and internal radiation exposure via plant uptake (Matsunaga et al. 2013). Radiocesium was shown to be strongly retained on the soil surface and this immobilization increases with increasing soil clay content (Smolders and Tsukada 2011). In Fukushima prefecture, sampling and testing data showed that radiocesium in the affected soils tends to be retained in the top 5 cm of surface soil with much reduced amounts in the depths below 5 cm (Nakano and Yong 2013).
A few months after the FNPP accident in 2011, the Japanese government performed an early decontamination trial for some contaminated farmlands. When the contaminated topsoil layer was removed, concentrations of radioactive Cs in soil were reduced by 75 %. Similarly, reversal tillage and interchanging topsoil with subsoil resulted in a redistribution of the radiocesium and its dilution in the 0–30 cm soil layer (Ministry of Agriculture, Forestry and Fisheries (MAFF) of Japan 2013). In the case of the off-limit or exclusion areas with relatively high contamination levels, the above trials were very effective to decrease γ-ray radiations in farmer’s fields because radioactive Cs was predominately retained in surface soil layer of the fields without ploughing. However, in the areas outside of the exclusion zone, practices such as removing highly contaminated top soil seem to be practically very difficult provided the volume of contaminated soil to be removed and the necessity of empty lands for safe disposal of such soil. Moreover, in these areas where low and middle contamination levels occur, farmers have already cultivated their fields to grow several crops, therefore radioactive Cs is being distributed in the entire plow layer. In this situation, to decrease γ-ray radiations, we also need to remove the entire plowed soil from the contaminated fields which is also practically very difficult because of the enormous amounts of contaminated soil.
Cs has been observed to exhibit many similarities to potassium (K) with respect to plant uptake; consequently, they can be taken up by plants and transferred into animals and human. The ability of plants to take up Cs is summarized in the transfer factor (TF) expressed as the ratio of Cs concentration in the plant tissues to that in the surrounding soil (Cook et al. 2007). TF is regarded as an important way to predict radionuclides activity concentrations in agricultural crops, when estimating the dose intake by human via food chain (Luksiene et al. 2013). In 2011, MAFF tried to remove radioactive Cs through cultivation of sunflower, regarded as one of best plants for radioactive Cs phytoremediation in arable lands. However, radiocesium TF obtained from the sunflower cultivation was very low about 0.0005; meaning that phytoremediation of radioactive Cs using this plant is not appropriate. Consequently, the phytoremediation trial using this plant was abandoned for most contaminated arable lands in Fukushima prefecture (MAFF 2013). The low Cs TF found for the sunflower might be explained by the fact that this plant develops a very deep root system; making for very low root uptake of radioactive Cs, especially in the undisturbed or uncultivated lands where radioactive Cs is retained in the surface layer of soil. After the Fukushima nuclear accident, many field investigations have been conducted to assess the transfer of radioactive Cs to some plants species such as rice (Endo et al. 2013; Ohmori et al. 2014). Some other studies tried to find plants that accumulate high amounts of radiocesium (Kobayashi et al. 2014; Mimura et al. 2014; Tanaka et al. 2013; Yoshihara et al. 2013), thus far, no hyperaccumulator plant has been found.
The greater part of plants species are associated with plant growth promoting rhizobacteria (PGPR) that belong to many genera such as Agrobacterium, Azospirillum, Bacillus, Enterobacter, Pseudomonas, Rhizobium (Choudhary and Johri 2009; Gray and Smith 2005; Hurek and Reinhold-Hurek 2003; Tripathi et al. 2002; Schmidt et al. 2012). Several species of these genera promote root and shoot growth, since they are able to increase the uptake of nutrients to interact with host plants. Therefore, in order to remove radioactive Cs from contaminated Fukushima soils, utilization of interactions between accumulator plants and microbes might result in an increase of radiocesium accumulation in plants. However, there are no reports characterizing the effects of the plant-bacteria interactions on radiocesium accumulation in plants under field conditions.
In our previous study (Djedidi et al. 2014); inoculation of certain plant species such as Japanese mustard spinach (komatsuna) with PGPR resulted in an increase of biomass and uptake of the stable isotope 133Cs in pot experiments. It has been shown that plants do not discriminate between stable versus radioactive isotopes of Cs and TFs of the stable and radioactive isotopes were well correlated (Tsukada et al. 2002). Therefore, to verify the results found in the previous work for stable isotope, further investigations under realistic field conditions in Fukushima prefecture and with radioactive Cs-contaminated soil are needed.
In this study, we attempt to evaluate the possibility of using plant–microbe interactions to stimulate radioactive Cs accumulation into plants using a contaminated field in Nihonmatsu city, Fukushima prefecture.
Materials and methods
Study site and determination of radioactive Cs in the soil
The test field, located in Nihonmatsu City approximately 50 km northwest the FNPP (Fig. 1), was selected with the cooperation of its owner. The Soil type in this location is brown forest soil. The GPS coordinates of this field are 37°33′26.6″N, 140°35′22.3″E. It was utilized for tobacco cultivation before the FNPP accident. In October 2011, the air dose rate was determined at 1 m above the soil surface and ranged from 1.53 to 1.8 µSv h−1 (micro Sievert). Before plowing, three soil cores (30 cm-deep) were sampled from the contaminated field and divided into three portions (0–5, 5–15 and 15–30 cm) to record the vertical distribution profile of radioactive Cs. Furthermore, after plowing, to determine radioactive Cs activity at the test field, soil samples were collected from the plow layer (0–15 cm). The total area of the experimental field was approximately 160 m2 (16 m × 10 m) and in every 15 m2, five samples were collected to result in a total of 75 soil sample. Thereafter each set of five samples were mixed to get composites samples (15 samples) that were analyzed for Cs radioactivity. All the soil samples were air dried for 28 days at a glass house and then passed through a 2-mm sieve. Prior to analysis, the soil samples were dried in an oven for 24 h at 105 °C. The radioactivity of 134Cs and 137Cs in the soil samples were determined by gamma spectrometry. Gamma ray emissions at energies of 604.7 keV (134Cs) and 661.6 keV (137Cs) were determined using a high-purity germanium coaxial detector system (Ortec, GEM 20-70) coupled with a multi-channel analyzer (Ortec, DSPEC jr 2.0). Each sample was measured for 1,800 s. Activities were expressed in Bq kg dry weight (DW)−1 and were decay corrected to the respective sampling dates.
Plant material and bacterial inoculation
The plants species used in this study were Japanese mustard spinach or komatsuna (Brassica rapa L. var. perviridis Bailey; Brassicaceae), Indian mustard (Brassica juncea (L.) Czern, Brassicaceae) and buckwheat known as soba in Japan (Fagopyrum esculentum Moench; Polygonaceae). These plants were selected for field trial because they showed high TF value when they were inoculated with two PGPR strains in a pot experiment using 133Cs (Djedidi et al. 2014). In case of komatsuna, we selected four cultivars named as follows: Nikko, Kahoku, Kyosumi and Shousai which can grow in Fukushima Prefecture.
Bacterial inoculation was performed using one Azospirillum sp. strain TS13 and one Bacillus pumilus strain TUAT1 and the bacterial cultures were prepared as described by Djedidi et al. (2014).
Seeds were germinated in plastic cell-trays (56.4 cm × 28.2 cm) containing 72 cells/tray. One seed per cell was sown to a depth of 1 cm. Sowing was performed on 19 May 2012, at this time the first inoculation was applied by pouring 1 L of the bacterial suspensions (108 CFU mL−1) in each tray. Inoculation was repeated three other times; 24, 30 May and 2 June 2012 by applying the same bacterial concentrations. Transplanting of 15-days-old seedlings was performed on 3 June 2012, and at that time inoculation was also performed by applying the bacterial suspensions directly to the plants in the field.
Experiment layout
Before the trial, the field was plowed and an experimental plot was established. The soil within this experimental field had a sandy clay loam texture, based on the USDA system of textural classification, and 3.9 % organic matter content. Other properties of the soil used are provided in Table 1. To improve soil fertility of the test field, ammonium sulfate (687.5 kg ha−1) and Calcium superphosphate (875 kg ha−1) were applied as sources of nitrogen and phosphate, thereafter, soil was mixed well using a power tiller. Potassium fertilizer was not applied.
The experiment layout consisted of a split plot design replicated in three blocks separated by a distance of approximately 0.5 m. The main plot factor was the bacterial inoculation where three treatments were developed: uninoculated control plants, Bacillus-inoculated plants and Azospirillum-inoculated plants. In the sub-plot factor, six plant cultivars, four Komatsuna, Indian mustard and buckwheat, were transplanted. Each block (one replication) was divided into 18 (three treatments for six plant cultivars) equal plots measuring 0.9 m on a side and separated by a distance of about 0.6 m. In each plot, 25 seedlings were transplanted in five rows of five seedlings separated by a distance of about 15 cm.
Sequential extraction of cesium
Soil samples of 10 g, from the 0–5 cm portions of the core samples mentioned previously, were used for this experiment. These samples were air dried and passed through a 2 mm sieve and kept in closed plastic bottles until use. Prior to extraction, radioactivity in the samples was checked. Sequential extraction procedure was carried out following the scheme shown in Table 2. To keep high efficiency of extraction, we applied a soil:solution ratio of 1:10 for all extractants. At each step, the solid and liquid phases were separated by filtering through a membrane filter (Whatman 0.45 µm). The solid phase was then washed with deionized water and the resulting liquid was added to the liquid phase. Before starting the extraction of the following fractions; F3, F4, F5 and F6, the residual soil from the corresponding previous fraction was dried at 105 °C for 2–3 h. Radioactivities of 134Cs and 137Cs in the resulting liquid phase from each step were determined by gamma spectrometry using a germanium detector, as mentioned previously for the soil samples. The activities in the extracts were expressed as a percent from the original activity in the soil.
Plant sampling and determination of 137Cs activity
Plants were harvested on 13 July 2012. At harvest time, five plants from each of the 18 plots were randomly selected and harvested. The number of harvested plants was 15 plants/treatment/cultivar. In total, 270 plant samples (15 plants × 3 treatments × 6 cultivars) were sampled. The plants were cut a few centimeters above the soil surface to prevent soil contamination. The harvested plants were air dried for more than 30 days in a drying room followed by drying in an oven at 72 °C for 24 h, prior to dry weight measurement. Thereafter, all samples were pulverized into a fine homogenous powder by a stainless steel cutter blender. Before analyzing 137Cs activity, subsamples from the dried samples were loaded into 20-ml vials, after being weighted. 137Cs activity was determined by counting gamma emissions for 1,200 s per sample. We used an automatic gamma counter (2480 Wizard2, Perkin-Elmer, Japan) equipped with a well-type NaI detector, with lead shielding, coupled to a multi-channel analyzer calibrated for the energy range 15–248 keV with a maximum dead time of 2.5 µs. The detector efficiency for 137Cs was 47 % and the energy resolution was <10 %. The detector was calibrated using blank background samples and a reference 137Cs standard (Spectrum Techniques, USA) with an activity of 10.878 kBq. Activities in plants were expressed in Bq kg DW−1 and were decay corrected to the sampling date.
The soil-to-plant TF of 137Cs was calculated as the ratio of radioactivity of the dried plants to that of dried soil; TF = 137Cs (Plant) Bq kg DW−1/137Cs (Soil) Bq kg DW−1.
Statistical analysis
Each treatment consisted of 15 replicates. Statistical analysis was performed using the KyPlot 4.0 (KyensLab Inc., Tokyo, Japan). The data were analyzed through analysis of variance (ANOVA). To uncover the statistical significance of differences (P < 0.05) between means, the Tukey test was performed.
Results and discussion
Soil contamination with radiocesium
Radioactivity concentrations of 134Cs and 137Cs before plowing are shown in Fig. 2a. In the 0–5 cm portion, 134Cs and 137Cs activities were 2,161.7 and 3,556 Bq kg DW−1, respectively. In the 5–15 cm portion, 127.7 Bq kg DW−1 for 134Cs and 224 Bq kg DW−1 for 137Cs were found. In the remaining portion, very low radioactivities of 134Cs and 137Cs were detected; 36.8 and 23.0 Bq kg DW−1, respectively. The highest relative proportion of total Cs (134Cs + 137Cs) was found in the top 5 cm with 93.3 % of the entire core activity. The remaining percentage was distributed as follows: 5.75 % in the 5–15 cm soil layer and a negligible proportion below 15 cm. This result shows that radiocesium remains predominantly within the top layer (5 cm) of the soil. Furthermore, it indicates that the post-contamination migration of Cs seems to be slow or limited due to the adsorption of the fallout radionuclide by the surface soil. This finding is in agreement with many other studies where radiocesium was shown to be mostly retained on the soil surface in contaminated areas in Fukushima (Endo et al. 2012, 2013; Ohno et al. 2012; Sakai et al. 2014).
Radioactivity of 134Cs and 137Cs within the plow layer are shown in Fig. 2b. In this case, activities of both isotopes in the 0–15 cm layer varied from 1,022 to 1,563 Bq kg DW−1 for 134Cs and from 1,014 to 1,713 Bq kg DW−1 for 137Cs. Total radiocesium activity varied from around 2,000 Bq kg DW−1 to 3,300 Bq kg−1 DW. This result shows that plowing, as an agricultural practice, resulted in a decrease or dilution of the radiocesium activity in the surface soil. Generally plowing is meant to decrease the transferable pool of radiocesium by mixing highly contaminated top soil with deeper layers that have low contamination (Camps et al. 2004). In a study conducted on contaminated paddy fields at Minami-Soma city in Fukushima prefecture, Endo et al. (2012) found that radiocesium was uniformly redistributed in the soil of a cultivated field. The same depth distribution profile of 137Cs and its redistribution due to agricultural practices including plowing was also reported for some Mediterranean soils (Gaspar and Navas 2013) and for undisturbed and cultivated soils affected by the Chernobyl accident, even several years after the deposition (Askbrant et al. 1996).
Radiocesium deposition (kBq) per unit area in the plow layer (0–15 cm) was estimated for the experimental field using the following formula:
where, mass of soil (kg) per unit area = area (1 m2) × bulk density (kg m−3) × plow layer depth (m). Deposition of 137Cs in the plow area estimated for different locations in the field, using the activity of the 15 soil samples mentioned previously, varied from 197.8 to 302.6 kBq for 134Cs and from 196.3 to 331.6 KBq for 137Cs. Average Cs deposition values were about 245.1 ± 31.2 and 265.1 ± 38.0 kBq, for 134Cs and 137Cs, respectively.
The results found here suggest that countermeasures aiming to decrease the radiocesium activity concentration in the soil surface and therefore decrease the risk of exposure and the soil-to-plant transfer of Cs can rely on practices such as plowing.
Chemical availability of Cs
When evaluating the amount of radiocesium that can be transferred from soil to plant parts, it is very important to know the proportion of radioactive Cs that exist in water soluble and exchangeable fractions in the soil as plants are mainly absorbing this isotope in those fractions.
Results of the extraction of 137Cs using different reagents are shown in Fig. 3. The percentage of plant available 137Cs (F1 + F2) was fewer than 10 % of the total radioactive Cs amount. In the case of the water soluble (Fraction 1), the amount of 137Cs was relatively higher (7.8 % for 137Cs) than those of previous reports regarding this fraction. For instance, Matsunaga et al. (2013) found that water-extracted Cs was not detected in soil contaminated with FNPP fallout. Livens and Baxter (1988) suggested that Chernobyl-derived 137Cs was relatively mobile, soon after the contamination. This observation could justify the existence of radiocesium in the water soluble fraction. Regarding the amount of 137Cs in this Fraction 1 of the tested soil, we need further investigation to understand the reasons of its existence. Despite the fact that the ammonium ion is very effective in desorbing Cs (Spezzano 2005), the amount of extractable 137Cs by ammonium acetate was not detected in Fraction 2 (Fig. 3). Similar low extractability for this fraction was found in the study performed by Spezzano (2005). These results may be due to the low CEC of the soil used here as shown in Table 1, since a strong positive correlation between ammonium acetate-extracted Cs and CEC has been described (Koarashi et al. 2012; Matsunaga et al. 2013). Additionally, the predominant clay mineral of the tested soil is illite; therefore, the absence of 137Cs in Fraction 2 extracted by ammonium acetate may be caused by its tight fixation to illite clay. Fraction 3 bound to Fe–Mn oxides was extracted by hydroxylamine with 25 % of acetic acid, and Fraction 4 bounded to Al/Fe-(hydr) oxides (Nakamaru and Uchida 2008) was extracted by ammonium oxalate. Radioactive Cs in these fractions has the possibility to be absorbed by plants because of the potential desorption action of organic acids secreted by plant roots. Consequently, the sum of the plant available 137Cs would be around 22.1 %, as the sum of detected radiocesium in Fractions 1–5. The remaining part can be qualified as an unavailable fraction as it was evidently split between the strongly fixed and the residual fractions. This finding is in accordance with those found previously in many reports (Forsberg and Strandmark 2001; Matsunaga et al. 2013; Rigol et al. 1999; Tsukada et al. 2008), where most of the radiocesium was found to be bound to persistent and residual fractions.
Except for the water soluble fraction, the overall results of sequential extraction can be considered as typical for the extractability of radiocesium as shown in almost all previous investigations.
Effect of bacterial inoculation on plant growth
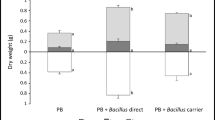
The effects of bacterial inoculation on biomass production varied according to the plant cultivar as shown in Fig. 4. Except for komatsuna Nikko and Indian mustard, inoculation with Bacillus and Azospirillum strains resulted in an increase of biomass production by the tested plants. The highest shoot dry weight was recorded for Azospirillum-inoculated Indian mustard which was about 1,780 g m−2, although it was not significantly different compared to that of the control uninoculated treatment. For the same plant, inoculation with Bacillus strains significantly decreased the shoot dry weight. Additionally, for komatsuna Kyosumi, komatsuna Shousai and buckwheat, Azospirillum TS13 strain showed clear inoculation effects on plant growth (Fig. 4). In the case of Bacillus TUAT1 strain, inoculation of the three komatsuna cultivars Kahoku, Kyosumi and Shousai induced significantly higher shoot dry weights compared to the control treatment. Regarding inoculation effects, inoculation with the Bacillus strain gave 38, 32 and 24 % as percent of dry weigh increase for komatsuna Kahoku, komatsuna Kyosumi and komatsuna Shousai, respectively. Similarly the percent of dry weight increase triggered by Azospirillum inoculation were 42, 51 and 40 % respectively for komatsuna Kahoku, komatsuna Kyosumi and komatsuna Shousai. The percent of shoot dry weight change for these three cultivars were relatively high and suggest a beneficial effect of bacterial inoculation on biomass production by these cultivars.
Biomass production of the tested plants as influenced by the bacterial treatment. Errors bars indicate standard deviation for n = 15. For each plant cultivar, different small letters indicate significant difference in shoot dry weight between the uninoculated, Bacillus and Azospirillum-inoculated plants based on the Tukey test. Two-way ANOVA was performed to determine the influence of bacterial inoculation (Inoculation) and the plant cultivar (plant) as well as their interaction (Interaction). The results of Two-way ANOVA are shown as: NS non-significant at the level P < 0.05; *significant at the level P < 0.05; **significant at the level P < 0.01; ***significant at the level P < 0.001
By comparison to this positive effect of inoculation, negative effects were recorded for some cultivars when inoculated by the Bacillus strain. For komatsuna Nikko, inoculation with this strain slightly decreased the shoot dry weight (decrease by 1.5 %) and for Indian mustard, the decrease was more pronounced; about 23 %. Regardless this negative effect, the results found here suggest that bacterial inoculation can positively influence plant growth and development, as suggested by Glick (1995). The effects of various treatments and soil amendments on biomass production of plant species grown on radiocesium-contaminated soils were previously investigated. Fuhrmann et al. (2003) found that ammonium and manure amendments did not significantly enhance the biomass production of cabbage, Indian mustard, pigweed and amaranth plants grown on Cs-contaminated soil in field experiments. On the other hand, in a greenhouse experiment, inoculation with arbuscular mycorrhizal fungi had a positive effect on plant biomass and was accompanied by an increase of 137Cs uptake (Vinichuk et al. 2013). Microbial inoculant and arbuscular mycorrhizal fungi are being widely used to improve plant growth under controlled and natural field conditions (Nadeem et al. 2013). Regarding Cs-contaminated soils, only a few studies were performed to assess the effect of bacterial inoculation. For instance, Tang et al. (2011) used Burkholderia strains as inoculant for Phytolacca americana and Amaranthus cruentus and found a significant increase of biomass production of plants grown on stable Cs-spiked soil. The same authors suggested that the inoculation with PGPR could be used as an effective way to promote plant growth and improve radionuclide uptake by plants.
Effect of bacterial inoculation on radiocesium uptake
In our previous work (Djedidi et al. 2014), stable 133Cs TF was increased in komatsuna, grown in an Andosol, when inoculation with an Azospirillum and Bacillus strains was applied. This result suggests a possibility to stimulate the soil-to-plant transfer of radiocesium through bacterial inoculation, for remediation purposes. After the FNPP accident in 2011, field investigations have begun to predict the soil-to-plant transfer of 137Cs, though, to our knowledge, there are no reports evaluating the effects of bacterial inoculation on radioactive Cs accumulation into plants. Under these circumstances, we were attempting to evaluate the potential use of plant–microbe interactions to stimulate radiocesium accumulation into plants in a contaminated field to verify the results of the previous work using the stable isotope 133Cs.
The radiocesium concentrations in the above-ground parts of the plants are shown in Fig. 5. Variability in terms of 137Cs activity concentration was recorded for the tested plants. Factorial analysis (2 way Anova) revealed a significant (P < 0.001) influence of the bacterial inoculation, plant cultivar, or both on 137Cs concentration in the above-ground parts of the plants. Regardless of the bacterial treatment, 137Cs activity concentration ranged from 19 to 121 Bq kg DW−1. The lowest concentrations were found for buckwheat, with a maximum of 28.2 Bq kg DW−1 in the Azospirillum inoculation treatment. The highest concentrations were recorded for komatsuna Nikko (Fig. 5). For this cultivar, Bacillus inoculation gave the maximum concentration of 121 Bq kg DW−1, while Azospirillum inoculation slightly decreased the concentration compared to the control treatment, although not significantly.
Radiocesium activity concentrations in the above-ground parts of tested plants. Errors bars indicate standard deviation for n = 15. Different small letters indicate significant difference between treatments based on the Tukey test. The results of Two-way ANOVA are shown as: NS non-significant at the level P < 0.05; *significant at the level P < 0.05; **significant at the level P < 0.01; ***significant at the level P < 0.001
For the three remaining komatsuna cultivars, Kahoku, Kyosumi and Shousai, 137Cs activity concentrations were lower than 60 Bq kg DW−1. Like biomass production, the responses of the plant cultivars, in terms of 137Cs activity concentrations, to the two bacterial strains were different. For instance, for Indian mustard and buckwheat, Azospirillum-inoculated plants gave the highest radioactivity concentrations that were significantly different than those of the remaining treatments. While for komatsuna Kyosumi, the two bacterial strains had similar effects on 137Cs concentrations, which were significantly higher than that of the control treatment. Moreover, for komatsuna Kahoku and Shousai, inoculation with the Azospirillum strain resulted in the lowest 137Cs concentrations, while plants inoculated with Bacillus strain showed similar concentrations to the uninoculated plants (Fig. 5). This variability in terms of Cs concentrations in the above-ground parts of plants without bacterial inoculation was found in previous reports that demonstrated that plants differ in their abilities to take up Cs in their natural habitats (Varskog et al. 1994; Willey and Martin 1995; Cook et al. 2007), in Cs-spiked soils or solutions (Cook et al. 2009; Tang et al. 2011) and in contaminated fields (Dushenkov et al. 1999; Fuhrmann et al. 2003).
Regarding the effects of bacterial inoculation on growth and radioactive Cs uptake in the tested plants, two main groups can be distinguished based on Figs. 4 and 5. The first one corresponds to Bacillus-inoculated komatsuna Nikko and Indian mustard with Azospirillum inoculation. For these two associations, inoculation induced an increase of Cs activity concentration without an increase of shoot dry weight. The second group contains komatsuna Kahoku with Bacillus inoculation, komatsuna Kyosumi with both strains, and Buckwheat with Azospirillum inoculation. In this group, inoculation increased concentrations of radioactive Cs in the above-ground part that was accompanied with an increase of shoot dry weight. Concerning differences between these two groups, we need further work to confirm factors elucidating this observation. However, our findings regarding the increase of 137Cs activity concentration by bacterial inoculation may be the first report, for field investigations.
Regarding the effects of soil microorganisms and their abilities to mobilize radioactive Cs in plant rhizosphere, numerous studies focused on the effect of arbuscular mycorrhizal fungi on Cs uptake by plants (De Boulois et al. 2006; Entry et al. 1999; Rogers and Williams 1986; Vinichuk et al. 2013). Conversely, the effects of soil bacteria on Cs bioavailability for plant uptake have been investigated less, although some studies focused on the characterization of Cs accumulating bacteria (Kuwahara et al. 2005, 2011; Tomioka et al. 1992) and the effect of bacterial exudates on Cs desorption (Wensling et al. 2005). For the two bacterial strains Azospirillum TS13 and Bacillus TUAT1 used here, further work about their abilities to mobilize radiocesium is required.
Soil-to-plant TF is provided in Table 3. Both bacterial inoculation and plant cultivar significantly influenced the 137Cs TF as demonstrated by Two-way Anova factorial comparisons (Table 3). TF for uninoculated buckwheat was the lowest, 0.014 among the tested plants. For the control treatments, the highest TF value was 0.055 in komatsuna Nikko. In the case of plants without inoculation, TF found in this study approximately ranged from 0.014 to 0.055. For inoculated plants, TF varied from 0.015 to 0.092 as shown in Table 3. This means, depending on the extent of compatibility between the plant cultivar and the bacterial strain, inoculation increased radioactive Cs uptake by plants. The same observation was previously described for the inoculation with arbuscular mycorrhizal fungi and it was suggested that the result of inoculation may be related to the interaction between the plant, the inoculant and the environment (Vinichuk et al. 2013). For most species, typical values for 137Cs transfer range between 0.02 and 0.1 as described in Fuhrmann et al. (2003). On the other hand, it has been shown that for agricultural plants TF values are normally lower in loam and clay soil, while TFs in organic or sandy soils can exceed the value at 1 or even greater (Zhu and Smolders 2000). In our previous pot experiment using stable isotope 133Cs in an Andosol, inoculation of komatsuna with the Azospirillum TS13 triggered a very high TF value that was around 3.5. In this context, it has been suggested that TFs of Cs observed under laboratory conditions were usually higher that those under agricultural conditions (Ishikawa et al. 2008). Furthermore, the soil-to-plant transfer of Cs is controlled by many factors including its availability for plant uptake. As mentioned previously, CEC is likely an important parameter influencing the plant available Cs. In our case, the differences recorded in terms of Cs TF in the pot experiment with Andosol and the field experiment with brown forest soil could be related to the difference in CEC for the two kinds of soil. The CEC for Andosol in the pot experiment (Djedidi et al. 2014) was much greater than that of the brown forest soil used in this study. Besides, the availability of Cs in soil is governed by micaceous clay minerals such as illite. These clay particles contain sorption sites with high sorption selectivity for alkali elements including Cs and K. These sites are called the frayed edge sites (FES) (Vandenhove and Sweeck 2011).
Therefore one possible reason that explains the low Cs TF found in the brown forest soil used here is the nature of the clay mineral which is illite. To characterize the sorption of Cs by soils, Radiocesium interception potential (RIP) (Cremers et al. 1988) has been used and has proven to be useful in predicting radiocesium mobility in the soil. Generally, soils with high RIP values have lower Cs mobility and hence a lower soil-to-plant transfer can be expected (Vandenhove and Sweeck 2011). Moreover, it has been suggested that Andosols, characterized by low illite content, show generally low Cs sorption or low RIP values (Vandebroek et al. 2009) associated with high Cs availability that could result in high soil-to plant transfer. Brown forest soils, widely found in Japan, are characterized by high RIP values (Vandebroek et al. 2009) and therefore low soil-to-plant transfer of Cs because of its low mobility in the soils. These differences in terms of Cs retention (RIP) between the brown forest soil used here and the Andosol used in our previous study would explain the difference found for the Cs TF values for the two types of soil.
For the tested plans, the removal of 137Cs from soil with the harvested biomass was estimated according to the formula described by Vandenhove (2013):
Similar to the transfer factor, the crop removal varied with the plant cultivars and inoculation strains (Table 3). The maximum removed quantity was obtained for the combination komatsuna Nikko-Bacillus strain, which was about 131.5 Bq m−2. Inoculation of Indian mustard with Azospirillum strain also resulted in a significant increase of 137Cs removal.
To put the results of this study into context, despite the beneficial effect of inoculation on biomass production for some cultivars, concentrations of 137Cs in the edible parts of the tested plants were not very high. The highest TF value was only 0.092 obtained for Nikko with Bacillus inoculation. Additionally, based on the values of the crop removal (Table 3), the percentage of removed 137Cs from the soil would be extremely low to consider restoration of such agricultural soil during one cropping season. McGrath and Zhao (2003) suggested that phytoremediation depends on two key factors, biomass production and transfer factor, and plants with a transfer factor greater than one could be successful for phytoremediation.
Conclusion
Using a brown forest soil in Nihonmatsu city, Fukushima prefecture, the soil-to-plant transfer of 137Cs was investigated in three agricultural crops; Japanese mustard spinach, Indian mustard and buckwheat in association with two bacterial inoculants identified as Bacillus and Azospirillum species.
Both positive and negative effects of inoculation in terms of growth and 137Cs uptake were recorded.
For some tested plants, inoculation increased 137Cs TF, with a highest value of 0.092 obtained for komatsuna Nikko-Bacillus association. In a similar way, the removed amount of radiocesium within the harvested biomass was increased especially in komatsuna Nikko-Bacillus and Indian mustard-Azospirillum associations. However, despite a positive effect of inoculation, the TF and crop removal values were not very high and thus, the removal of 137Cs from soil would be very low.
Accordingly, to develop an effective method to remove radioactive Cs from contaminated farm lands based on plant–microbe interaction in Fukushima prefecture, we need further investigation to find higher Cs accumulator plants. Similarly, exploration of a good combination between such plants and bacterial strains having higher abilities to accelerate radioactive Cs uptake, would be of great importance.
References
Askbrant S, Melin J, Sandalls J, Rauret G, Vallejo R, Hinton T, Cremers A, Vandecastelle C, Lewyckyj N, Ivanov YA, Firsakova SK, Arkhipov NP, Alexakhin RM (1996) Mobility of radionuclides in undisturbed and cultivated soils in Ukraine, Belarus and Russia six years after the Chernobyl fallout. J Environ Radioact 31:287–312. doi:10.1016/0265-931X(95)00054-E
Bunzl K, Schimmack W, Belli M, Riccardi M (1997) Sequential extraction of fallout radiocesium from the soil: small scale and large scale spatial variability. J Radioanal Nucl Chem 226:47–53
Camps M, Rigol A, Hillier S, Vidal M, Rauret G (2004) Quantitative assessment of the effects of agricultural practices designed to reduce 137Cs and 90Sr soil-plant transfer in meadows. Sci Total Environ 332:23–38. doi:10.1016/j.scitotenv.2004.04.008
Chino M, Nakayama H, Nagai H, Terada H, Katata G, Yamazawa H (2011) Preliminary estimation of release amounts of 131I and 137Cs accidentally discharged from the Fukushima Dai-ichi Nuclear Power Plant into the atmosphere. J Nucl Sci Technol 48:1129–1134
Choudhary DK, Johri BN (2009) Interactions of Bacillus spp. and plants—with special reference to induced systemic resistance (ISR). Microbiol Res 164:493–513. doi:10.1016/j.micres.2008.08.007
Cook LL, Inouye RS, McGonigle TP, White GJ (2007) The distribution of stable cesium in soils and plants of the eastern Snake River Plain in southern Idaho. J Arid Environ 69:40–64. doi:10.1016/j.jaridenv.2006.08.014
Cook LL, Inouye RS, McGonigle TP (2009) Evaluation of four grasses for use in phytoremediation of Cs-contaminated arid land soil. Plant Soil 324:169–184. doi:10.1007/s11104-009-9942-z
Cremers A, Elsen A, De Preter P, Maes A (1988) Quantitative analysis of radiocaesium retention in soils. Nature 335:247–249
De Boulois H, Voets L, Delvaux B, Jakobsen I, Declerck S (2006) Transport of radiocaesium by arbuscular mycorrhizal fungi to Medicago truncatula under in vitro conditions. Environ Microbiol 8:1926–1934. doi:10.1111/j.1462-2920.2006.01070.x
Djedidi S, Kojima K, Yamaya H, Ohkama-Ohtsu N, Bellingrath-Kimura SD, Watanabe I, Yokoyama T (2014) Stable cesium uptake and accumulation capacities of five plant species as influenced by bacterial inoculation and cesium distribution in the soil. J Plant Res 127:585–597. doi:10.1007/s10265-014-0647-x
Dushenkov S, Mikheev A, Prokhnevsky A, Ruchko M, Sorochinsky B (1999) Phytoremediation of radiocesium-contaminated soil in the vicinity of Chernobyl, Ukraine. Environ Sci Technol 33:469–475
Endo S, Kimura S, Takatsuji T, Nanasawa K, Imanaka T, Shizuma K (2012) Measurement of soil contamination by radionuclides due to Fukushima Dai-ichi Nuclear Power Plant accident and associated cumulative external dose estimation. J Environ Radioact 111:18–27. doi:10.1016/j.jenvrad.2011.11.006
Endo S, Kajimoto T, Shizuma K (2013) Paddy-field contamination with 134Cs and 137Cs due to Fukushima Dai-ichi Nuclear Power Plant accident and soil-to-rice transfer coefficients. J Environ Radioact 116:59–64. doi:10.1016/j.jenvrad.2012.08.018
Entry JA, Watrud LS, Reeves M (1999) Accumulation of 137Cs and 90Sr from contaminated soil by three grass species inoculated with mycorrhizal fungi. Environ Pollut 104:449–457. doi:10.1016/S0269-7491(98)00163-8
Forsberg S, Strandmark M (2001) Migration and chemical availability of 137Cs and 90Sr in Swedish long-term experimental pastures. Water Air Soil Poll 127:157–171
Fuhrmann M, Lasat M, Ebbs S, Cornish J, Kochian L (2003) Uptake and release of cesium-137 by five plant species as influenced by soil amendments in field experiments. J Environ Qual 32:2272–2279
Gaspar L, Navas A (2013) Vertical and lateral distributions of 137Cs in cultivated and uncultivated soils on Mediterranean hillslopes. Geoderma 207–208:131–143. doi:10.1016/j.geoderma.2013.04.034
Glick BR (1995) The enhancement of plant growth by free-living bacteria. Can J Microbiol 41:109–117
Gray EJ, Smith DL (2005) Intracellular and extracellular PGPR: commonalities and distinctions in the plant–bacterium signaling processes. Soil Biol Biochem 37:395–412. doi:10.1016/j.soilbio.2004.08.030
Hamada N, Ogino H (2012) Food safety regulations: what we learned from the Fukushima nuclear accident. J Environ Radioact 111:83–99. doi:10.1016/j.jenvrad.2011.08.008
Hurek T, Reinhold-Hurek B (2003) Azoarcus sp. strain BH72 as a model for nitrogen-fixing grass endophytes. J Biotechnol 106:169–178. doi:10.1016/j.jbiotec.2003.07.010
Ishikawa NA, Tagami k, Uchida S (2008) Estimation of 137Cs plant root uptake using naturally existing 133Cs. J Nucl Sci Technol Supplement 6:146–151
Koarashi J, Atarashi-Andoh M, Matsunaga T, Sato T, Nagao S, Nagai H (2012) Factors affecting vertical distribution of Fukushima accident-derived radiocesium in soil under different land-use conditions. Sci Total Environ 431:392–401. doi:10.1016/j.scitotenv.2012.05.041
Kobayashi D, Okouchi T, Yamagami M, Shinano T (2014) Verification of radiocesium decontamination from farmlands by plants in Fukushima. J Plant Res 127:51–56. doi:10.1007/s10265-013-0607-x
Kuwahara C, Fukumoto A, Ohsone A, Furuya N, Shibata H, Sugiyama H, Kato F (2005) Accumulation of radiocesium in wild mushrooms collected from a Japanese forest and cesium uptake by microorganisms isolated from the mushroom-growing soils. Sci Total Environ 345:165–173. doi:10.1016/j.scitotenv.2004.10.022
Kuwahara C, Fukumoto A, Nishina M, Sugiyama H, Anzai Y, Kato F (2011) Characteristics of cesium accumulation in the filamentous soil bacterium Streptomyces sp. K202. J Environ Radioact 102:138–144. doi:10.1016/j.jenvrad.2010.11.004
Livens FR, Baxter MS (1988) Particle size and radionuclide levels in some west Cumbrian soils. Sci Total Environ 70:l–17. doi:10.1016/0048-9697(88)90248-3
Luksiene B, Marciulioniene D, Gudeliene I, Schönhofer F (2013) Accumulation and transfer of 137Cs and 90Sr in the plants of the forest ecosystem near the Ignalina nuclear power plant. J Environ Radioact 116:1–9. doi:10.1016/j.jenvrad.2012.09.005
Matsunaga T, Koarashi J, Atarashi-Andoh M, Nagao S, Sato T, Nagai H (2013) Comparison of the vertical distributions of Fukushima nuclear accident radiocesium in soil before and after the first rainy season, with physicochemical and mineralogical interpretations. Sci Total Environ 447:301–314. doi:10.1016/j.scitotenv.2012.12.087
McGrath SP, Zhao FJ (2003) Phytoextraction of metals and metalloids from contaminated soils. Curr Opin Biotechnol 14:277–282. doi:10.1016/S0958-1669(03)00060-0
Mimura T, Mimura M, Kobayashi D, Komiyama C, Sekimoto H, Miyamoto M, Kitamura A (2014) Radioactive pollution and accumulation of radionuclides in wild plants in Fukushima. J Plant Res 127:5–10. doi:10.1007/s10265-013-0599-6
Ministry of Agriculture, Forestry and Fisheries (MAFF) of Japan (2013). http://www.maff.go.jp/mobile/kinkyu/tohoku_saigai/08/2011/1109/110914/110914_gijutu_betu01.html and http://www.s.affrc.go.jp/docs/press/pdf/110914-09.pdf. In Japanese
Morino Y, Ohara T, Nishizawa M (2011) Atmospheric behavior, deposition, and budget of radioactive materials from the Fukushima Dai-ichi Nuclear Power Plant in March 2011. Geophys Res Lett 38:L00G11. doi:10.1029/2011GL048689
Nadeem SM, Ahmad M, Zahir ZA, Javaid A, Ashraf M (2013) The role of mycorrhizae and plant growth promoting rhizobacteria (PGPR) in improving crop productivity under stressful environments. Biotech Adv 32:429–448. doi:10.1016/j.biotechadv.2013.12.005
Nakamaru Y, Uchida S (2008) Distribution coefficients of tin in Japanese agricultural soils and the factors affecting tin sorption behavior. J Environ Radioact 99:1003–1010. doi:10.1016/j.jenvrad.2007.11.012
Nakano M, Yong RN (2013) Overview of rehabilitation schemes for farmlands contaminated with radioactive cesium released from Fukushima power plant. Eng Geol 155:87–93. doi:10.1016/j.enggeo.2012.12.010
Ohmori Y, Inui Y, Kajikawa M et al (2014) Difference in cesium accumulation among rice cultivars grown in the paddy field in Fukushima Prefecture in 2011 and 2012. J Plant Res 127:57–66. doi:10.1007/s10265-013-0616-9
Ohno T, Muramatsu Y, Miura Y, Oda K, Inagawa N et al (2012) Depth profiles of radioactive cesium and iodine released from the Fukushima Dai-ichi Nuclear Power Plant in different agricultural fields and forests. Geochem J 46:287–295. doi:10.2343/geochemj.2.0204
Puhakainen M, Riekkinen I, Heikkinen T, Jaakkola T, Steinnes E, Rissanen K, Suomela M, Thørring H (2001) Effect of chemical pollution on forms of 137Cs, 90Sr and 239,240Pu in Arctic soil studied by sequential extraction. J Environ Radioact 52:17–29
Rigol A, Roig M, Vidal M, Rauret G (1999) Sequential extractions for the study of radiocesium and radiostrontium dynamics in mineral and organic soils from western Europe and Chernobyl area. Environ Sci Tech 33:887–895
Rogers RD, Williams SE (1986) Vesicular arbuscular mycorrhizal—influence on plant uptake of cesium and cobalt. Soil Biol Biochem 18:371–376
Sakai M, Gomi T, Nunokawa M, Wakahara T, Onda Y (2014) Soil removal as a decontamination practice and radiocesium accumulation in tadpoles in rice paddies at Fukushima. Environ Pollut 187:112–115. doi:10.1016/j.envpol.2014.01.002
Schmidt CS, Alavi M, Cardinale M, Müller H, Berg G (2012) Stenotrophomonas rhizophila DSM14405T promotes plant growth probably by altering fungal communities in the rhizosphere. Biol Fertil Soils 48:947–960. doi:10.1007/s00374-012-0688-z
Smolders E, Tsukada H (2011) The transfer of radiocesium from soil to plants: mechanisms, data, and perspectives for potential countermeasures in Japan. Integrat Environ Assess Manag 7:379–381
Spezzano P (2005) Distribution of pre- and post-Chernobyl radiocaesium with particle size fractions of soils. J Environ Radioact 83:117–127. doi:10.1016/j.jenvrad.2005.02.002
Tanaka K, Iwatani H, Sakaguchi A, Takahashi Y, Onda Y (2013) Local distribution of radioactivity in tree leaves contaminated by fallout of the radionuclides emitted from the Fukushima Daiichi Nuclear Power Plant. J Radioanal Nucl Chem 295:2007–2014
Tang S, Liao S, Guo J, Song Z, Wang R, Zhou X (2011) Growth and cesium uptake responses of Phytolacca americana Linn. and Amaranthus cruentus L. grown on cesium contaminated soil to elevated CO2 or inoculation with a plant growth promoting rhizobacterium Burkholderia sp. D54, or in combination. J Hazard Mater 198:188–197. doi:10.1016/j.jhazmat.2011.10.029
Tomioka N, Uchiyama H, Yagi O (1992) Isolation and characterization of cesium accumulating bacteria. Appl Environ Microbiol 58:1019–1023
Tripathi AK, Nagarajan T, Verma SC, Le Rudulier D (2002) Inhibition of biosynthesis and activity of nitrogenase in Azospirillum brasilense Sp7 under salinity stress. Curr Microbiol 44:363–367. doi:10.1007/s00284-001-0022
Tsukada H, Hasegawa H, Hisamatsu S, Yamasaki S (2002) Transfer of 137Cs and stable Cs from paddy soil to polished rice in Aomori Japan. J Environ Radioact 59(3):351–363. doi:10.1016/S0265-931X(01)00083-2
Tsukada H, Takeda A, Hisamatsu S, Inaba J (2008) Concentration and specific activity of fallout 137Cs in extracted and particle-size fractions of cultivated soils. J Environ Radioact 99:875–881. doi:10.1016/j.jenvrad.2007.11.014
Vandebroek L, Van Hees M, Delvaux B, Spaargaren O, Thiry Y (2009) Acid extraction as a predictive tool of radiocaesium interception potential (RIP) in a worldwide scale. Radioprotection 44:635–638
Vandenhove H (2013) Phytoremediation options for radioactively contaminated sites evaluated. Ann Nucl Energy 62:596–606. doi:10.1016/j.anucene.2013.02.005
Vandenhove H, Sweeck L (2011) Soil vulnerability for cesium transfer. Integr Environ Assess Manag 7:374–378
Varskog P, Naeumann R, Steinnes E (1994) Mobility and plant availability of radioactive Cs in natural soil in relation to stable Cs, other alkali elements and soil fertility. J Environ Radioact 22:43–53. doi:10.1016/0265-931X(94)90034-5
Vinichuk M, Mårtensson A, Ericsson T, Rosén K (2013) Effect of arbuscular mycorrhizal (AM) fungi on 137Cs uptake by plants grown on different soils. J Environ Radioact 115:151–156. doi:10.1016/j.jenvrad.2012.08.004
Wensling LA, Harsh JB, Ward TE, Palmer CD, Hamilton MA, Boyle JS, Flury M (2005) Cesium desorption from illite as affected by exudates from rhizosphere bacteria. Environ Sci Technol 39:4505–4512
Willey NJ, Martin MH (1995) Annual patterns of Cs-133 concentration in British upland vegetation. Chemosphere 30:717–724. doi:10.1016/0045-6535(94)00437-Y
Yoshihara T, Matsumura H, Hashida S, Nagaoka T (2013) Radiocesium contaminations of 20 wood species and the corresponding gamma-ray dose rates around the canopies at 5 months after the Fukushima nuclear power plant accident. J Environ Radioact 115:60–68. doi:10.1016/j.jenvrad.2012.07.002
Zhu YG, Smolders E (2000) Plant uptake of radiocesium: a review of mechanisms, regulation and application. J Exp Bot 51:1635–1645
Acknowledgments
This study was supported by the Special Research Fund of the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan titled “Research and development of security and safe crop production to reconstruct agricultural lands in Fukushima Prefecture based on novel techniques to remove radioactive compounds using advanced bio-fertilizer and plant protection strategies”. This work was also supported by a Grant-in-Aid for Scientific Research (B):24380176 from the Japan Society for the Promotion of Science (JSPS).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Djedidi, S., Terasaki, A., Aung, H.P. et al. Evaluation of the possibility to use the plant–microbe interaction to stimulate radioactive 137Cs accumulation by plants in a contaminated farm field in Fukushima, Japan. J Plant Res 128, 147–159 (2015). https://doi.org/10.1007/s10265-014-0678-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10265-014-0678-3