Abstract
Phospholipase D (PLD) and its product phosphatidic acid play important roles in the regulation of plant growth, development, and stress responses. The genome database analysis has revealed PLD family in Arabidopsis, rice, poplar and grape. In this study, we report a genomic analysis of 18 putative soybean (Glycine max) PLD genes (GmPLDs), which exist in the 14 of 20 chromosomes. GmPLDs were grouped into six types, α(3), β(4), γ, δ(5), ε(2), and ζ(3), based on gene architectures, protein domains, evolutionary relationship, and sequence identity. These GmPLDs contained two HKD domains, PX/PH domains (for GmPLDζs), and C2 domain (for the other GmPLDs). The expression patterns analyzed by quantitative reverse transcription PCR demonstrated that GmPLDs were expressed differentially in various tissues. GmPLDα1, α2, and β2 were highly expressed in most tissues, whereas GmPLDδ5 was only expressed in flowers and GmPLDζ1 was predominantly expressed in flowers and early pods. The expression of GmPLDα1 and α2 was increased and that of GmPLDγ was decreased by salt stress. GmPLDα1 protein expressed in E. coli was active under the reaction conditions for both PLDα and PLDδ, hydrolyzing the common membrane phospholipids phosphatidylcholine, phosphatidylethanolamine, and phosphatidylglycerol. The genomic analysis for soybean PLD family provides valuable data for further identity and characterization of their functions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Phospholipase D (PLD) hydrolyzes membrane phospholipids to generate a free head and phosphatidic acid (PA), which is an important messenger in plants, microorganisms and mammals (Hong et al. 2010; Munnik 2001). Since the first eukaryotic cDNA of PLD was cloned from castor bean (Wang et al. 1994), several PLD cDNAs have been cloned from higher plants, including Arabidopsis thaliana (Hong et al. 2008, 2009; Qin et al. 1997, 2006; Zhang et al. 2003), Oryza sativa (Li et al. 2007; Ueki et al. 1995), Zea mays (Qin et al. 1997), Nicotiana tabacum (Lein and Saalbach 2001), Lycopersicon esculentum (Whitaker et al. 2001), and Brassica oleracea (Kim et al. 1999). Up to date, genome analysis for PLD family has been done in Arabidopsis, Oryza sativa (rice), Populus tremula (poplar), and Vitis vinifera (grape) (Li et al. 2007; Liu et al. 2010; Qin and Wang 2002; Wang 2005; Yamaguchi et al. 2009). 12 Arabidopsis PLD members are grouped into six classes, α(3), β(2), γ(3), δ, ε, and ζ(2) based on sequence similarity and biochemical properties (Qin and Wang 2002). In rice, 17 PLD members are divided into six groups, α(8), β(2), δ(3), ζ(2), κ, and φ, according to gene and domain structure analysis (Li et al. 2007). All plant PLDs found contain two HKD domains, which have catalytic functions (Qin and Wang 2002). Most plant PLDs contain a C2 domain which is for Ca2+—or other effectors (including phospholipids, inositol phosphates and proteins)—binding (Nalefski and Falke 1996; Rizo and Sudhof 1998; Zheng et al. 2001). PLDζ1 and PLDζ2 include PX/PH domains. While OsPLDφ, PtPLDφ (PtPLD11), and VvPLDφ (VvPLD3) contain a signal peptide at N-terminus, instead of C2 domain and PX/PH domains (Li et al. 2007; Liu et al. 2010). PX domain binds to phosphoinositides and SH3 domain (Cheever et al. 2001; Hiroaki et al. 2001; Ponting 1996). PH domain is a protein domain composed of about 120 amino acids. In animal cells, this domain plays a role in recruiting the proteins to membranes by binding to phosphatidylinositol lipids (such as phosphatidylinositol (3,4,5)-trisphosphate and phosphatidylinositol (4,5)-bisphosphate) or proteins such as G proteins (Lemmon and Ferguson 2000; Qin and Wang 2002). Both PX and PH domains function in cells by targeting proteins to appropriate cellular compartments or enabling them to interact with other components of the signal transduction pathways.
Plant PLDs are involved in mediating the growth, development, and response to environmental stimuli (Hong et al. 2010; Li et al. 2009; Testerink and Munnik 2005; Wang 2005). In Arabidopsis, PLDα1, the most abundant PLD, has been found to regulate salt and osmotic tolerance, abscisic acid signaling, and seed aging (Li et al. 2009). PLDα1 mediates these processes through down targets mediated by PA. For example, PA plays an important role in promoting ABA-mediated guard cell closure by the interaction with both ABI1 (Zhang et al. 2004) and G protein (Mishra et al. 2006). In salt stress response, PA recruits MPK6 to plasma membrane where it phosphorylates SOS1 antiporter (Yu et al. 2010). PLDα3 sharing 70% amino acid sequence similarity to PLDα1, positively regulates plant responses to hyperosmotic stresses by the regulation of PA signaling-mediated TOR and AGC2.1 expression (Hong et al. 2008).
PLDδ is an oleate-stimulated PLD, which connects microtubules with plasma membrane (Dhonukshe et al. 2003). The knockout of PLDδ makes plants sensitive to freezing and oxidative stresses (Li et al. 2004; Zhang et al. 2003). While PLDβ1 has been found to participate in wounding and pathogen response (Li et al. 2009; Wang et al. 2000).
PLDζs, similar with animal PLDs in structure, have unique functions in root growth. PLDζ1-deficient plants specifically show altered patterns of root hair formation, whereas PLDζ1 overexpression results in branched and swollen root hairs (Ohashi et al. 2003). PLDζ2 regulates auxin-mediated root gravitropism by affecting cycling of PIN2-containing vesicles and hence auxin transport and distribution (Li and Xue 2007). The knockout of both PLDζ1 and PLDζ2 reduces the growth of primary roots (Li et al. 2006). During phosphorus starvation, these PLDs hydrolyze phospholipids to supply phosphate and diacylglycerol moieties for galactolipid synthesis, therefore maintaining membrane stability (Li et al. 2006). Knockout of PLDε reduces root hair elongation and primary root growth under nitrogen but not phosphate deprivation (Hong et al. 2009).
PLDs have been investigated in other plant species, including rice (Li et al. 2007; McGee et al. 2003; Yamaguchi et al. 2009), tomato (Laxalt et al. 2001), poplar, and grape (Liu et al. 2010). Using RNAi methods, OsPLDβ1 is been found to function as a positive regulator in the ABA-mediated seed germination, but as a negative regulator of disease resistance (Li et al. 2007; Yamaguchi et al. 2009).
A PLD protein with molecular mass of 92 kDa purified from soybean suspension-cultured cells shows similar biochemical properties with PLDα in Arabidopsis (Abousalham et al. 1995). Up to date, the PLD family has not been reported in soybean. In this study, we carried out genomic analysis of PLD family in soybean based on the draft genome sequences (Schmutz et al. 2010). We examined the transcript patterns of GmPLDs in different tissues and in response to NaCl stress. GmPLDαs were further functionally characterized using the recombinant proteins.
Materials and methods
Genomic search and sequence analysis
The blast search was used with AtPLD, OsPLD, PtPLD, and VvPLD in the Phytozome (http://www.phytozome.net/soybean/) to identify PLD genes in soybean. The keyword “phospholipase D” was also used in browse tool in the Phytozome, NCBI (http://www.ncbi.nlm.nih.gov/) and RIKEN (http://rsoy.psc.riken.jp). The multiple sequence alignments were constructed by MEGA4.1 from the website (http://www.megasoftware.net/) with the default parameters.
The conserved domain sequences of PLD proteins were identified by SMART (http://smart.embl-heidelberg.de/) and PFAM (http://pfam.sanger.ac.uk/search) tools with the default parameters.
Plant growth conditions
Soybean (Glycine max (L.) Merr.) cultivar “Nannong 99-10” was grown in the natural environment from June to October in Nanjing, China. The harvested seeds were germinated for 2 days and transferred to pots containing soils and vermiculites. Roots, leaves, and stems were harvested after transferring for 50 days. Early soybean pods were harvested 1 week after flowering, and late soybean pods and immature seeds were harvested 3 weeks after flowering. The mature seeds were harvested about 6 weeks after flowering.
Salt stress treatment
The soybean seedlings were grown in a growth room at 160 μmol m−2s−1 of light intensity and 14 h/10 h (30°C/23°C) day/night regimes. The 20-day-old seedlings were transferred to Hogland solution with or without 250 mM NaCl for 4 or 8 h. The leaves were harvested for the isolation of RNA and detection of gene expression.
qRT-PCR
Transcript expression of PLD genes was quantified by quantitative reverse transcription PCR (qRT-PCR). Total RNA was extracted from harvested materials described above, using the Trizol method as the manufacturer described (Takara, Japan). 1 μg RNA was used for reverse transcription (Takara SYBR PrimeScript RT-PCR Kit for Perfect Real Time). One PCR reaction system included 25 μL cDNA mixture corresponding to 5 ng of total RNA. The reaction was carried out according to the method offered by the manufacturer (Takara SYBR PrimeScript RT-PCR Kit for Perfect Real Time) in a qRT-PCR equipment (MJ Research Opticon 2.0). The specific-primers for GmPLD genes were shown in Supplemental table 1. The PCR products were sequenced for confirming. The standard calibration curve for each gene was obtained by performing the PCR by 4–5 dilutions of the cDNA fragments. The specificity of the individual PCR product was examined by the heat dissociation protocol from 55 to 95°C. The cons6 was used as a standard gene for different gene expressions (Libault et al. 2008).
Cloning of GmPLDαs
The blast tools in NCBI and RIKEN were used to search ESTs and cDNAs of GmPLDα1, 2, 3 with AtPLDα. The specific primers used for cloning are shown in Supplemental table 1 (PLDα1, 2, 3-C). The RNA was isolated from the leaves, and the genes were cloned with LA Taq from Takara.
Protein expression and western blotting
The coding sequences of GmPLDα1,2, 3, inserting between BamHI and SalI sites, were cloned into pGEMT-Easy vectors with the specific primers shown in Supplemental table 1 (PLDα1, 2, 3-E). The plasmids were digested with BamHI and SalI and ligated into pGEX-4T-1 vector to produce GmPLDα1, α2, and α3 with glutathione S-transferase (GST) fused at N-terminus, and were transformed into E. coli BL21. The recombinant GmPLDα proteins were expressed according to the method as follows: GmPLDα1, α2, and PLDα3 were induced by 0.5 mM isopropyl-1-thio-β-d-galactopyranoside (IPTG) at 26°C for 8 h. The bacteria were precipitated, and lysed by sonication in phosphate-balanced solution (PBS, pH 7.4) with 1 mM phenylmethanesulfonyl fluoride (PMSF). Bacterial lysate was centrifuged at 12,000g for 10 min, and the upper phase was purified by GST beads (Zhao and Wang 2004). The purified proteins were separated by 12% SDS-PAGE. The proteins were transferred to a polyvinylidene difluoride membrane after electrophoresis. The membrane was blotted with GST antibody (1:2,000) followed by incubation with a second antibody (1:2,000) conjugated to alkaline phosphatase. The protein bands were visualized by alkaline phosphatase reaction.
PLD activity assay
PLDα activity was determined according to the method by Wang et al. (1993). The reaction mixture (100 μL) contained 100 mM MES (pH 6.5), 0.6 mM SDS, 25 mM CaCl2 (or as indicated in the legend of Fig. 6c), 1 mM PC (containing 3H-PC), and 5 μg purified proteins. The reaction conditions for different PLDs and substrate preparation were according to the published methods (Hong et al. 2008).
Results and discussion
PLD family in soybean
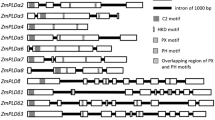
The soybean genome sequences have been released recently (Schmutz et al. 2010). To identify the members of PLD family in soybean (Glycine max), the corresponding sequence information from Arabidopsis, rice, polar, and gape was used to perform multiple searches of the relevant DNA databases, the keyword ‘‘phospholipase D’’ searches, and protein domain searches. 27 genes were identified in soybean using these approaches. The obtained sequences were further analyzed for an encoding PLD using the programs PFAM and SMART. The results indicated that these 27 genes include four pseudogenes, two genes encoding putative proteins without HKD, C2, or PXPH domain, one gene encoding a putative protein including one HKD domain and C2 domain, two genes coding encoding putative proteins with two HKD domains but without C2 or PH/PX domains, and 18 genes encoding putative PLDs with two HKD domains and C2 domain (or PH/PX domains). These 18 PLD genes (GmPLDs) are located in 14 of 20 chromosomes (Fig. 1a; Supplemental table 2). The GmPLD family was grouped into six types, named as GmPLDα(3), β(4), γ, δ(5), ε(2), ζ(3), based on gene architectures (Fig. 1b), protein domains (Fig. 2), evolutionary relationship (Fig. 3), and sequence identity (Fig. 3; Supplemental table 3).
The phylogenetic analysis for PLD family in soybean, Arabidopsis, and rice. The tree was constructed by the UPGMA method in the MEG4.1 soft from the website (http://www.megasoftware.net/) with the default parameters
The gene structure among the GmPLD family is diverse. There are four exons in GmPLDα1/α2 and GmPLDεs, which is same to AtPLDα and AtPLDε, whereas, there are three exons in GmPLDα3. GmPLDβ3 contains 10 exons, which is same to the case in AtPLDβs, but GmPLDβ1 and GmPLDβ2 include 13 exons, and GmPLDβ4 contains 18 exons. GmPLDγ is consisted of 10 exons, which is same to AtPLDγ. GmPLDδs, the biggest group in GmPLD family, contain 10–12 exons. The GmPLDζs contain the highest amount of exons, 21 exons for GmPLDζ1and 20 exons for GmPLDζ2 and ζ3. Compared with AtPLD, GmPLD family has more members of PLDβ (2 vs. 4) and δ (1 vs. 5), but less members of PLDγ (3 vs. 1).
There are PLD gene clusters in Arabidopsis (PLDγ1–PLDγ2–PLDγ3 in chromosome 4) and rice (PLDα4–PLDα5–PLDα6 in chromosome 6) (Li et al. 2007). However, no PLD cluster is found in soybean. Interestingly, several pairs of GmPLDs show high identity in amino acid sequences. For example, there is 97.9% identity between GmPLDα1 and GmPLDα2; 94.1% between β1 and β2; 96.4% between δ1 and δ2; 95% between δ4 and δ5; and 97.9% between ζ2 and ζ3 (Supplemental table 3). In the evolutionary history of soybean, genome duplications occurred, which were followed by gene diversification and loss, and numerous chromosome rearrangements (Shultz et al. 2006; Schmutz et al. 2010). These events might contribute to the high identity between the pairs of GmPLDs in soybean.
Protein domains in GmPLDs
In general, most of plant PLDs can be grouped into two subfamilies, C2-PLDs and PX/PH-PLDs, based on protein structures (Li et al. 2009). In rice, there is a SP-PLD, which harbors a signal peptide instead of C2 or PH/PX domains at the N-terminus (Li et al. 2007), although the function of this domain keeps unclear. 15 of 18 GmPLDs (3αs, 4βs, γ, 5δs, 2εs) are C2-PLD, and three GmPLDζs are PH/PX-PLDs (Fig. 2). The C2 domains are autonomously folded protein modules that generally act as Ca2+ and phospholipid binding domains. Ca2+ binding domain is coordinated by four to five amino acid residues in bipartite loops within the C2 domain (Qin and Wang 2002). Compared with cPLA2, GmPLDα1 and GmPLDα2 lack one potential Ca2+-binding residues, and GmPLDα3 has two residues substituted (Supplemental Fig. 1). Similar lacking for Ca2+-binding residues has been found in Arabidopsis PLDα (Qin and Wang, 2002). GmPLDβ4 has a Ca2+-binding residue, while GmPLDε1 and ε2 totally loss all Ca2+ binding residues (Supplemental Fig. 1).
The conserved PX/PH domains are present in GmPLDζs. GmPLDζs have a shorter PX domain (29 residues) than AtPLDζs (107 and 158 residues for PLDζ1 and PLDζ2, respectively) and OsPLDζs (128 and 187 residues for PLDζ1 and PLDζ2, respectively). These plants have similar PH domains in the length (91–129 residues) except OsPLDζ1, which has only 25 residues.
The highly conserved domains in eukaryotic PLDs are two HKD domains that contain HXKXXXXD/E motif (Qin and Wang 2002). All GmPLDs contain two HxKxxxxD motifs, except that K is mutated to N in the second HxKxxxxD motif in GmPLDε1, which was confirmed by amplifying a short genomic DNA fragment containing this mutated site and sequencing (Supplemental Fig. 2). In OsPLDθ, both H and K in the second HxKxxxxD motif were mutated to N (Elias et al. 2002). We found that there are 35–39 amino acids in the first HKD domain of C2-GmPLDs, but only 27 amino acids of PX/PH-GmPLDs. The second HKD domain is composed of 27 amino acids in both C2-GmPLDs and PX/PH-GmPLDs. Two HKD motifs are separated by approximately 300 amino acids in C2-PLDs, but by about 400 amino acids in PX/PH-PLDs (Supplemental Fig. 2).
It should be noticed that there are two additional potential PLDs which are not included in the 18 GmPLDs. One is GmPLDα4 (phytozome locus Glyma08g20710.1 with 650 amino acids) showing 56% identity with GmPLDα1, and the other is GmPLDε3 (phytozome locus Glyma06g07220.1 with 666 amino acids) showing 83% identity with GmPLDε1. These GmPLDs contain two HKD domains, without either C2, PH/PX, or SP domain (Supplemental Fig. 3), which exists in reported PLDs. When these proteins were expressed in E. coli, they showed no activity in the presence or absence of Ca2+. Further identity is necessary to determine whether these genes encode functional PLDs.
GmPLDs expression in tissues
To investigate the steady-state expression patterns of GmPLD genes in soybean, qRT-PCR was used to detect the transcript levels in roots, stems, leaves, flowers, early pods, late pods, immature seeds, and mature seeds. As shown in Fig. 4, individual GmPLD exhibited different and overlapping patterns of expression. In general, the expression of GmPLDαs in tested tissues was higher than that of other GmPLDs. Especially, GmPLDα1 was most abundant in all tissues except mature seeds. Similar expressing patterns of PLDα1 have been found in Arabidopsis (Li et al. 2006; Zhang et al. 2010) and rice (Li et al. 2007; Yamaguchi et al. 2009). Unlike the other PLDαs in Arabidopsis and rice, which displayed absolutely lower expression as compared with PLDα1 (Li et al. 2007; Yamaguchi et al. 2009), GmPLDα2 and α3 showed only little lower expression in most tissues than GmPLDα1 (Fig. 4). The results suggest that GmPLDαs might have more extensive functions in the growth and development in soybean than in other plant species.
The expression of GmPLDs in tissues. Total RNA from tissues of soil-grown plants were used for qRT-PCR. The values were standardized using cons6 gene in soybean. The values show a representative result of three independent experiments. The error bars indicate SD of three qRT-PCR samples from the same cDNA archive (see Supplemental table 4 for digital data)
GmPLDβ2 and β3 also displayed high expression in most tested tissues, and their expressions were much higher than those of GmPLDβ1 and β4 (Fig. 4). In rice, OsPLDβ1was highly expressed in leaf sheaths, blades, and immature seeds, showing much higher expression than OsPLDβ2 (Yamaguchi et al. 2009). The expression of AtPLDβ1 in rosettes and roots was higher and that of AtPLDβ2, but much lower than that of AtPLDα1 (Li et al. 2006).
Some GmPLDs showed special expression patterns in the tissues. For example, GmPLDδ5 expressed predominately in flowers, GmPLDζ3 mainly in early pods, and GmPLDζ1 majorly in flowers and early pods (Fig. 4). These results may imply that these GmPLDs have special functions in the tissues.
Effect of salt treatment on GmPLDs expression
We then tested the transcription changes of GmPLDs under NaCl stress. Figure 5 showed the GmPLDs transcriptions whose changes induced by NaCl were more than 3-fold as compared with the control, except GmPLDα3. The GmPLDα1 and α2 transcripts increased with the duration of NaCl treatment, their transcript increased ~8 and ~30-fold at 8 h of NaCl treatment as compared with their control, respectively. The expression of GmPLDδ3 and δ4 increased ~3-fold under NaCl treatment for 4 h, and decreased to initial level for 8 h. The transcript level for GmPLDγ was decreased to one tenth of the control under NaCl treatment for 8 h (Fig. 5). Although the expression of GmPLDα3 was increased by NaCl less than 3-fold, it was shown in Fig. 5 because AtPLDα3 (it shares 60% identity with GmPLDα3) has been reported to regulate salt tolerance positively (Hong et al. 2008). OsPLDα was recently reported to be involved in rice salt tolerance by the mediation of H+-ATPase activity and transcription (Shen et al. 2011). It would be essential to investigate whether and which GmPLDs regulates salt tolerance in soybean.
Expression of GmPLDs under NaCl treatment. 20-day-old seedlings were treated with Hogland solution with or without 250 mM NaCl. The leaves were harvested 0, 4, and 8 h after NaCl treatment. The expression of GmPLDs was standardized by that of cons6, and the expression level of control was arbitrarily set to 1. The values show the averages of three biological replicates, each with three qRT-PCR samples from the same cDNA archive. The bars indicate SD of three replicates (see Supplemental table 5 for digital data). Data were compared using LSD test. The asterisks indicate that the mean value is significantly different from that of the control. *P < 0.05 and **P < 0.01
Recombinant GmPLDα1 shows activity towards to common membrane phospholipids
To determine whether GmPLDs encode functional PLDs, three GmPLDα cDNAs cloned from the leaves were constructed into pGEX-4T-1 vector with GST tag and expressed in Escherichia coli. As shown in Fig. 6a, the bands with about 120 KDa were found in GmPLDα1, α2, and α3 tagged with GST. The purified recombinant proteins were used to determine PLD activity. GmPLDα1 showed the highest activity under PLDα reaction conditions that included 25 mM Ca2+, SDS, and single-lipid-class vesicles (Fig. 6b). When Ca2+ concentration in the reaction medium was below 1 mM, PLD activity was sharply decreased (Fig. 6c). GmPLDα1 displayed a low activity under PLDδ conditions that included micromolar levels of Ca2+ and oleic acid. Although no comparison was found for AtPLDα1 activity under PLDα and PLDδ conditions, AtPLDα3 activity under PLDδ conditions was only 1/8 of that under PLDα conditions (Hong et al. 2008). Whereas, GmPLDα1 activity under PLDδ conditions made up 1/2 of that under PLDα conditions (Fig. 6b). GmPLDα1 was nearly inactive under PLDβ(γ), or -ζ conditions, which included phosphatidylinositol 4,5-bisphosphate (PIP2), phosphatidylethanolamine (PE), and micromolar levels or no Ca2+ in the reaction mixtures (Fig. 6b). The results suggest that GmPLDα1 activity shows lower requirement for Ca2+ in the presence of oleic acid.
GmPLDα protein expression and activity assay. a The western blotting of GmPLDα proteins. The purified proteins from E. coli BL21 with GST tag were used. b GmPLDαs activity under PLDα, -β(γ), -δ, and -ζ conditions using dipalmitoylglycero-3-phospho-(methyl-3H) choline as a substrate. c The activity of GmPLDα1 in a Ca2+-dependent manner. d The GmPLDαs hydrolytic activity toward to substrates PC, PE, PG, and PS. NBD-fluorescence labeled lipids were used as the substrates. The error bars in b, c indicate SD of three biological replicates. Data were compared using LSD test. The asterisks in b indicate that the mean value is significantly different from that of the condition of PLDα activity. The asterisks in c indicate that the mean value is significantly different from that of the condition in the presence of 25 mM Ca2+. *P < 0.05 and **P < 0.01
GmPLDα1 hydrolyzed the common membrane phospholipids phosphatidylcholine (PC), PE, and phosphatidylglycerol (PG). But it showed much low activity towards to the substrate phosphatidylserine (PS) (Fig. 6d). Similar patterns were reported for AtPLDα3 (Hong et al. 2008).
When the same amount of protein with GmPLDα1 was used, GmPLDα2 and α3 activities were hardly detected under the tested conditions (Fig. 6b, d). There are only 16 out of 809 amino acids different between GmPLDα1 and GmPLDα2. The great difference in the activity between two proteins may be due to (1) enzymatic character changes caused by the 16 amino acid mutations; (2) the difference in post-transcriptional modifications between GmPLDα1 and GmPLDα2 (or GmPLDα3). Further research is needed to determine biochemical characters of these GmPLDαs.
In conclusion, we report here soybean PLD family, including their gene and protein structures, and their expression patterns in tissues and under salt stress conditions. Together with the GmPLDα activity characterization, our results suggest that GmPLDs show their unique properties as compared with other reported PLDs.
Accession numbers
Sequence data from this article can be found on the Phytozome website, Arabidopsis Genome Initiative database and GenBank website. Accession numbers for soybean PLDs are listed in Supplemental table 2. For Arabidopsis PLDs, please refer to Qin and Wang (2002), and for rice PLDs, please refer to Li et al. (2007). Poplar and grape PLDs sequences can be found from Liu et al. (2010). Other sequences, which can be found on the GenBank Website, are listed as follows: cons6 (CD397253), PLCδ1 (P10688.1), cPLA2 (NP_077734.1), and MPK6 (AEC10325.1).
References
Abousalham A, Teissere M, Gardies AM, Verger R, Noat G (1995) Phospholipase D from soybean (Glycine max L.) suspension-cultured cells: purification, structural and enzymatic properties. Plant Cell Physiol 36:989–996
Cheever ML, Sato TK, de Beer T, Kutateladze TG, Emr SD, Overduin M (2001) Phox domain interaction with PtdIns(3)P targets the Vam t-SNARE to vacuole membranes. Nat Cell Biol 3:613–618
Dhonukshe P, Laxult AM, Goedhart J, Gadella TWJ, Munnik T (2003) Phospholipase D activation correlates with microtubule remorganization in living plant cells. Plant Cell 15:2666–2679
Elias M, Potocky M, Cvrckova F, Zarsky V (2002) Molecular diversity of phospholipase D in angiosperms. BMC Genomics 3:2
Hiroaki H, Ago T, Ito T, Sumimoto H, Kohda D (2001) Solution structure of the PX domain, a target of the SH3 domain. Nat Struct Biol 8:526–530
Hong Y, Pan X, Welti R, Wang X (2008) Phospholipase Dα3 is involved in the hyperosmotic response in Arabidopsis. Plant Cell 20:803–816
Hong Y, Devaiah SP, Bahn SC, Thamasandra BN, Li M, Welti R, Wang X (2009) Phospholipase Dε and phosphatidic acid enhance Arabidopsis nitrogen signaling and growth. Plant J 58:376–387
Hong Y, Zhang W, Wang X (2010) Phospholipase D and phosphatidic acid signalling in plant response to drought and salinity. Plant Cell Environ 33:627–635
Kim DU, Roh TY, Lee J, Noh JY, Jang YJ, Hoe KL, Yoo HS, Choi MU (1999) Molecular cloning and functional expression of a phospholipase D from cabbage (Brassica oleracea var capitata). Biochim Biophys Acta 1437:409–414
Laxalt AM, ter Riet B, Verdonk JC, Parigi L, Tameling WI, Vossen J, Haring M, Musgrave A, Munnik T (2001) Characterization of five tomato phospholipase D cDNAs: rapid and specific expression of LePLDbeta1 on elicitation with xylanase. Plant J 26:237–247
Lein W, Saalbach G (2001) Cloning and direct G-protein regulation of phospholipase D from tobacco. Biochim Biophys Acta 1530:172–183
Lemmon MA, Ferguson KM (2000) Signal-dependent membrane targeting by pleckstrin homology (PH) domains. Biochem J 350:1–18
Li G, Xue HW (2007) Arabidopsis PLDzeta2 regulates vesicle trafficking and is required for auxin response. Plant Cell 19:281–295
Li W, Li M, Zhang W, Welti R, Wang X (2004) The plasma membrane-bound phospholipase Ddelta enhances freezing tolerance in Arabidopsis thaliana. Nat Biotechnol 22:427–33
Li M, Welti R, Wang X (2006) Quantitative profiling of Arabidopsis polar glycerolipids in response to phosphorus starvation: role of phospholipase Dζ1 and ζ2 in phosphatidylcholine hydrolysis and digalactosyldiacylglycerol accumulation in phosphorus-starved plants. Plant Physiol 142:750–761
Li G, Lin F, Xue H (2007) Genome-wide analysis of the phospholipase D family in Oryza sativa and functional characterization of PLDβ1 in seed germination. Cell Res 17:881–894
Li M, Hong Y, Wang X (2009) Phospholipase D- and phosphatidic acid-mediated signaling in plants. Biochim Biophys Acta 1791:927–935
Li Q, Zhang C, Yang Y, Hu X (2010) Genome-wide and molecular evolution analyses of the phospholipase D gene family in poplar and grape. BMC Plant Biol 10:117–131
Libault M, Thibivilliers S, Bilgin DD, Radwan O, Benitez M, Clough SJ, Stacey G (2008) Identification of four soybean reference genes for gene expression normalization. Plant Genome 1:44–54
McGee JD, Roe JL, Sweat TA, Wang X, Guikema JA, Leach JE (2003) Rice phospholipase D isoforms show differential cellular location and gene induction. Plant Cell Physiol 44:1013–1026
Mishra G, Zhang W, Deng F, Zhao J, Wang X (2006) A bifurcating pathway directs abscisic acid effects on stomatal closure and opening in Arabidopsis. Science 312:264–266
Munnik T (2001) Phosphatidic acid: an emerging plant lipid second messenger. Trends Plant Sci 6:227–233
Nalefski EA, Falke JJ (1996) The C2 domain calcium-binding motif: structural and functional diversity. Protein Sci 5:2375–2390
Ohashi Y, Oka A, Rodrigues-Pousada R, Possenti M, Ruberti I, Morelli G, Aoyama T (2003) Modulation of phospholipid signaling by GLABRA2 in root-hair pattern formation. Science 300:1427–1430
Ponting CP (1996) Novel domains in NADPH oxidase subunits, sorting nexins, and PtdIns 3-kinases: binding partners of SH3 domains. Protein Sci 5:2353–2357
Qin C, Wang X (2002) The Arabidopsis phospholipase D family. Plant Physiol 128:1057–1068
Qin W, Pappan K, Wang X (1997) Molecular heterogeneity of phospholipase D (PLD). Cloning of PLDgamma and regulation of plant PLDgamma, -beta, and -alpha by polyphosphoinositides and calcium. J Biol Chem 272:28267–28273
Qin C, Li M, Qin W, Bahn SC, Wang C, Wang X (2006) Expression and characterization of Arabidopsis phospholipase Dγ2. Biochim Biophys Acta 1761:1450–1458
Rizo J, Sudhof TC (1998) C2-domain, structure and function of a universal Ca2+-binding domain. J Biol Chem 273:15879–15882
Schmutz J, Cannon SB, Jackson SA et al (2010) Genome sequence of the palaeopolyploid soybean. Nature 463:178–183
Shen P, Wang R, Jing W, Zhang W (2011) Rice phospholipase Dα is involved in salt tolerance by the mediation of H+-ATPase activity and transcription. J Integr Plant Biol 53:289–299
Shultz JL, Kurunam D et al (2006) The soybean genome database (SoyGD): a browser for display of duplicated, polyploid, regions and sequence tagged sites on the integrated physical and genetic maps of Glycine max. Nucleic Acids Res 34:D758–D765
Testerink C, Munnik T (2005) Phosphatidic acid: a multifunctional stress signaling lipid in plants. Trends Plant Sci 10:368–375
Ueki J, Morioka S, Komari T, Kumashiro T (1995) Purification and characterization of phospholipase D (PLD) from (Oryza sativa L.) and cloning of cDNA for PLD from rice and maize (Zea mays L.). Plant Cell Physiol 36:903–914
Wang X (2005) Regulatory functions of phospholipase D and phosphatidic acid in plant growth, development, and stress responses. Plant Physiol 139:566–573
Wang X, Dyer JH, Zheng L (1993) Purification and immunological analysis of phospholipase D from castor bean endosperm. Arch Biochem Biophys 306:486–494
Wang X, Xu L, Zheng L (1994) Cloning and expression of phosphatidylcholine-hydrolyzing phospholipase D from Ricinus communis L. J Biol Chem 269:20312–20317
Wang C, Zien CA, Afitlhile M, Welti R, Hildebrand DF, Wang X (2000) Involvement of phospholipase D in wound-induced accumulation of jasmonic acid in Arabidopsis. Plant Cell 12:2237–2246
Whitaker BD, Smith DL, Green KC (2001) Cloning, characterization and functional expression of a phospholipase D alpha cDNA from tomato fruit. Physiol Plant 112:87–94
Yamaguchi T, Kuroda M, Yamakawa H, Ashizawa T, Hirayae K, Kurimoto L, Shinya T, Shibuya N (2009) Suppression of a phospholipase D gene, OsPLDβ1, activates defense responses and increases disease resistance in rice. Plant Physiol 150:308–319
Yu L, Nie J, Cao C, Jin Y, Yan M, Wang F, Liu J, Xiao Y, Liang Y, Zhang W (2010) Phosphatidic acid mediates salt stress response by regulation of MPK6 in Arabidopsis thaliana. New Phytol 188:762–773
Zhang W, Wang C, Qin C, Wood T, Olafsdottir G, Welti R, Wang X (2003) The oleate-stimulated phospholipase D, PLDδ, and phosphatidic acid decrease H2O2-induced cell death in Arabidopsis. Plant Cell 15:2285–2295
Zhang W, Qin C, Zhao J, Wang X (2004) Phospholipase Dα1-derived phosphatidic acid interacts with ABI1 phosphatase 2C and regulates abscisic acid signaling. Proc Natl Acad Sci USA 101:9508–9513
Zhang W, Wan X, Hong Y, Li W, Wang X (2010) Plant Phospholipase D. In: Munnik T (ed) Lipid signaling in plants. Plant Cell Monographs, vol 16. Springer, Berlin, pp 39–62
Zhao J, Wang X (2004) Arabidopsis phospholipase Dα1 interacts with the heterotrimeric G-protein α-subunit through a motif analogous to the DRY motif in G-protein-coupled receptors. J Biol Chem 279:1794–1800
Zheng L, Krishnamoorthi R, Zockievoshi M, Wang X (2001) Distinct Ca2+ binding properties of novel C2 domains of plant phospholipase Dα and β. J Biol Chem 275:19700–19706
Acknowledgment
The work is supported by grants from Ministry of Science and Technology in China, Ministry of Education in China (KYT201001), and Jiangsu province (200910 and PAPD) to W. Zhang.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhao, J., Zhou, D., Zhang, Q. et al. Genomic analysis of phospholipase D family and characterization of GmPLDαs in soybean (Glycine max). J Plant Res 125, 569–578 (2012). https://doi.org/10.1007/s10265-011-0468-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10265-011-0468-0