Abstract
In three temperature-sensitive mutants of Arabidopsis, root redifferentiation 1 (rrd1), rrd2, and root initiation defective 4 (rid4), formation of fasciated lateral roots was previously observed under high temperature conditions of 28°C. When lateral roots were induced from explants of very young seedlings of these mutants by culture with exogenously supplied auxin at 28°C, expansion of lateral root primordia leading to lateral root fasciation occurred reproducibly and semi-synchronously with a high frequency. This experimental system allowed us to examine how radial organization of root tissues is altered in association with expansion of primordia. Analysis with various tissue-specific reporter genes indicated that in the fasciated lateral roots, cell files of the stele are increased markedly while the numbers of cortical and epidermal cell layers are not changed. This suggests that radial organization during root primordium development involves a mechanism that makes outer layer patterning more robust than inner layer patterning against unusual enlargement of the morphogenetic field.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Root tissues of vascular plants are arranged concentrically from the periphery to the center, in the order of epidermis, ground tissue consisting of parenchymatous cortex and endodermis, and stele. The stele comprises vascular tissues and pericycle surrounding them. Mechanisms underlying generation of this radial pattern have been studied extensively in a model plant, Arabidopsis. One of striking outcomes from these studies was identification of two GRAS family transcription factors, SCARECROW (SCR) and SHORT ROOT (SHR), as key regulators of differentiation of the endodermis (Di Laurenzio et al. 1996; Helariutta et al. 2000). Detailed analyses have revealed how SCR and SHR cooperate to spatially control endodermis differentiation (Nakajima et al. 2001; Cui et al. 2007). SHR is produced in the stele and moves outward into the adjacent ground tissue, where SHR forms a complex with SCR and SCR transcription is up-regulated by the SCR/SHR complex-dependent positive feedback loop. Formation of the SCR/SHR complex sequesters SHR in the nucleus and prevents SHR movement beyond the innermost layer of the ground tissue. These interactions between SCR and SHR provide a molecular basis for endodermis differentiation confined to a single layer of cells surrounding the stele (Cui et al. 2007). Recently it has been shown that microRNAs, miR165 and miR166, which are produced in the endodermis by the SCR/SHR function, move inward into the stele cells and build a gradient acting as positional cues for tissue differentiation inside the stele (Carlsbecker et al. 2010; Miyashima et al. 2011). Additionally these microRNAs have been also implicated in the control of radial pattering of the ground tissue (Miyashima et al. 2009, 2011).
As mentioned above, many aspects of radial pattern formation in the root are now being understood in relation to SCR and SHR. However, there remain several issues of radial patterning that is not accounted for by the SCR/SHR-dependent mechanism. For example, it is still unknown how the framework radial pattern arises with the birth of roots and determines a border between primary interior and exterior domains, i.e., SHR- and SCR-expressing domains. Generally the nature of a basic system generating morphological patterns can be reflected on the responses of patterning to changes in the size of morphogenetic field (Meinhardt 1982). Therefore, if the initial size of root primordia can be increased or decreased, radial tissue organization of the roots developed from such root primordia would be informative about the framework system of radial patterning of the root.
We isolated many temperature-sensitive mutants of Arabidopsis by screening with root formation in tissue culture as an index phenotype (Konishi and Sugiyama 2003; Sugiyama 2003). Of these mutants, root redifferentiation defective 1 (rrd1), rrd2, and root initiation defective 4 (rid4) were characterized by formation of fasciated lateral roots under high temperature conditions, which is called temperature-dependent fasciation (TDF) hereafter. The peculiar feature of the TDF mutants has offered a unique opportunity to examine radial organization of root tissues influenced by the expansion of root primordia. The results obtained show that outer layer patterning is much more robust than inner layer patterning is, which we discuss for getting possible insights into the framework system of root radial patterning.
Materials and methods
Plant materials and growth condition
The TDF mutants, rrd1, rrd2, and rid4 were derived from an ethyl methanesulfonate-mutagenized population of the Landsberg erecta (Ler) strain of Arabidopsis thaliana (Konishi and Sugiyama 2003; Sugiyama 2003). Green fluorescent protein (GFP) and β-glucuronidase (GUS) reporter lines used were as follows. The SCR::GFP line originated from Wassilewskija (Ws) was described in Wysocka-Diller et al. (2000). The SHR::GFP and WOX5::GUS lines, both of which were originated from Columbia (Col), were described in Helariutta et al. (2000) and Ohbayashi et al. (2011), respectively. QC184 was the promoter trap line of Ws that had been selected by Sabatini et al. (1999) from the INRA collections (Bechtold et al. 1993). The DR5::GUS line was the Col strain carrying the DR5(X7)-GUS transgene (Ulmasov et al. 1997). The PIN1::PIN1:GFP line corresponded to pPIN1::PIN1EGFP described by Xu et al. (2006), and the PIN2::PIN2:GFP line corresponded to PIN2-EGFP described by Xu and Scheres (2005). Each of the TDF mutations was introduced into these reporter lines by crossing.
Seedlings were aseptically grown on Murashige–Skoog medium supplemented with 1.0% (w/v) sucrose, buffered to pH 5.7 with 0.05% (w/v) 2-morpholinoethanesulfonic acid (MES), and solidified with 1.5% (w/v) agar under continuous light (10–15 μmol m−2 s−1) at 22°C.
Lateral root induction
As described in Ohtani et al. (2010), explants were prepared from 4-day-old seedlings, and cultured on root-inducing medium (RIM) under continuous light (15–25 μmol m−2 s−1) for the induction of semi-synchronous formation of lateral roots. RIM was B5 medium supplemented with 2.0% (w/v) glucose and 0.5 mg l−1 indole-3-butyric acid, buffered to pH 5.7 with 0.05% (w/v) MES, and solidified with 0.25% (w/v) agar Culture temperature was set to 22°C for the permissive condition and to 28°C for the restrictive condition.
Histological analysis
For whole-mount observation, tissue samples were fixed in 25 mM sodium phosphate buffer (pH 7.0) containing 2% (w/v) formaldehyde and 1% (w/v) glutaraldehyde, rinsed with 100 mM sodium phosphate buffer (pH 7.0), and cleared with an 8:1:2 (w/v/v) mixture of chloral hydrate, glycerin, and water. For highlighting cell organization, the method of Malamy and Benfey (1997) was instead employed for tissue fixation and clearing. Observations were made with a microscope equipped with Nomarski optics (BX50-DIC; Olympus, Tokyo, Japan) to obtain differential interference contrast (DIC) images.
For histological observation of transverse and longitudinal sections of lateral roots, tissue samples were fixed in FAA [50% (v/v) ethanol, 10% (v/v) formalin, 5% (v/v) acetic acid], dehydrated in an ethanol series, and embedded in Technovit 7100 resin (Heraeus Kulzer, Wehrheim, Germany). The resin blocks were cut into 8 μm sections and stained with 0.1% (w/v) aqueous solution of toluidine blue solution.
Reporter expression analysis
For histochemical detection of GUS reporter expression, tissue samples were fixed in 90% (v/v) acetone overnight at −20°C, rinsed with 100 mM sodium phosphate (pH 7.0), and incubated in X-Gluc solution [0.5 mg ml−1 5-bromo-4-chloro-3-indolyl β-d-glucuronide cyclohexylammonium salt, 0.5 mM potassium ferricyanide, 0.5 mM potassium ferrocyanide, 100 mM sodium phosphate (pH 7.4)] overnight at 37°C. After rinsing with 100 mM sodium phosphate buffer (pH 7.0), the samples were mounted on glass slides with an 8:1:2 (w/v/v) mixture of chloral hydrate, glycerin, and water, and then subjected to DIC microscopy.
To examine the fluorescence signals from GFP reporters, samples were observed by using a confocal laser scanning microscope (LSM510; Carl Zeiss, Oberkochen, Germany).
Mathematical model
A simple Turing model based on the interaction of two diffusible factors, activator and inhibitor, was considered. The reaction–diffusion dynamics of the activator (a) and inhibitor (b) was described by a modified and discretized version of the standard equations of Meinhardt (1982) as follows:
This indicates that the activator is produced autocatalytically, decays at the rate of μ a , diffuses with the coefficient of D a while the inhibitor is produced in dependence on the activator, decays at the rate of μ b , and diffuses with the coefficient of D b and that the autocatalytic production of the activator is suppressed by the inhibitor. Effects of the activator concentration on the production of activator and inhibitor were assumed to be saturable, where smaller values of α cause easier saturation of the activator effects. k a and k b gave the rates of basal production of the activator and inhibitor, respectively. It was also assumed that the interaction of the activator and inhibitor is active only in a limited area in the one-dimensional morphogenetic field, which represents a lateral root primordium region in the pericycle. The morphogenetic field consisted of linearly arranged 40 cells, and a group of cells where the activator-inhibitor dynamics was active were located at the center of the field. Boundaries between this active area and its flanking regions were open for diffusion of the activator and inhibitor. At the leftmost and rightmost ends of the filed, a reflecting boundary condition was used. Numerical calculation with a small random fluctuation was started from the spatially uniform state and repeated to obtain stable patterns of activator distribution. Calculation and graph drawing were performed with Mathematica 7.0 (Wolfram Research, Champaign, IL, USA).
Results
Fasciated lateral root formation in the TDF mutants
We previously found that primary root explants of the TDF mutants formed fasciated lateral roots with a certain frequency when cultured on RIM at the restrictive temperature (Konishi and Sugiyama 2003; Sugiyama 2003). To investigate fasciated lateral roots of the TDF mutants in more detail, in the present study, we used the semi-synchronous lateral root induction system (Ohtani et al. 2010), in which almost de novo lateral root formation was induced from explants of very young seedlings upon RIM culture. In this system, formation of fasciated lateral roots occurred reproducibly with a very high frequency in the TDF mutant explants at 28°C (Fig. 1a) but not at 22°C. In the cases of rrd1, rrd2, and rid4, approximately a half, a quarter, and two thirds of lateral roots formed at 28°C were fasciated roots, respectively (see Fig. 2). In both the wild-type and any mutant explants at either 22 or 28°C, cell division was activated to initiate development of lateral root primordia within the first 12 h of culture, and primordium development was completed and most of lateral root primordia emerged from the parent root epidermis within the next 48 h (data not shown; for the time course of lateral root development in the wild type, see Ohtani et al. 2010). Before this time, expansion of lateral root primordia, probably leading to fasciation, was already obvious in the TDF mutant explants cultured at 28°C (Fig. 1b). These primordia had much more cells along the base and were thus much wider as compared to normal primordia at the same developmental stage.
Formation of fasciated lateral roots on the TDF mutant explants in the semi-synchronous lateral root induction system. a Typical views of fasciated lateral roots formed on the TDF mutant explants in comparison with a normal lateral root formed on the wild-type (WT) explant. Explants of young seedlings were cultured on RIM at 28°C for 6 days, and processed by the standard method for DIC microscopy. Scale bar represents 100 μm. b Early developmental stages of lateral roots formed on the wild-type (WT) and mutant explants. Explants were cultured on RIM for 48 h at 22 or 28°C, and processed by the method of Malamy and Benfey (1997) for DIC microscopy. Scale bar represents 50 μm
Effects of temperature shift on the formation of fasciated lateral roots in culture of the TDF mutant explants. In the temperature shift-down experiment, cultures of the wild-type (WT) and mutant explants were started at 28°C, and the culturing temperature was lowered from 28 to 22°C at various times during culture. In the shift-up experiment, cultures were started at 22°C, and the temperature was raised from 22 to 28°C at various times. After a total of 6 days in culture, the explants were examined for the proportion of fasciated lateral roots to total lateral roots. For each data point, 12–18 explants were used
Determination of critical periods for the TDF phenotype
To determine when the TDF mutations affect the development of lateral roots, we shifted the culture temperature from 28 to 22°C (shift down) or from 22 to 28°C (shift up) at various times during culture and examined effects of these treatments on fasciated root formation. In the shift-down experiment, all the mutant explants formed more fasciated lateral roots as the temperature was shifted down to 22°C at later times, and the temporal exposure to 28°C for only 1 day at the beginning of culture was fairly effective in inducing lateral root fasciation (Fig. 2). Inversely, in the shift-up experiment, the mutant explants formed less fasciated roots as the temperature was shifted up to 28°C at later times, and culture at 22°C for only 1 day before the shift up significantly suppressed the occurrence of lateral root fasciation (Fig. 2). In brief, temperature conditions during the first day of culture were critical for lateral root morphogenesis in the TDF mutants. This means that the TDF mutations strongly influences the early developmental stages of lateral root primordia and consequently cause lateral root fasciation.
It is of note here that, in the above temperature shift experiments, no lateral roots showed chimeric morphology such that only the basal or apical part was fasciated. This means that the lateral root phenotype was irreversibly determined at the initial stage of primordial development and not changed during the subsequent process of development and growth.
Radial tissue organization of fasciated lateral roots
For histological analysis of fasciated lateral roots of the TDF mutants, we first observed the transverse and longitudinal sections of fasciated roots. Comparison of these sections with sections of normal lateral roots of the wild type showed that in the fasciated roots, cell files of the stele were preferentially increased without significant changes in the numbers of the outer tissue layers (Fig. 3a). In some fasciated roots that were extremely broadened, the concentric arrangement of tissue layers was somewhat distorted, but even in such cases, we could see the tendency of preferential increase of the stele cell files (Fig. 3a).
Radial tissue organization of fasciated lateral roots formed on the TDF mutant explants. a Longitudinal and transverse sections of lateral roots. Lateral root formation was induced from the wild-type (WT) and rid4 explants by culture on RIM at 28°C for 6 days. Sections were prepared from normal lateral roots of the wild type and fasciated lateral roots of rid4. Scale bars represent 50 μm. b Reporter expression patterns in lateral roots. Lateral root formation was induced from explants of the SHR::GFP, SCR::GFP, WOX5::GUS, and QC184 reporter lines carrying one or none (WT) of the TDF mutations by culture on RIM at 28°C. The explants were collected after 4 days (SCR::GFP), 6 days (SHR::GFP and WOX5::GUS), or 8 days (QC184) of culture, and processed for histochemical detection of GUS activity or immediately examined for GFP fluorescence. Scale bar represents 50 μm
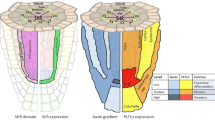
Next we examined tissue organization of the fasciated lateral roots with various reporter genes. Reporters used were SHR::GFP for marking the stele and stele initial (Helariutta et al. 2000), SCR::GFP for the endodermis, quiescent center, and ground tissue initial (Sabatini et al. 2003), and WOX5::GUS for the quiescent center (Sarkar et al. 2007). The promoter trap line QC184, in which GUS is expressed in dependence on the PLETHORA (PLT) activity, was also used to mark the quiescent center (Sabatini et al. 2003; Aida et al. 2004). The interior region expressing SHR::GFP was significantly expanded in the fasciated roots, demonstrating the increase of cell files of the stele (Fig. 3b). However, SCR::GFP expression was confined to a single cell layer in the fasciated roots as well as in normal roots (Fig. 3b). Additionally there was no or little fasciation-associated alteration in the number of cell layers outside the SCR::GFP-expressing layer (Fig. 3b). These results indicated that patterns of the epidermal, cortical, and endodermal layers were substantially unchanged by the fasciation. GUS expressions in the fasciated roots of the WOX5::GUS and QC184 lines were strikingly elevated and expanded as compared to those in normal roots, which were indicative of an increase of cells with the identity of the quiescent center (Fig. 3b). As the region where these GUS expression and SCR::GFP expression were overlapped should point the quiescent center more strictly, our data suggested that the quiescent center region was expanded radially but not longitudinally in the fasciated roots.
Auxin distribution in the fasciated lateral roots
During lateral root development, auxin gradient is generated with the aid of auxin efflux facilitators PINs and plays an essential role in the establishment of the apical–basal axis (Benková et al. 2003). To test the possibility that the auxin gradient generation is affected in the fasciated lateral roots of the TDF mutants, we examined expression patterns of auxin-related reporter genes: DR5::GUS as an auxin indicator (Ulmasov et al. 1997) and two PIN reporters, PIN1::PIN1:GFP (Xu et al. 2006) and PIN2::PIN2:GFP (Xu and Scheres 2005). The PIN1::PIN1:GFP expression in the fasciated roots was observed in increased cell files of the stele, and there PIN1:GFP was localized on the apical side of the stele cells (Fig. 4). The expression pattern of DR5::GUS visualized a tip-to-base gradient of auxin in the fasciated roots (Fig. 4). These were substantially similar to the data from normal roots, and indicated that the auxin-dependent system establishing the apical-basal axis was working almost normally in the fasciated roots. Differences between normal and fasciated lateral roots were found in the expression levels of DR5::GUS and PIN2::PIN2:GFP. In the fasciated roots, the DR5::GUS expression was relatively high and the PIN2::PIN2:GFP expression was very low (Fig. 4). It can be inferred from this result that the reduced efficiency of the basipetal transport of auxin by PIN2 may bring about a higher-level accumulation of auxin at the apex in the fasciated roots.
Auxin distribution and PIN expression patterns in fasciated lateral roots formed on the TDF mutant explants. Lateral root formation was induced from explants of the PIN1::PIN1:GFP, PIN2::PIN2:GFP, and DR5::GUS reporter lines carrying one or none (WT) of the TDF mutations by culture on RIM at 28°C. The explants were collected after 6 days of culture, and processed for histochemical detection of GUS activity or immediately examined for GFP fluorescence. Scale bar represents 50 μm
Discussion
In the semi-synchronous root induction system, all of three TDF mutants, rrd1, rrd2, and rid4, formed fasciated lateral roots with a considerably high frequency when exposed to the restrictive temperature during the first day of culture, at which time development of lateral root primodia was starting or had just started. Morphologically the sign of root fasciation could be observed in young developing primordia, in which the base width increased due to the increased number of cells. From these results, we can conclude that the lateral root fasciation in the TDF mutants is the consequence of the unusual expansion of lateral root primordia at the initial stage of development. The origin of a lateral root primordium is a small group of short cells produced by limited rounds of asymmetric, anticlinal cell division, termed formative division, in the pericycle (De Smet et al. 2006). It is likely that, in the TDF mutants, additional rounds of formative cell division occur to increase primordial cells and eventually cause lateral root fasciation. This is in contrast to the case of well-known fasciation mutants, fasciata 1 (fas1) and fas2, of which root fasciation is attributed to the instability of the maintenance of root meristems (Kaya et al. 2001).
Our findings show that in the fasciated lateral roots of the TDF mutants, meristem establishment and tissue differentiation take place in already broadened root primordia. This feature enabled us to study the problem of how radial tissue patterning of the root responds to the expansion of the field for patterning. Given a morphogenesis system that generates a concentric arrangement of outer and inner domains, what changes can be imagined to occur in this radial pattern when the morphogenetic field is enlarged? Generally possible changes may be: (1) proportional enlargement of both the outer and inner domain regions, (2) enlargement of only the outer domain region, (3) enlargement of only the inner domain region, (4) emergence of new centers and multiplication of concentric patterns, and (5) others. The results of histological analysis using various reporters demonstrated that in the fasciated lateral roots of any of the TDF mutants, as compared to normal lateral roots, cell files of the stele were increased markedly while layers of the epidermis, cortex, and endodermis were not increased. This is correspondent to the case (3) and indicates that outer layer patterning is more robust against unusual enlargement of the morphogenetic field than inner layer patterning is, which should be a reflection of the nature of unidentified mechanisms controlling basic radial patterns in the plant root.
Since the pioneering work by Turing (1952), Turing-type models based on reaction–diffusion dynamics of two or more chemical factors have been argued as candidates of autonomous mechanisms of biological pattern formation (e.g., Meinhardt 1982). Turing-type models are now not just theoretical, but there has been increasing evidence to support their real involvement in diverse aspects of biological pattern formation (Kondo and Miura 2010). Recently a Turing-type mathematical model for the shoot apical meristem has been built by incorporating interactions between CLAVATA3 (CLV3) and WUSCHEL (WUS) and successfully applied for explaining the meristem homeostasis (Fujita et al. 2011). In view of these trends, it may be reasonable to consider a Turing-type model as a first candidate of potential framework models for basic radial patterning of the root. In such model, as described in Meinhardt (1982), it depends on model dynamics and parameter settings how patterns change in response to changes in the size of morphogenetic field. For example, simulations under different values of the saturability parameter often return different pattern changes upon the enlargement of the field (Fig. 5). Inversely, relationships between pattern and field size can give constraints on model dynamics and parameter settings. The robustness of the root outer layer patterning shown by the present study will provide this kind of helpful information for the future modeling of the framework system of basic radial organization in the root.
Spatial distribution of the activator in different size of active areas under different settings of the saturability parameter. The reaction–diffusion dynamics described in "Materials and methods" was assumed to be active in the region of the 17th to 24th cells, 15th to 26th cells, or 13th to 28th cells. The saturability parameter α was set to be 10 or 100. All other parameters were fixed as follows: A = 1, B = 1, β = 0.1, μ a = 0.01, μ b = 0.02, D a = 0.02, D b = 0.4, k a = 0.0005, k b = 0. Rectangles above the graphs indicate interior (in) and exterior (ex) domain regions when assuming that the activator concentrations determine interior or exterior domains with a threshold of about 2.4. Note that as the morphogenetic field is expanded, the exterior domain region is expanded at α = 100 while the interior domain region is expanded at α = 10
References
Aida M, Beis D, Heidstra R, Willemsen V, Blilou I, Galinha C, Nussaume L, Noh YS, Amasino R, Scheres B (2004) The PLETHORA genes mediate patterning of the Arabidopsis root stem cell niche. Cell 119:109–120
Bechtold N, Ellis J, Pelletier G (1993) In planta Agrobacterium mediated gene transfer by infiltration of adult Arabidopsis thaliana plants. C R Acad Sci Paris Life Sci 316:1194–1199
Benková E, Michniewicz M, Sauer M, Teichmann T, Seifertová D, Jürgens G, Friml J (2003) Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115:591–602
Carlsbecker A, Lee JY, Roberts CJ, Dettmer J, Lehesranta S, Zhou J, Lindgren O, Moreno-Risueno MA, Vatén A, Thitamadee S, Campilho A, Sebasian J, Bowman JL, Helariutta Y, Benfey PN (2010) Cell signalling by microRNA165/6 directs gene dose-dependent root cell fate. Nature 465:316–321
Cui H, Levesque MP, Vernoux T, Jung JW, Paquette AJ, Gallagher KL, Wang JY, Blilou I, Scheres B, Benfey PN (2007) An evolutionarily conserved mechanism delimiting SHR movement defines a single layer of endodermis in plants. Science 316:421–425
De Smet I, Vanneste S, Inzé D, Beeckman T (2006) Lateral root initiation or the birth of a new meristem. Plant Mol Biol 60:871–887
Di Laurenzio L, Wysocka-Diller J, Malamy JE, Pysh L, Helariutta Y, Freshour G, Hahn MG, Feldmann KA, Benfey PN (1996) The SCARECROW gene regulates an asymmetric cell division that is essential for generating the radial organization of the Arabidopsis root. Cell 86:423–433
Fujita H, Toyokura K, Okada K, Kawaguchi M (2011) Reaction–diffusion pattern in shoot apical meristem of plants. Plos One 6:e18243
Helariutta Y, Fukaki H, Wysocka-Diller J, Nakajima K, Jung J, Sena G, Hauser MT, Benfey PN (2000) The SHORT–ROOT gene controls radial patterning of the Arabidopsis root through radial signalling. Cell 101:555–567
Kaya H, Shibahara K, Taoka K, Iwabuchi M, Stillman B, Araki T (2001) FASCIATA genes for chromatin assembly factor-1 in Arabidopsis maintain the cellular organization of apical meristems. Cell 104:131–142
Kondo S, Miura T (2010) Reaction–diffusion model as a framework for understanding biological pattern formation. Science 329:1616–1620
Konishi M, Sugiyama M (2003) Genetic analysis of adventitious root formation with a novel series of temperature-sensitive mutants of Arabidopsis thaliana. Development 130:5637–5647
Malamy JE, Benfey PN (1997) Organization and cell differentiation in lateral roots of Arabidopsis thaliana. Development 124:33–44
Meinhardt H (1982) Models of biological pattern formation. Academic Press, London
Miyashima S, Hashimoto T, Nakajima K (2009) ARGONAUTE1 acts in Arabidopsis root radial pattern formation independently of the SHR/SCR pathway. Plant Cell Physiol 50:626–634
Miyashima S, Koi S, Hashimoto T, Nakajima K (2011) Non-cell-autonomous microRNA165 acts in a dose-dependent manner to regulate multiple differentiation status in the Arabidopsis root. Development 138:2303–2310
Nakajima K, Sena G, Nawy T, Benfey PN (2001) Intercellular movement of the putative transcription factor SHR in root patterning. Nature 413:307–311
Ohbayashi I, Konishi M, Ebine K, Sugiyama M (2011) Genetic identification of Arabidopsis RID2 as an essential factor involved in pre-rRNA processing. Plant J 67:49–60
Ohtani M, Demura T, Sugiyama M (2010) Particular significance of SRD2-dependent snRNA accumulation in polarized pattern generation during lateral root development of Arabidopsis. Plant Cell Physiol 51:2002–2012
Sabatini S, Beis D, Wolkenfelt H, Murfett J, Guilfoyle T, Malamy J, Benfey P, Leyser O, Bechtold N, Weisbeek P, Scheres B (1999) An auxin-dependent distal organizer of pattern and polarity in the Arabidopsis root. Cell 99:463–472
Sabatini S, Heidstra R, Wildwater M, Scheres B (2003) SCARECROW is involved in positioning the stem cell niche in the Arabidopsis root meristem. Genes Dev 17:354–358
Sarkar AK, Luijten M, Miyashima S, Lenhard M, Hashimoto T, Nakajima K, Scheres B, Heidstra R, Laux T (2007) Conserved factors regulate signalling in Arabidopsis thaliana shoot and root stem cell organizers. Nature 446:811–814
Sugiyama M (2003) Isolation and initial characterization of temperature-sensitive mutants of Arabidopsis thaliana that are impaired in root redifferentiation. Plant Cell Physiol 44:588–596
Turing A (1952) The chemical basis of morphogenesis. Phil Trans B 237:37–72
Ulmasov T, Murfett J, Hagen G, Guilfoyle TJ (1997) Aux/lAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. Plant Cell 9:1963–1971
Wysocka-Diller JW, Helariutta Y, Fukaki H, Malamy JE, Benfey PN (2000) Molecular analysis of SCARECROW function reveals a radial patterning mechanism common to root and shoot. Development 127:595–603
Xu J, Scheres B (2005) Dissection of Arabidopsis ADP-RIBOSYLATION FACTOR 1 function in epidermal cell polarity. Plant Cell 17:525–536
Xu J, Hofhuis H, Heidstra R, Sauer M, Friml J, Scheres B (2006) A molecular framework for plant regeneration. Science 311:385–388
Acknowledgments
We thank Dr. Tom J. Guilfoyle (University of Missouri) for providing the DR5::GUS line, Drs. Philip N. Benfey (Duke University), Hidehiro Fukaki (Kobe University), and Minako Ueda (Nara Institute of Science and Technology) for the SCR::GFP and SHR::GFP lines, and Drs. Ben Scheres (Utrecht University), Jirí Friml (Ghent University), Masahiko Furutani (Nara Institute of Science and Technology) for the PIN reporter lines. This work was supported by a Grant-in-Aid for Scientific Research on Priority Areas (No. 19060001 to M.S.) of the Ministry of Education, Culture, Sports, Science and Technology, Japan and also by a Grant-in-Aid for JSPS Fellows (No. 218676 to K.O.) from the Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Otsuka, K., Sugiyama, M. Tissue organization of fasciated lateral roots of Arabidopsis mutants suggestive of the robust nature of outer layer patterning. J Plant Res 125, 547–554 (2012). https://doi.org/10.1007/s10265-011-0471-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10265-011-0471-5