Abstract
Primula nutans Georgi is widely distributed in hummock-and-hollow wetlands on the Qinghai-Tibetan Plateau. To assess the ecophysiology of this species in responding to microenvironments, we examined the photosynthetic characteristics and individual carbon gain of plants growing in different microsites from a hummock-and-hollow wetland on the Qinghai-Tibetan Plateau and under laboratory conditions. Plants from wetland hummock microsites showed significantly higher light-saturated photosynthetic CO2 uptake (A max) than those from microsites in hollows at a controlled temperature of 15°C in leaf chamber. Leaf dark respiration rate (R) was only significantly higher in plants from hummocks than hollows at the measuring temperature of 35°C. Optimum temperature for A max was 15°C for all plants in the field despite different microsites. In plants growing under laboratory conditions differing in light and temperature, both A max and R were significantly higher under higher growth light (photosynthetic photon flux density, PPFD: 800 or 400 μmol m−2 s−1) than low light of 90 μmol m−2 s−1. No statistically significant differences in A max and R existed in plants differing in growing temperatures. Estimates derived from the photosynthetic parameters of field plants, and microsite environmental measures including PPFD, air temperature and soil temperature showed that the optimum mean daily temperature for net daily carbon gain was around 10°C and the net daily carbon gain was largely limited under lower daily total PPFD. These results suggest that the differences in A max and R in P. nutans are strongly affected by growing light regimes but not by temperature regimes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Environments for plant growth such as light and temperature can be highly heterogeneous in space and in time. The influence of spatial heterogeneity of environment on plant performance can occur at very small scales, e.g., at centimeter or even smaller scales (e.g., Lechowicz and Bell 1991; Silvertown 1981; Pearcy 1983). To cope with a spatially heterogeneous environment, a plant, being not able to relocate itself, relays often on its morphological plasticity and physiological adjustment (e.g., Givnish 1988; Pearcy and Sims 1994). The spatial variations in microsite light and temperature regimes are therefore expected to result in the changes of physiological response, morphology, growth, and then distribution of individual plants. Bazzaz (1979) suggests that the distribution of plant species reflects the capacities of individuals of a plant species to maintain function under diverse environmental conditions. An important implication of this principle is thus that patterns of individual physiological response to environment would be a major element in the realized ecological breadth of species (e.g., Field 1988; Chazdon et al. 1996; Sultan et al. 1998).
Photosynthetic performance is one of the most critical aspects of physiological function for plants to respond to micro-environmental changes. Many studies have examined photosynthetic response by focusing on a single limiting resource, e.g., on light resource in most cases in order to explain the growth performance and abundance distribution of individual plants in different microsites (e.g., Chapin 1991; Pearcy and Sims 1994; Nicotra et al. 1997; Germino and Smith 2001). Most of these studies demonstrated that photosynthetic acclimation to light regimes play an important role for plant’s growth and survive in microsites with different light availability. In alpine environments, low temperature and high radiation are often the important factors affecting leaf photosynthesis in responding to microsite environments (Germino and Smith 2001; Cui et al. 2003, 2004). There is, however, little detailed information on how alpine plants adapt physiologically to their microsite environments, which are often found within a harsh macro environment, in particular in terms of strong radiation and low temperature (Körner 2003).
Primula nutans Georgi is found in a wide range of micro-habitats in wetlands on the Qinghai-Tibetan Plateau, which is the highest plateau with extremely sever physical environments in the world. These wetlands are characterized by hummock-and-hollow microtopography (Shen et al. 2006). Our previous studies have revealed how the species would change its individual distribution, morphology, and growth in response to the micro-environmental variation (Shen et al. 2006). In these studies, we found that both photosynthetic photon flux density (PPFD) and air temperature vary considerably in the grassland microsites of P. nutans. We do not know, however, what are the consequences of these environmental variations to the growth and distribution of P. nutans. To explain the mechanisms that underlie the growth and distribution response of P. nutans to its microsite, we need to understand its ecophysiological responses, in particular the photosynthetic responses, to its micro-environmental changes.
There is no detailed information available on the photosynthetic characteristics of P. nutans. Even for the genus Primula there are only a few reported studies dealing with photosynthesis (Whale 1983; Combe et al. 1993; Noda et al. 2004, 2007). Whale (1983) reported that the maximum photosynthetic rate of three Primula species declined steadily as the growing season advanced; this suggests the importance of early spring in the carbon economy of these species. Noda et al. (2004, 2007) found that photosynthetic and respiration characteristics of Primula sieboldii are highly dependant on environmental temperature. Knowledge about the characteristics of CO2 exchange in response to changes in light and temperature conditions may provide insights into explaining the distribution of this species over different micro-environments.
Our aims were therefore (1) to clarify the photosynthetic characteristics of P. nutans plants in the field and the laboratory in response to contrasting light and temperature conditions; and (2) to evaluate the potential influences of changes in temperature and light regimes on the carbon economics of this species.
Materials and methods
Plant materials and study site
Plant material. Primula nutans Georgi, a perennial herb, has a circumpolar distribution from North America to Europe and Asia (Richards 2003). The annual ramet of P. nutans expands their leaves in early spring. On the Qinghai-Tibetan Plateau, the above-ground parts of P. nutans emerge in late April, and the leaves fully expand in early May. Flowering occurs immediately after leaf expansion in early June. The rosette-form ramet has two to five leaves. The leaves fall off in September, and the parent ramet dies back; only the below-ground gemmae survive from autumn through the winter to produce new ramets at the start of the following growing season. Our previous study showed high morphological plasticity of the species in response to hummocky environments in an alpine wetland, e.g., individual ramets had large leaves and high peduncle growing in the hollow microsite (Shen et al. 2006).
Study site. Wetlands are widely distributed on the Qinghai-Tibetan Plateau, covering more than 5% of the total land area. We conducted our investigation in a typical alpine wetland, the Luanhaizi wetland (lat 37°36′N, long 101°21′E, 3,250 m a.s.l.), at the northeastern edge of the plateau. The wetland lies on the northwestern side of a small lake, where the ground tend to have hummocky microtopography, a typical spatially heterogeneous topography featuring elevated mounds (hummocks) alternating with flatter hollows. Hummock microsites are likely to have higher light availability, higher temperature, and lower soil moisture than hollow microsites. The relatively sparse vegetation is dominated by Carex allivescens and Kobresia tibetica. P. nutans is the most abundant of the broadleaved species in the wetland. [See Shen et al. (2006) for other detailed descriptions of the site.]
Environmental measurements
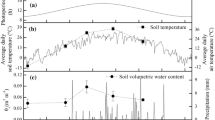
In early spring 2004 we randomly established four quadrats (each 1×1 m, including both hummock and hollow microsites) in the wetland to permit long-term measurement of environmental parameters. In each quadrat, we installed two quantum sensors (GaAsP Photodiode G1118, Hamamatsu Ltd, Hamamatsu, Japan) 5 cm above the ground surface, and one temperature sensor (a copper-constantan thermocouple) at a depth of 5 cm in the soil of the hummock and hollow microsites, respectively. We also set one temperature sensor 5 cm above the vegetation surface to measure air temperature. All quantum sensors were calibrated under sunlight and artificial shade against a standard quantum sensor (Li-Cor Model 190S, Li-Cor, Lincoln, NE, USA; Tang and Washitani 1995). We recorded PPFD and soil temperatures at 1-min intervals using a datalogger (Thermic 2300A, Eto Denki Ltd, Tokyo, Japan), during the period from 1 June to 31 August 2004.
Measurement of photosynthesis of plants from the field
We obtained light–photosynthesis curves to examine the characteristics of CO2 exchange of P. nutans. The sampled plants were growing in the hummocky wetland. All the measurements were made on fully expanded intact leaves. Measurements were made in August 2004, when newly expanded leaves had reached their maximum leaf area on the individual ramets on the hummocks and in the hollows; we used a portable gas-exchange measurement system equipped with an Arabidopsis chamber (LI-6400, Li-Cor, Inc. Lincoln, Nebraska, USA). Since the leaves of P. nutans grow almost prostrate on the ground surface, we dug up the ramets with sufficient soil so that plants could survive in a good state during the measurement and took them, in pots, to the laboratory at Haibei Research Station, Chinese Academy of Sciences, about 3 km from the study site. The measurement was conducted immediately after taking the plants to the laboratory. We measured the CO2 gas exchange of three or four plants from each of the two contrasting microtopographies: hummocks and hollows under a range of PPFD values from 0 to 2,600 μmol m−2 s−1 and at four temperatures: 7, 15, 25, and 35°C in the sample chamber. During these measurements, we maintained the CO2 concentration at 400 μmol mol−1 and the relative humidity between 60 and 70% by adjusting entering air through a water or desiccant. We illuminated the leaves with an artificial metal halide light source (LA-180Me, Hayashi, Korin Electronic Co., Ltd, Hong Kong, China) at each light level for at least 20 min before recording the photosynthetic rate. We fitted the photosynthesis–PPFD curves according to the equation of Thornley (1976), as below:
where A is the net CO2 assimilation rate on a leaf area basis, I is the PPFD, R is the dark respiration rate that was measured after the photosynthesis measurement by darkening the chamber with black vinyl, α is the apparent quantum yield of the light–photosynthesis curve, GA max is the light-saturated rate of gross photosynthesis, and θ is the convexity factor.
Simulation of individual net carbon gain
To assess the effects of the photosynthetic characteristics of P. nutans on individual carbon gain, we conducted a simulation to estimate the net carbon gain of the plants under various microsite light and temperature environments. The data used for the simulation were: (1) the physiological parameters, including those for the light–photosynthesis response and temperature–respiration response of the leaves, which were obtained from plants taken from the field from hummock and hollow microsites, respectively; (2) the root respiration rate under different temperature conditions, which was estimated from the ratio of leaf dark respiration to root respiration of congeneric species Primula sieboldii derived from Noda et al. (2007) and from the leaf dark respiration rate obtained from our field plants; (3) the ratio of leaf to root obtained in our previous study (Shen et al. 2006); and (4) our measurement data of the temporal microsite variations in light and temperature.
To simulate a daily net carbon gain for an averaged individual plant of P. nutans, we (1) obtained the daily total carbon gain (GPP) by summing up instantaneous leaf photosynthesis that was calculated from measured diurnal course of PPFD and air temperature, and PPFD–photosynthesis curves measured under different temperatures; (2) calculated the leaf carbon loss (R leaf) from leaf dark respiration by assuming that daytime and nighttime respirations were the same function of temperature; (3) estimated the carbon loss due to root respiration (R root) from the mean root dry weight and soil temperature measured in the study, by using the respiration–temperature relationship from Noda et al. (2007) under the assumption that a mass-based respiration is the same in P. nutans and P. sieboldii. The daily net carbon gain was obtained by GPP − R leaf − R root. We ignored the shoot (stem) respiration because that the leaves were compacted within an extremely short shoot in P. nutans. The effects of daily mean temperature and PPFD on daily net carbon gain were examined for the period from June to August.
The ratio of below-ground to above-ground biomass is about 2.5 and similar in the two species. If we assume that root respiration rate is also similar in the two species and that both photosynthetic capacity and maintenance respiration rate per unit biomass do not significantly differ either among the species or between below-ground and above-ground biomass within the same species, the current simulation should not be effective for our discussion of P. nutans in response to different microsites.
Measurement of photosynthesis of plants in the laboratory
Growth conditions. At the end of the growing period in September 2004, we carefully harvested 40 ramets, including all below-ground plant mass. The ramets were kept in a refrigerator at about 5°C to maintain them in dormancy until March 2005. At the beginning to sprout, the ramets were removed from the refrigerator, and planted into plastic pots with vermiculite, 10.5 cm in diameter. The pots were placed in a growth cabinet (KG, Koito Corp., Tokyo, Japan) at the National Institute for Environmental Studies, Japan. Light was provided by 1,000-W microwave lamps and 400-W metal halide lamps (Toshiba Corp., Tokyo, Japan) with a 14-h photoperiod and three light regimes (800, 400, and 90 μmol m−2 s−1, respectively), and each light regime was divided into three temperature regimes (the day/night temperature is 27/15, 22/10, and 17/5°C, respectively). To avoid any influence due to sudden increase of light and temperature, we increased or reduced PPFD and temperature gradually within 3 h. Plants were watered daily and fertilized once a week with Magamp-K nutrient solution (Hyponex, Tokyo, Japan) diluted 1:1,000. Air humidity was maintained at around 70% and CO2 concentration at around 400 μmol mol−1.
Photosynthetic response to PPFD. To clarify the physiological characteristics of plants growing under various combinations of light and temperature conditions, we measured the light–photosynthetic curves for three or four plants under in situ temperature conditions at each growth temperature. Other details of the photosynthetic measurement procedures were the same as described in the previous section on measurement of photosynthesis of field plants.
Data analysis
All measurements were made three to six times using leaves from three or four plants. The results are presented as means (M) ± standard deviations (SD).
We used independent-sample t tests and KaleidaGraph software (Synergy Software, Inc., PA, USA) to assess the differences of photosynthetic parameters in plants between the hummocks and hollows. Analysis of variance (ANOVA) was conducted to test the effects of light and temperature regimes on the photosynthetic parameters of P. nutans by using the software program StatView (Abacus Concepts Inc., Berkeley, CA, USA).
Results
Gas exchange response of leaves in field plants
Photosynthetic rate was light saturated at a PPFD of about 300–500 μmol m−2 s−1 under a controlled temperature of 7 and 35°C, but it was saturated at a PPFD of around 1,000 μmol m−2 s−1 at the two other measurement temperatures for the ramets both from hummock and hollow microsites (Fig. 1). The light-saturated photosynthetic rate was the highest at the temperature of 15°C and decreased rapidly with decreasing or increasing the measurement temperatures for plants both from hummocks and hollows (Fig. 2a). The dark respiration rate of P. nutans leaves increased with increasing measurement temperature (Fig. 2b); it was about three times higher at 35°C than at 7°C in plants both from hummocks and hollows.
Photosynthetic rate at different air temperatures by leaves of Premula nutans growing on hummocks (open circle) and in hollows (filled circle), as a function of photosynthetic photon flux density (PPFD). Values represent means ± standard deviations of measurements from three or four individuals. Student’s t test was conducted to compare the photosynthetic rate between two microsites under each measuring light regime. The asterisk indicates significant differences between the contrasting microsites at P < 0.05
Light-saturated photosynthetic rate (A max, a) and dark respiration rate (R, b) as a function of air temperature in Premula nutans growing on hummocks (open circle) and in hollows (filled circle). Values represent means ± standard deviations of three or four samples. Student’s t-test results for significant differences between the contrasting microsites are shown as asterisks for P < 0.05
The maximum rate of photosynthetic was significantly higher in the leaves from the hummocks than from the hollows when the measurement temperature was set at 15 or 25°C (Fig. 2a). The leaf dark respiration rate (R) at low temperatures was rather similar for the plants obtained from the two contrasting microtopographies, but it was significantly higher (by about 30%) in the hummock plants than in the hollow plants at 35°C (Fig. 2b).
Gas exchange response of plants growing under laboratory conditions
The maximum photosynthetic rates (A max) of plants growing under the light regimes of 400 and 800 μmol m−2 s−1 in the growth chamber were similar, but significantly higher than in the plants raised under 90 μmol m−2 s−1 (Fig. 3). Under all light regimes, A max was the highest at the growth temperature of 17°C and tended to decrease at higher growing temperatures, in particular for the plants raised under the lowest light condition, but there is not statistical difference (Table 1).
Light-saturated photosynthetic rate (A max, a), dark respiration rate (R, b), light compensation point (LCP, c), and initial slope of light–photosynthesis curve (d) of Premula nutans growing under different light and temperature regimes (circle: high light, 800 μmol m−2 s−1; square: medium light, 400 μmol m−2 s−1; triangle: low light, 90 μmol m−2 s−1). Measurements were conducted under in situ growth temperature and light regimes. Values represent means ± standard deviations of three or four samples
Dark respiration rate (R) and light compensation point (LCP) showed similar trends under growth temperature/light conditions (Fig. 3). Both reached the highest at the medium temperature (22°C). The initial slope of light–photosynthesis curves was not significantly different among different light or temperature conditions (Fig. 3). Both the light and the temperature regimes had significant influences on R and LCP, while A max is only affected by light regime (Table 1).
Microsite light and temperature regimes and simulated CO2 uptake
Detailed continuous measurements of light and temperature at P. nutans microsites in the alpine wetland showed considerable spatial and temporal variation (Supplementary Fig. 1, Supplementary Fig. 2). The summed proportion of time in which PPFD exceeded 100 μmol m−2 s−1 during the day (from 6:00 to 21:00 in Beijing time) decreased from 80% in June on the hummocks to about 40% in August in the hollows (Supplementary Fig. 1). However, the mean and peak microsite temperature, including air temperature and soil temperature, increased from June to August both on the hummocks and in the hollows (Supplementary Fig. 2).
Using the microsite light and temperature regimes given above, we simulated the net carbon budget for individual plants on the basis of our measured photosynthetic responses in order to examine the physiological effects on plant carbon economics for both hummock and hollow microsite, separately. The diurnal course of net photosynthetic rate closely followed that of light change (Fig. 4). The net photosynthetic rate increased rapidly with the increase in light and air temperature during the morning and reached the highest values at around 9:00–13:00 (Beijing time) on clear day, but at around 15:00 on overcast day (Fig. 4). Air temperature peaked at around 15:00 on both cloudy and clear days.
Examples of diurnal changes in PPFD (a), temperature (b), and simulate a net carbon gain (c) on a typical hummock (HU, gray line) and in its neighboring hollow (HO, black line) on an overcast day (left column) and a clear day (right column). Air temperature (dotted line) represents the temperature about 5 cm above the surface of the vegetation, and soil temperature represents the temperature at a soil depth of 5 cm
The simulated daily net carbon budget was highly correlated with microsite daily total PPFD, but less related to microsite temperature (Fig. 5). Individual plants showed negative CO2 gain when microsite total PPFD was lower than about 10 mol m−2 day−1, and when the daily mean air temperature of microsites was less than 5°C or higher than 16°C. The optimum daily mean temperature for the maximum of the simulated daily net carbon gain was around 10°C (Fig. 5).
Simulated daily net carbon gain of individual ramets in response to different microsite light (a) and air temperature regimes (b) and their compound effect (c). CO2 uptake was calculated from microsite light and temperature data measured at 1-min intervals from June to August and from the photosynthetic parameters obtained under different light and temperature conditions of plants growing in the field. Root respiration was estimated from the leaf dark respiration rate in this study and respiration ratio of leaf to root derived from Noda et al. (2007) and the biomass ratio of leaf to root obtained by Shen et al. (2006). Data of 44 days from 16 sites (five sites from hummock and 11 sites from hollow) were used for the simulation
The contour map showed clearly that at the same temperature, photosynthetic carbon gain was highly correlated to the microsite daily total PPFD. This effect was particularly clear at temperatures above 10°C. Relatively high carbon gain (more than 0.8 mmol g−1 day−1) was attained at the daily total PPFD above 35 mol m−2 day−1 under temperatures below the mean daily 12°C.
Discussion
Photosynthetic acclimation and carbon budget to light regimes
The major conclusion of this study is that photosynthetic acclimation to light regimes play a more important role than to temperature in P. nutans in responding to microsite environments. This can be explained at the following aspects. Firstly, in the field, apart from the significantly higher photosynthetic light-saturation points in hummock plants than in hollow plants at a chamber temperature of 15°C, the photosynthetic responses of P. nutans from the two contrasting topographical microsites—hummocks and hollows—were similar under very high or low temperature. This lack of difference in A max under high or low temperatures perhaps suggests that acclimatization of photosynthetic capacity in response to the light regimes may play a trivial role for leaf carbon gain of P. nutans in the hollows and on the hummocks, at least under stressful temperature environments (Fig. 2). However, we described marked morphological plasticity in the plants from the two extreme microsite regimes (Shen et al. 2006), which perhaps indicates that morphological plasticity plays a more important role for the species in the acclimatization to micro-environmental conditions. Moreover, the photosynthetic acclimatization to light was further demonstrated by the laboratory experiments, which indicates that plants grown under light regimes of 400 and 800 μmol m−2 s−1 had similar photosynthetic parameters each other (Fig. 3). This confirms the field observation mentioned above that light acclimatization in photosynthetic response is not significant under relatively high light regimes, i.e., PPFD above 400 μmol m−2 s−1. Photosynthetic rate, however, was significantly lower under the lowest light regime than the other two light regimes (Fig. 3). That is, extremely low light availability may result in significant reduction of carbon gain in the plants. Much less occurrence of the ramets in the hollows than on the hummocks can be partly explained by such the limitation of low light availability on biomass production in some of the hollow microsites (Shen et al. 2006).
Although radiation is often high but temperature is low in alpine environments, as confirmed by many previous studies, the major limitation of alpine plant photosynthesis is the photosynthetic photon flux density, but not temperature (see review by Körner 2003). Photosynthetic acclimation to light regimes should be therefore important for alpine plants growing in different microsites with contrasting light availability. Such acclimation has been observed in many studies in tropical forests (e.g., Pearcy 1987; Chazdon et al. 1996), but few in herbaceous canopies in particular those in alpine ecosystems (Körner 1982).
Finally, the results in the simulation suggest that microsite daily total PPFD can be a more important predictable variable than microsite daily mean temperature in estimating microsite net carbon gain for P. nutans (Fig. 5). This is because the correlation of microsite daily total carbon gain showed much smaller scattering with microsite daily total PPFD than microsite daily mean temperature. Moreover, at the same microsite mean temperature, the daily net carbon gain showed a relatively distinct increasing pattern with increasing microsite daily total PPFD.
The temperature optimum of photosynthesis
It is still in argument for the optimum temperature of photosynthesis in alpine plants. Many believe that alpine plants have much lower temperature optima than comparable low elevation plants (Pisek et al. 1973; Todaria 1988; Cabrera et al. 1998). Some, however, argued that the temperature optimum of photosynthesis in alpine plants is high (Körner and Diemer 1987; Körner 2003). An optimum temperature of 22°C is “typical” for alpine plants and the optima can even be as high as 27°C (Körner and Diemer 1987). Körner (2003) summarized that the optima for photosynthesis in alpine plants are adjusted to prevailing leaf temperatures during periods of high light.
However, P. nutans showed very low temperature optimum photosynthesis. The photosynthetic response of P. nutans in the natural habitat suggested that the optima for A max are around 15°C (Fig. 2). The results of the laboratory measurements suggest that the optimum temperature is less than 17°C (Fig. 3). The model simulation, however, indicates that the optimum microsite mean daily temperature for net daily carbon gain is even lower, at around 10°C (Fig. 5), which is mainly because that the mean daily temperature considered the nighttime temperature also. If only daytime temperature is considered the optimum temperature for the daily net CO2 uptake was close to 15°C. Temperature optima can be as low as 12–16°C in treeline conifers (Pisek et al. 1973) and forbs (Cabrera et al. 1998). Körner (2003), however, argued that the low optima are because these plants were in shade environments, while in sunny habitats the temperature optima of photosynthesis are not very different between alpine and lowland plants. P. nutans, however, showed little difference in optima of A max in plants from different light regimes.
It is not quite clear currently why P. nutans showed so low temperature optima of photosynthesis, nor why the optima was similar in plants from different light regimes (Fig. 3). The fact that the mean daytime temperature is close to the optima suggests that mean daytime temperature may play an important role in determining the temperature optimum of photosynthesis of the species. Moreover, in the diurnal course of photosynthesis, the peak CO2 uptake often occurred in the morning, when PPFD had reached the photosynthetic light-saturated point but the temperature was not at its highest. By the time the temperature had peaked at around 15:00, the PPFD had often decreased due to short-period clouding (Fig. 4). That is, the maximums of PPFD and temperature during daytime were decoupled in the alpine ecosystem, which may play an important role to the temperature acclimation of photosynthetic characteristics. Physiologically, the photosynthetic response to temperature varies among species and also within the same species under different growth temperatures (e.g., Berry and Bjorkman 1980). In gas-exchange measurements, the optimum temperature for light-saturated photosynthesis has been reported to change with growth temperature (e.g., Hikosaka et al. 1999; Bunce 2000). A recent study indicated that the shift of temperature dependency is due to a change in the optimum temperature of ribulose-1,5-bisphosphate (RuBP) carboxylation (Yamori et al. 2005).
Possible contribution of phenology to simulated carbon budget
In this study we examined the photosynthetic characteristics of P. nutans late in the growing period. The photosynthetic capacity may reach its maximum around July and then decline with progress of growing season. The measurement performed in August would thus not reflect necessarily the maximum photosynthetic capacity if the late changed in parallel with leaf growth. However, the current measurement could still provide us with insights into our understanding of photosynthetic acclimation to different microsite environments if we assume that any physiological difference including those related with phenology in the same species is due to the variation of microsite environments. The difference of photosynthetic acclimation in different microsites could be more evident in August than in earlier growing stages.
In the simulation, we did not consider the effects of seasonal changes and microsite acclimation in leaf life-span, seasonal changes of A max and N concentration on carbon gain per individual plant. Leaf leaf-span is one of the critical parameters in estimating individual carbon gain (Kikuzawa 1995, 2003), while A max changes often with leaf aging, in particular in leaves in the open (e.g., Mediavilla and Escurdero 2003). Leaf nitrogen content determining largely the leaf photosynthetic capacity decreases in general with leaf aging (Field 1983). We assumed that instantaneous photosynthetic responses to PFD and temperature were constant as those obtained in August during the period from June to August. The leaf carbon gain might thus be underestimated if the actual photosynthetic rate decreased with leaf aging. Other Primula species tend to exhibit large seasonal variation in photosynthetic capacity (Whale 1983; Combe et al. 1993). However, since P. nutans can be considered as a simultaneous species, which all the leaves open as a flush during a short period, the carbon gain of individual ramets may not change so much because simultaneous leaf-open species tend to have a slow decrease of canopy photosynthesis during leaf aging (Kikuzawa 2003).
References
Bazzaz FA (1979) The physiological ecology of plant succession. Annu Rev Ecol Syst 10:351–371
Berry J, Bjorkman O (1980) Photosynthetic response and adaptation to temperature in higher plants. Annu Rev Plant Physiol 31:491–543
Bunce JA (2000) Acclimation of photosynthesis to temperature in eight cool and warm climate herbaceous C3 species: temperature dependence of parameters of a biochemical photosynthesis model. Photosynth Res 63:59–67
Cabrera HM, Rada F, Cavieres L (1998) Effects of temperature on photosynthesis of two morphologically contrasting plant species along an altitudinal gradient in the tropical high Andes. Oecologia 114:145–152
Chapin FS (1991) Integrated response of plants to stress. Biosience 41(1):29–36
Chazdon R, Pearcy RW, Lee DW, Fetcher N (1996) Photosynthetic responses of tropical forest plants to contrasting light environments. In: Mulkey SS, Chazdon RL, Smith AP (eds) Tropical forest plant ecophysiology. Chapman & Hall, New York, pp 5–55
Combe L, Bertolini JM, Quetin P (1993) Photosynthese de la primevere (Primula obconica Hance): effets du gaz carbonique et de l’eclairement. Can J Plant Sci 73:1149–1161
Cui XY, Tang YH, Gu S, Nishimura S, Shi SB, Zhao XQ (2003) Photosynthetic depression in relation to plant architecture in two alpine herbaceous species. Environ Exp Bot 50(2):125–135
Cui XY, Tang YH, Gu S, Shi SB, Nishimura S, Zhao XQ (2004) Leaf orientation, incident sunlight, and photosynthesis in the alpine species Suassurea superba and Gentiana straminea on the Qinghai-Tibetan plateau. Arct Antarct Alp Res 36(2):219–228
Field C (1983) Allocating leaf nitrogen for the maximization of carbon gain: leaf age as a control on the allocation program. Oecologia 56:341–347
Field CB (1988) On the role of photosynthetic responses in contrasting the habitat distribution of rainforest plants. In: Evans JR, von Caemmerer S, Adams WW (eds) Ecology of photosynthesis in sun and shade. CSIRO, Melbourne, pp 343–358
Germino MJ, Smith WK (2001) Relative importance of microhabitat, plant form and photosynthetic physiology to carbon gain in two alpine herbs. Funct Ecol 15(2):243–251
Givnish TJ (1988) Adaptation to sun and shade: a whole-plant perspective. Aust J Plant Physiol 15:63–92
Hikosaka K, Murakami A, Hirose T (1999) Balancing carboxylation and regeneration of ribulose bisphosphate in leaf photosynthesis: temperature acclimation in an evergreen tree, Quercus myrsinaefolia. Plant Cell Environ 22:841–849
Kikuzawa K (1995) Leaf phenology as an optimal strategy for carbon gain in plants. Can J Bot 73:158–163
Kikuzawa K (2003) Phenological and morphological adaptation to the light environment in two woody and two herbaceous plant species. Funct Ecol 17:29–38
Körner Ch (1982) CO2 exchange in the alpine sedge Carex curvula as influenced by canopy structure, light and temperature. Oecologia 53:98–104
Körner Ch (2003) Alpine plant life, functional plant ecology of high mountain ecosystems, 2nd edn. Springer, Berlin, Heidelberg, New York, New York
Körner Ch, Diemer M (1987) In situ photosynthetic responses to light, temperature and carbon dioxide in herbaceous plants from low and high altitude. Funct Ecol 1:179–194
Lechowicz MJ, Bell G (1991) The ecology and genetics of fitness in forest plants. II. Microspatial heterogeneity of the edaphic environment. J Ecol 79:687–696
Mediavilla S, Escudero A (2003) Photosynthetic capacity, integrated over the lifetime of a leaf, is predicted to be independent of leaf longevity in some tree species. New Phytol 159:203–211
Nicotra AB, Chazdon RL, Schlichting CD (1997) Patterns of genotypic variation and phenotypic plasticity of light response in two tropical piper (Piperaceae) species. Am J Bot 84(11):1542–1552
Noda H, Muraoka H, Washitani I (2004) Morphological and physiological acclimation responses to contrasting light and water regimes in Primula sieboldii. Ecol Res 19: 331–340
Noda H, Muraoka H, Tang Y, Washitani I (2007) Phenological changes in rate of respiration and annual carbon balance in a perennial herbaceous plant, Primula sieboldii. J Plant Res 120(3):375–383
Pearcy RW (1983) The light environment and growth of C3 and C4 tree species in the understory of a Hawaiian forest. Oecologia 58:19–25
Pearcy RW (1987) Photosynthetic gas exchange responses of Australian tropical forest tress in canopy, gap and understory micro-environments. Funct Ecol 1:169–178
Pearcy RW, Sims DA (1994) Photosynthetic acclimation to changing light environments: scaling from the leaf to the whole plant. In: Cardwell MM, Pearcy RW (eds) Exploitation of environmental heterogeneity by plants. Academic Press, San Diego, pp 145–174
Pisek A, Larcher W, Vegis A, Napp-Zinn K (1973) The normal temperature range. In: Precht H, Christophersen J, Hensel H, Larcher W (eds) Temperature and life. Springer, Berlin, Heidelberg, New York, pp 102–194
Richards J (2003) Primula. Timber Press, Portland
Shen H, Tang Y, Washitani I (2006) Morphological plasticity of Primula nutans to hummock-and-hollow microsites in an alpine wetland. J Plant Res 119:257–264
Silvertown JW (1981) Micro-spatial heterogeneity and seedling demography in species-rich grassland. New Phytol 88:117–128
Sultan SE, Wilczek AM, Bell DL, Hand G (1998) Physiological response to complex environments in annual Polygonum species of contrasting ecological breath. Oecologia 115:564–578
Tang YH, Washitani I (1995) Characteristics of small-scale heterogeneity in light availability within a Miscanthus sinensis canopy. Ecol Res 10:189–197
Thornley JHM (1976) Mathematical models in plant physiology. Academic Press, London
Todaria NP (1988) Ecophysiology of mountain plants: I. Photosynthesis. Acta Physiol Plant 10:199–226
Whale DM (1983) Seasonal variation in the gas exchange characteristics of Primula species. Oecologia 59:377–383
Yamori W, Noguchi K, Terashima I (2005) Temperature acclimation of photosynthesis in spinach leaves: analyses of photosynthetic components and temperature dependencies of photosynthetic partial reactions. Plant Cell Environ 28:536–547
Acknowledgments
This study was supported by the Twenty-first Century COE Program of the University of Tokyo, “Biodiversity and Ecosystem Restoration,” sponsored by the Japan Society for the Promotion of Science; the Global Environment Research Program S1-I (2); and a study grant from the Ministry of the Environment of Japan for the early detection and prediction of global warming on the Tibetan Plateau. The authors thank Hibiki Noda, Xiaoyong Cui, Shengbo Shi, Song Gu, Yingnian Li, and Xinquan Zhao for their discussion.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10265_2008_146_MOESM1_ESM.eps
Supplementary Fig. 1. Frequency distributions of PPFD on a typical hummock (a, c, e) and in its neighboring hollow (b, d, f) in different months (a, b: June; c, d: July; e, f: August). The percentage time of PPFD > 100 μmol m−2 s−1 in the daytime is given in each chart. (EPS 516 kb)
10265_2008_146_MOESM2_ESM.eps
Supplementary Fig. 2. Frequency distributions of daytime air temperature (a, d, g) and soil temperature on a typical hummock (b, e, h) and in its neighboring hollow (c, f, i) in different months (a, b, c: June; d, e, f: July; g, h, i: August). Air temperature was measured about 5 cm above the surface of the vegetation, and soil temperature was measured at a soil depth of 5 cm. (EPS 655 kb)
Rights and permissions
About this article
Cite this article
Shen, H., Tang, Y., Muraoka, H. et al. Characteristics of leaf photosynthesis and simulated individual carbon budget in Primula nutans under contrasting light and temperature conditions. J Plant Res 121, 191–200 (2008). https://doi.org/10.1007/s10265-008-0146-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10265-008-0146-z