Abstract
It is widely believed that plastid and mitochondrial genomes are inherited through the maternal parent. In plants, however, paternal transmission of these genomes is frequently observed, especially for the plastid genome. A male gametic trait, called potential biparental plastid inheritance (PBPI), occurs in up to 20% of angiosperm genera, implying a strong tendency for plastid transmission from the male lineage. Why do plants receive organelles from the male parents? Are there clues in plastids that will help to elucidate the evolution of plants? Reconstruction of the ancestral state of plastid inheritance patterns in a phylogenetic context provides insights into these questions. In particular, a recent report demonstrated the unilateral occurrence of PBPI in angiosperms. This result implies that nuclear cytoplasmic conflicts, a basic driving force for altering the mode of organelle inheritance, might have arisen specifically in angiosperms. Based on existing evidence, it is likely that biparental inheritance may have occurred to rescue angiosperm species with defective plastids.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Soon after the establishment of the Mendelian laws of inheritance, it was reported that traits in plant leaves may be inherited in a non-Mendelian fashion. The first reported example involved reciprocal crosses between green and variegated plants of Pelargonium zonale (Baur 1909). The F1 progeny comprised various proportions of individuals with green, white and variegated leaves. The leaf colors of both parents were transmitted to the progeny, although the ratio of transmission was essentially unequal. This phenomenon is termed biparental inheritance. Another example was reciprocal crosses between green and variegated plants of Mirabilis jalapa (Correns 1909). In this case, however, leaf color of the progeny always followed that of the female parent, indicating that the genetic trait was inherited only from the maternal parent. This pattern is therefore termed maternal inheritance. It is well established that the plastid genome exhibits non-Mendelian inheritance phenomena and, in a majority of angiosperm species, the genome exhibits maternal inheritance.
The mitochondrial genome is another independent carrier of genetic traits in eukaryotic cells. It is notable that inheritance of this genome in animals is consistently maternal. Exceptions showing paternal transmission have not been observed so far. However, like the biparental plastid inheritance found in plants, transmission of the mitochondrial genome through the male lineage is also observed in angiosperms. Examples include Musa acuminata (Fauré et al. 1994), Cucumis sativus and C. melo (Havey 1997; Havey et al. 1998). It appears the mode of organelle inheritance is less uniform in plants. In fact, based on the presence of detectable plastid DNA in the male gametic cell—a cytological trait called potential biparental plastid inheritance (PBPI, to be discussed below)—it is indicated that about 20% of angiosperms exhibit the potential for paternal plastid transmission (Corriveau and Coleman 1988; Zhang et al. 2003). The remaining 80% display maternal inheritance. Clearly, biparental plastid inheritance is a reasonably widespread trait in angiosperms. For mitochondria, the examples given above indicate paternal transmission in angiosperms, although this mode is much less frequently observed than in plastids (Mogensen 1996).
It is clear that a diversity of non-Mendelian inheritance exists in plants, in contrast to the uniform maternal inheritance found in animals. Unfortunately, however, the biological significance of paternal transmission in plants remains unknown. How does paternal transmission benefit plants? In this article, we discuss this question from a plant evolutionary perspective. Several recent reports suggest that biparental inheritance is derived in the evolution of angiosperms and this mode of plastid inheritance may have rescued angiosperms with defective nuclear cytoplasmic conflicts.
PBPI is an indicator for biparental plastid inheritance
The molecular mechanism that regulates plastid inheritance is unknown. However, it is clear that plastids are excluded from the male gametic cells of angiosperms displaying maternal plastid inheritance (Hagemann and Schröder 1989). Therefore, a common cytological tag for maternal inheritance is that the generative or sperm cells are free of plastids, and accordingly lack plastid DNA. Miyamura et al. (1987) first applied fluorescence microscopy to inspect male gametic plastid DNA. They successfully detected signals of plastid DNA in the male gametic cells of species known to display biparental inheritance, and verified that no such signals were detected in the male gametic cells of species exhibiting maternal inheritance (Fig. 1). This provides an easy and rapid technique for distinguishing between maternal and biparental plastid inheritance and subsequently the method has been widely used (Corriveau and Coleman 1988; Nagata et al. 1999; Zhang et al. 2003).
Modes of plastid inheritance can be detected with fluorescence microscopy. Sperm cells of Mirabilis jalapa [a species used by Correns (1909) to discover maternal inheritance], Pelargonium zonale [a species used by Baur (1909) in the discovery of biparental inheritance] and Linnaea borealis [a species we selected to show very weak potential biparental plastid inheritance (PBPI)] were stained with DAPI and examined with a fluorescence microscope. Small dotted fluorescence signals of plastid DNA (arrows) occur in cells of P. zonale and L. borealis, indicating PBPI for these species. The cells of M. jalapa lack such signals. Note that the signal intensity can be distinct between species with PBPI. It is obvious that P. zonale has a much stronger PBPI than L. borealis. Images are produced at the same magnification. SN Sperm nucleus. Bar 20 μm
It is apparent that the presence of plastid DNA in male gametic cells is a prerequisite for paternal transmission. This phenomenon is called potential biparental plastid inheritance (PBPI). Experimentally, all species exhibiting biparental plastid inheritance in genetic analyses show PBPI (Corriveau and Coleman 1988; Kuroiwa 1991). Conversely, however, in very few cases, plants with PBPI may not be identified genetically as showing biparental plastid inheritance (Corriveau and Coleman 1988). We predict this might be because PBPI is sometimes weak (Fig. 1). Paternal transmission cannot be easily traced when a very small proportion of male plastid DNA is contributed. Therefore, PBPI is a cellular indicator, and is possibly a more sensitive and accurate indicator than genetic analysis for biparental plastid inheritance.
Biparental inheritance is a primordial form of organelle inheritance
When two cells fuse, mixing of the cytoplasm occurs automatically in the daughter cell. It is not difficult to imagine that biparental inheritance would take place in this case if cytoplasm from either parent is not excluded after fusion. For this reason, biparental inheritance is believed to be the primitive form of organelle inheritance. The earliest sexual reproduction is presumed to have arisen with simple cell fusion and accompanied the origin of life (Sterrer 2002; Xu 2004). Early eukaryotic cells possessing endosymbionts (plastids) must have undergone such a fusion for sexual reproduction. It can thus be presumed that, during early sex, the daughter cell received endosymbionts from both parents. A primitive type of biparental inheritance, therefore, should be represented by organelle transmission in the earliest eukaryotic cells (see Birky 1995).
The majority of advanced eukaryotic organisms (e.g., animals and plants), however, exhibit maternal inheritance. This implies that the primitive biparental mode has been converted to a maternal mode during eukaryotic evolution. Before discussing biparental plastid inheritance in angiosperms, it is important to consider how primitive biparental inheritance was modified. We believe uniparental inheritance was required for the endosymbiotic origin of eukaryotic cells. An established event, i.e., the transfer of substantial genetic material from the endosymbiont to the host nuclear genome (Dyall et al. 2004), may hold the secret. Before the transfer, the host and the endosymbiont may have possessed genomes of equal size. The transfer enriched the host nuclear genome and decreased the endosymbiont genome size, contributing to the stability of the endosymbiont. This is elementary to evolution because host control is essential in eukaryotic cells. The primitive biparental inheritance existing at this time, however, enriched the endosymbiont genome by contributing different genomes to the daughter cell. This would impair host control and cell stability. We therefore believe that a decline in primitive biparental inheritance must be a prerequisite for eukaryotic origin and evolution (Fig. 2). Accordingly, a uniparental mode of plastid inheritance should have arisen as early as the late stage of endosymbiosis, and nuclear cytoplasm conflicts could have been the driving force for the occurrence of uniparental inheritance. Similarly, we postulate that the origin and evolution of maternal mitochondrial inheritance may have undergone the same process in the common ancestor of plants and animals at an earlier period.
Uniparental inheritance must have arisen during the endosymbiotic origin and evolution of eukaryotic cells. a Following the capture of a cyanobacterium, transfer of genetic material from the cyanobacterium to the host genome occurs to establish host control. This achieves cell stability to ensure neither loss nor overproliferation of the endosymbiont. b Biparental inheritance during the early evolution of sex contributes to enrichment of the endosymbiont (early chloroplast) genome, which is destructive to the established host control. c Uniparental inheritance arises by an unknown mechanism that reduces the endosymbiont (early chloroplast) genome of one parent. To illustrate genetic variation, the genomes of cell nuclei and early chloroplasts are indicated in blue, pink, and purple
Biparental inheritance is derived in angiosperms
Consistent with the above hypothesis, the earliest plants must have displayed uniparental inheritance. This is supported by a unicellular green alga, Chlamydomonas reinhardii, in which uniparental inheritance is observed for both plastid and mitochondrial genomes (Kuroiwa et al. 1982; Nishimura et al. 1999). The sporophytic plants subsequently examined, e.g., the liverwort Pellia, a representative of the earliest land plants (Pacak and Szweykowska-Kulinska 2003), a bryophyte (Natcheva and Cronberg 2007) and two pteridophytes (Gastony and Yatskievych 1992; Guillon and Raquin 2000), all exhibit uniparental (maternal) plastid inheritance. These findings support the idea that uniparental plastid inheritance arose and became predominant during early plant evolution. Accordingly, the widespread biparental plastid inheritance observed in angiosperms may be a derived trait that arose independently in later plant evolution.
Evidence for the reoccurrence of biparental plastid inheritance in angiosperms was first presented by Birky (1995). He overlaid plants with a known mode of plastid inheritance onto a plant phylogenetic tree. The results indicated the evolution of biparental inheritance from lineages possessing maternal inheritance. However, a problem with that study was the difficulty of relying on genetic data of plastid inheritance to classify a species as having a maternal or biparental inheritance mode. It is known that organelle inheritance is a quantitative trait. Possession of biparental inheritance does not mean an equal proportion of plastids are received from the two parents, nor does maternal inheritance mean that plastid transmission from the male parent never occurs. For instance, in Chlorophytum comosum, a species known to display biparental inheritance, paternal plastids are inherited at a rate of 2–8% (Collins 1922; Pandey and Blaydes 1957). In Nicotiana tabacum, a representative of species with maternal inheritance, 0.07 and 2.5% of the offspring inherit paternal plastids in interspecific and intraspecific crosses, respectively (Medgyesy et al. 1986). In fact, Birky’s study misclassified Antirrhinum, Borago, Petunia and Nicotiana, for example, as genera with biparental inheritance. This leads to the conclusion that plastid inheritance has alternated frequently between maternal and biparental modes during angiosperm evolution (see Birky 1995).
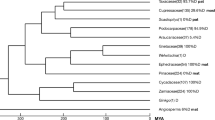
A recent study using PBPI as the indicator for biparental plastid inheritance resolved this problem and clarified the derivation of biparental plastid inheritance in angiosperms (Hu et al. 2008). The authors traced divergence in the modes of plastid inheritance in the traditionally delimited Caprifoliaceae and identified a switching point at which PBPI occurs in the early evolution of this family (Fig. 3). This provides direct evidence for the occurrence of biparental plastid inheritance in angiosperms. A similar case was reported by the author’s group in a study of Oleaceae (Liu et al. 2004). In order to obtain a common pattern for the reoccurrence of PBPI, Hu et al. (2008) implemented an extended analysis by overlaying 206 angiosperm genera with known (potential) mode of plastid inheritance onto the phylogenetic reconstruction of the Angiosperm Phylogeny Group (2003). Twenty-five switching points at which clades or branches with PBPI arose were discovered. However, differing largely from the suggestion by Birky (1995), all PBPI is indicated to be derived from maternal inheritance and appears as terminal clades or branches. The suggested change from biparental to maternal modes has not been observed. This implies that biparental plastid inheritance in angiosperms was derived unilaterally from the maternal mode during advanced angiosperm evolution.
Biparental plastid inheritance is derived in angiosperms. The example shows the order Dipsacales. Thin branches Genera, thick branches clades. Branches highlighted by the yellow square comprise genera of the Caprifoliaceae as traditionally delimited. Branches possessing maternal and (potential) biparental modes of plastid inheritance are indicated in green and red, respectively. See Hu et al. (2008) for further details
Biparental inheritance may rescue plants with defective plastid genome
If biparental plastid inheritance is the primitive form of organelle inheritance, why has it arisen independently in angiosperms? This question has puzzled us for a long time. A recent genetic study using Pisum sativum, a species displaying PBPI (see Corriveau and Coleman 1988), provides a clue to answer this question. When plants with deficient chlorophyll pigmentation caused by defective plastids are pollinated with wild-type pollen, a small proportion of green plants appears in the F1 progeny (Bogdanova 2007). It was further demonstrated that the green plants inherit normal plastids from the wild-type pollen, and these plastids restore the wild-type phenotype. Another study on Oenothera, a genus containing species all displaying PBPI (see Corriveau and Coleman 1988), yielded similar results (Chiu and Sears 1993). In both of these cases, transmission of normal plastids through the paternal lineage acts to rescue plants with defective plastid genomes.
It is known that organelle DNA is more prone to damage than nuclear DNA (Mambo et al. 2003). Serious mutations in the plastid genome may cause nuclear cytoplasmic conflict (often termed nuclear-plastid incompatibility in the case of higher plants), which is the most important driving force for altering the mode of organelle inheritance (Hurst et al. 1996). We hypothesize that events similar to those described above may have arisen late in the evolution of angiosperms. If this is the case, occurrence of biparental plastid inheritance may have occurred as a critical guarantee for the survival and radiation of ancestral species with defective plastids.
It is obvious that biparental plastid inheritance in angiosperms re-enriches the genetic variability of the plastid genome. However, this may not impair cell stability in higher plants because the plastid genome, after the transfer of genes from the cyanobacterial genome into the cell nucleus, is so small in higher plants that more than 5,000 nucleus-encoded gene products have to be reimported into the plastid to support its function (Martin and Herrmann 1998; Peltier et al. 2000). As plastids became reliant on the cell nucleus, selection for cell stability may have been relaxed in more-advanced angiosperms, enabling biparental inheritance to arise under an opposing selective force.
Perspectives
Biparental plastid inheritance is widespread in angiosperms. Such a biparental mode, however, is not observed in the mitochondria of both plants and animals. This implies a peculiarity of angiosperm plastids. The unilateral occurrence as indicated by Hu et al. (2008) implies that evolutionary pressure may be simple and straightforward. This is a novel idea largely different from that of Birky (1995). Based on existing evidence, we suggest that biparental plastid inheritance may have been derived to permit rescue of angiosperms with nuclear-plastid incompatibility caused by defective plastids. However, further experimental studies are required to test our hypothesis. For instance, verification that the plastid genome of angiosperms has been exposed to massive mutations in the past would provide convincing support. Furthermore, with reference to Wikström et al. (2001), PBPI among the 206 genera analyzed by Hu et al. (2008) may be estimated to have arisen at an increasing rate during the middle and late periods of angiosperm evolution from approximately 95 million to 5 million years ago (Fig. 4). An investigation into the paleoenvironmental conditions on Earth and the interaction between plant evolution and atmospheric fluctuations during this period is needed to identify any factors that might have induced plastid mutations.
PBPI is likely to have occurred at an increasing frequency during the middle and late periods of angiosperm evolution. From Wikström et al. (2001), we estimated the approximate time of divergence for the 206 genera analyzed by Hu et al. (2008). The bars show the relative occurrence of maternal inheritance (grey) and PBPI (black) during different periods of angiosperm evolution. The number of total/PBPI genera that occurred during each period is shown above the bars. The left-hand bar includes the early-diverging extant angiosperms Nymphaea, Illicium, Chloranthus, Aristolochia, Chimonanthus, Ceratophyllum, Nelumbo, Buxus, and Paeonia
It is well known that plastid and mitochondrial mRNAs are frequently modified in plants by RNA editing (Maier et al. 1996; Bock 2000), and that lack of editing leads to defective phenotypes (Bock et al. 1994; Schmitz-Linneweber et al. 2005). Might the editing sites correspond to basal mutations that occur in organelle genomes? If so, both biparental inheritance and RNA editing might be mechanisms to address organelle defects. An interesting phenomenon is that the mitochondrial genome possesses many more editing sites than the plastid genome (Maier et al. 1996). Could it be possible that the RNA editing mechanisms restore functioning of the mitochondrial genome, whereas the mechanisms in plastids are insufficient to cover all basal mutations, allowing biparental plastid inheritance to occur? In addition, RNA editing is indicated to be ubiquitous in land plants (more so in angiosperms than gymnosperms) but absent in algae and cyanobacteria (Freyer et al. 1997). Might there be atmospheric factors that have induced mutations in organelle genomes? We believe all these questions are relevant and require further investigation to elucidate the evolution of biparental plastid inheritance in plants.
References
Angiosperm Phylogeny Group (2003) An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG II. Bot J Linn Soc 141:399–436
Baur E (1909) Das Wesen und die Erblichkeitsverhältnisse der ‘arietates albomarginatae hort’ von Pelargonium zonale. Z Indukt Abstammungs-Vererbungsl 1:330–351
Birky CW Jr (1995) Uniparental inheritance of mitochondrial and chloroplast genes: mechanisms and evolution. Proc Natl Acad Sci USA 92:11331–11338
Bock R (2000) Sense from nonsense: how the genetic information of chloroplasts is altered by RNA editing. Biochimie 82:549–557
Bock R, Kossel H, Maliga P (1994) Introduction of a heterologous editing site into the tobacco plastid genome: the lack of RNA editing leads to a mutant phenotype. EMBO J 13:4623–4628
Bogdanova VS (2007) Inheritance of organelle DNA markers in a pea cross associated with nuclear-cytoplasmic incompatibility. Theor Appl Genet 114:333–339
Chiu WL, Sears BB (1993) Plastome–genome interactions affect plastid transmission in Oenothera. Genetics 133:989–997
Collins EJ (1922) Variegation and its inheritance in Chlorophytum elatum and C. comosum. J Genet 12:1–17
Correns C (1909) Vererbungsversuche mit blass(gelb)grünen und buntblättrigen sippen bei Mirabilis jalapa, Urtica pilulifera und Lunaria annua. Z Indukt Abstammungs-Vererbungsl 1:291–329
Corriveau JL, Coleman AW (1988) Rapid screening method to detect potential biparental inheritance of plastid DNA and results for over 200 angiosperms. Am J Bot 75:1443–1458
Dyall SD, Brown MT, Johnson PJ (2004) Ancient invasions: from endosymbionts to organelles. Science 304:253–257
Fauré S, Noyer JL, Carreel F, Horry JP, Bakry F, Lanaud C (1994) Maternal inheritance of chloroplast genome and paternal inheritance of mitochondria genome in bananas (Musa acuminate). Curr Genet 25:265–269
Freyer R, Kiefer-Meyer MC, Kossel H (1997) Occurrence of plastid RNA editing in all major lineages of land plants. Proc Natl Acad Sci USA 94:6285–6290
Gastony GJ, Yatskievych G (1992) Maternal inheritance of the chloroplast and mitochondrial genomes in cheilanthoid ferns. Am J Bot 79:716–722
Guillon JM, Raquin C (2000) Maternal inheritance of chloroplasts in the horsetail Equisetum variegatum (Schleich.). Curr Genet 37:53–56
Hagemann R, Schröder MB (1989) The cytological basis of the plastid inheritance in angiosperms. Protoplasma 152:57–64
Havey MJ (1997) Predominant paternal transmission of the mitochondrial genome in cucumber. J Hered 88:232–235
Havey MJ, McCreight J, Rhodes B, Taurick G (1998) Differential transmission of the cucurbit organellar genomes. Theor Appl Genet 97:122–128
Hu YC, Zhang Q, Rao GY, Sodmergen (2008) Occurrence of plastids in the sperm cells of Caprifoliaceae: biparental plastid inheritance in angiosperms is unilaterally derived from maternal inheritance. Plant Cell Physiol 49:958–968
Hurst LD, Atlan A, Bengtsson BO (1996) Genetic conflicts. Q Rev Biol 71:317–364
Kuroiwa T (1991) The replication, differentiation, and inheritance of plastids with emphasis on the concept of organelle nuclei. Int Rev Cytol 128:1–62
Kuroiwa T, Kawano S, Nishibayashi S, Sato C (1982) Epifluorescent microscopic evidence for maternal inheritance of chloroplast DNA. Nature 198:481–483
Liu Y, Cui HX, Zhang Q, Sodmergen (2004) Divergent potentials for cytoplasmic inheritance within the genus Syringa. a new trait associated with speciogenesis. Plant Physiol 136:2762–2770
Maier RM, Zeltz P, Kossel H, Bonnard G, Gualberto JM, Grienenberger JM (1996) RNA editing in plant mitochondria and chloroplasts. Plant Mol Biol 32:343–365
Mambo E, Gao X, Cohen Y, Guo Z, Talalay P, Sidransky D (2003) Electrophile and oxidant damage of mitochondrial DNA leading to rapid evolution of homoplasmic mutations. Proc Natl Acad Sci USA 100:1838–1843
Martin W, Herrmann RG (1998) Gene transfer from organelles to the nucleus: how much, what happens, and why? Plant Physiol 118:9–17
Medgyesy P, Pay A, Marton L (1986) Transmission of paternal chloroplasts in Nicotiana. Mol Gen Genet 204:195–198
Miyamura S, Nagata T, Kuroiwa T (1987) Quantitative fluorescence microscopy on dynamic changes of plastid nucleoids during wheat development. Protoplasma 133:66–72
Mogensen HL (1996) The hows and whys of cytoplasmic inheritance in seed plants. Am J Bot 83:383–404
Nagata N, Saito C, Sakai A, Kuroiwa H, Kuroiwa T (1999) The selective increase or decrease of organellar DNA in generative cells just after pollen mitosis one controls cytoplasmic inheritance. Planta 209:53–65
Natcheva R, Cronberg N (2007) Maternal transmission of cytoplasmic DNA in interspecific hybrids of peat mosses, Sphagnum (Bryophyta). J Evol Biol 20:1613–1616
Nishimura Y, Misumi O, Matsunaga S, Higashiyama T, Yokota A, Kuroiwa T (1999) The active digestion of uniparental chloroplast DNA in a single zygote of Chlamydomonas reinhardtii is revealed by using the optical tweezer. Proc Natl Acad Sci USA 96:12577–12582
Pacak A, Szweykowska-Kulińska Z (2003) Organellar inheritance in liverworts: an example of Pellia borealis. J Mol Evol 56:11–17
Pandey KK, Blaydes GW (1957) Cytoplasmic inheritance of plastids in Impatiens sultanii F., Petunia violacea Lindl. and Chlorophytum elatum. Ohio J Sci 57:135–147
Peltier J, Friso G, Kalume D, Roepstorff P, Nilsson F, Adamska I, van Wijk K (2000) Proteomics of the chloroplast: systematic identification and targeting analysis of lumenal and peripheral thylakoid proteins. Plant Cell 12:319–341
Schmitz-Linneweber C, Kushnir S, Babiychuk E, Poltnigg P, Herrmann RG, Maier RM (2005) Pigment deficiency in nightshade/tobacco cybrids is caused by the failure to edit the plastid ATPase a-subunit mRNA. Plant Cell 17:1815–1828
Sterrer W (2002) On the origin of sex as vaccination. J Theor Biol 216:387–396
Wikström N, Savolainen V, Chase MW (2001) Evolution of the angiosperms: calibrating the family tree. Proc R Soc Lond B Biol Sci 268:2211–2220
Xu JP (2004) The prevalence and evolution of sex in microorganisms. Genome 47:775–780
Zhang Q, Liu Y, Sodmergen (2003) Examination of the cytoplasmic DNA in male reproductive cells to determine the potential for cytoplasmic inheritance in 295 angiosperm species. Plant Cell Physiol 44:941–951
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Creative Research Group Program, no. 30421004; Key Program, no. 30430040) and the National Basic Research Program of China (Program 973, no. 2007CB108700).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, Q., Sodmergen Why does biparental plastid inheritance revive in angiosperms?. J Plant Res 123, 201–206 (2010). https://doi.org/10.1007/s10265-009-0291-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10265-009-0291-z