Abstract
This is the first report on γ-tubulin and microtubule arrays during microsporogenesis in a gymnosperm. Meiosis in Ginkgo biloba is polyplastidic, as is typical of the spermatophyte clade, and microtubule arrays are organized at various sites during meiosis and cytokinesis. In early prophase, a cluster of γ-tubulin globules occurs in the central cytoplasm adjacent to the off-center nucleus. These globules diminish in size and spread over the surface of the nucleus. A system of microtubules focused on the γ-tubulin forms a reticulate pattern in the cytoplasm. As the nucleus migrates to the center of the microsporocyte, γ-tubulin becomes concentrated at several sites adjacent to the nuclear envelope. Microtubules organized at these foci of γ-tubulin give rise to a multipolar prophase spindle. By metaphase I, the spindle has matured into a distinctly bipolar structure with pointed poles. In both first and second meiosis, γ-tubulin becomes distributed throughout the metaphase spindles, but becomes distinctly polar again in anaphase. In telophase I, γ-tubulin moves from polar regions to the proximal surface of chromosome groups/nuclei where interzonal microtubules are organized. No cell wall is deposited and the interzonal microtubules embrace a plate of organelles between the two nuclear cytoplasmic domains (NCDs) of the dyad. Following second meiosis, phragmoplasts that form between sister and non-sister nuclei fuse to form a complex six-sided structure that directs simultaneous cytokinesis. γ-Tubulin becomes associated with nuclei after both meiotic divisions and is especially conspicuous in the distal hemisphere of each young microspore where an unusual encircling system of cortical microtubules develops.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ginkgo biloba is a curiosity among seed plants. It represents the last remaining species of a distinct and widespread group that reached maximum diversity in the Cretaceous. Fossils assignable to the genus Ginkgo occur in the early Jurassic making it the oldest extant genus of seed plants and earning it the status of a “living fossil” (Hori et al. 1997; Royer et al. 2003). As such, Ginkgo is important to our understanding of the evolution of reproductive mechanisms in spermatophytes. The trees are dioecious, and sporogenesis results in haploid spores of two sizes and fates. Microspores develop into pollen grains and megaspores develop into large nutritive megagametophytes. Both male and female gametophyte development exhibit unique characteristics. Development of the male gametophyte culminates in release of two massive multiflagellate sperm that swim to archegonia at the surface of the mature megagametophyte. The megagametophyte develops as a syncytium before cellularization via the process of alveolation in a manner similar to development of the nuclear-type endosperm in flowering plants (Brown et al. 2002).
The process of meiosis in microsporogenesis in Ginkgo has been far less studied than the subsequent development of the male gametophyte (Friedman and Gifford 1997). The general process of microsporogenesis in gymnosperms (Pennell 1988; Kurmann 1990) follows that of angiosperms (Brown and Lemmon 1991a; Furness et al. 2002). The polyplastidic microsporocytes are isolated from surrounding sporophytic tissues by deposition of a special callose wall. Meiosis results in a tetrad of haploid microspores. As in other groups of plants (bryophytes, ferns, angiosperms) (Brown and Lemmon 1991a; Furness et al. 2002), both successive and simultaneous forms of meiotic cytokinesis occur in gymnosperms. In Taxus, for example, a dyad wall is deposited after meiosis I, but in many gymnosperms such as Pinus, brief bi-nucleate and four-nucleate syncytial stages occur before simultaneous cytokinesis separates the microsporocyte into a tetrad of microspores (Pennell 1988). In Ginkgo, meiotic cytokinesis occurs simultaneously after second division. After meiosis I, all of the plastids and mitochondria concentrate into a conspicuous band in the equatorial plane of first division and effectively partition the otherwise undivided cytoplasm into two nuclear cytoplasmic domains (NCDs). This organelle band was first described by Mann in 1924 and was reexamined ultrastructurally by Wolniak (1976) who showed that it also contains abundant endomembranes and microtubules.
There have been no reports on microtubule arrays driving microsporogenesis in gymnosperms. As part of an ongoing study on the evolution of sporogenesis in land plants, we undertook this study with the goal of providing data on the organization of the microtubule arrays responsible for the processes of meiosis and cytokinesis in this pivotal evolutionary group of ancient seed plants. It is becoming increasingly apparent that the nucleation of microtubules in plants without centrosomes is consistently associated with γ-tubulin as it is in animal cells (Liu et al. 1993; Joshi and Palevitz 1996; Vaughn and Harper 1998; Dibbayawan et al. 2001; Shimamura et al. 2004). γ-Tubulin has not yet been shown to form ring complexes at the minus ends of microtubules in plants, but it consistently occurs at the different sites of microtubule nucleation during both the mitotic and meiotic cell cycles (Shimamura et al. 2004; Brown and Lemmon 2004; Brown et al. 2004). In this study we use the G9 monoclonal antibody against γ-tubulin from fission yeast. This antibody has been thoroughly characterized and shown to recognize γ-tubulin in both seed plants (Ovenchkina and Oakley 2001) and bryophytes (Shimamura et al. 2004). Data from this study of triple-stained microsporocytes of Ginkgo show the relationship of γ-tubulin to the organization of microtubules in all nuclear stages of meiosis and cytokinesis.
Materials and methods
Microstrobili were collected from horticultural specimens growing in Lafayette Parish, Louisiana, where the pollen trees undergo meiosis and cytokinesis near the end of March. Developmental stages were determined by viewing cells from a single sporangium squashed in aceto-orcein. The remaining sporangia from the microstrobilus were prepared for study of the cytoskeleton by indirect immunofluorescence according to methods published by Brown and Lemmon (1995).
Briefly, microsporocytes were gently squeezed from the sporangia onto coverslips coated with Mayer’s egg albumen histological adhesive and fixed in a drop of 4% formaldehyde freshly prepared from paraformaldehyde in microtubule-stabilizing buffer overnight at 4°C. The preparations were allowed to dry and covered by a thin agarose-gelatin film. Walls were removed with enzymes and membranes permeabilized with Triton X-100. For double staining of microtubules and γ-tubulin, preparations were incubated with a mixture of primary antibodies, i.e. monoclonal antibody against yeast α-tubulin (YOL 1/34, Accurate Chemical and Scientific, Westbury, NY, USA) at a concentration of 1:100 and monoclonal antibody G9 against fission yeast γ-tubulin (gift of T. Horio, Tokushima University, Japan) at a concentration of 1:160,000, for 1 h at 37°C. After a thorough wash in buffer, preparations were incubated in secondary antibodies: anti-rat IgG conjugated to rhodamine red and anti-mouse IgG conjugated to fluorescein for 1 h at 37°C. Following several washes in d-H20, nucleic acids were stained with 1 μM To-Pro-3 iodide (Molecular Probes, Eugene, OR, USA) in d-H20 for 2 min and mounted in Prolong antifade reagent (Molecular Probes, Eugene, OR, USA). Fluorescence was examined by confocal laser scanning microscopy (CLSM). The digital files were transferred to a Macintosh PowerPC using NIH Image software for preparation of illustrations.
Results
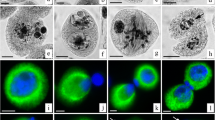
Triple localization by immunofluorescence shows a general scheme of polyplastidic meiosis and simultaneous cytokinesis (Figs. 1, 2, 3) that is typical of euphyllophytes, but with some interesting variations. In early meiotic prophase when the unusually large nucleus occupies an off-center position in the sporocyte (Fig. 1, 1 and 2), microtubules are arranged in a reticulate pattern over the nucleus and throughout the cytoplasm. This array is associated with globules of γ-tubulin adjacent to the nuclear envelope (Fig. 1, 1). These globules are first concentrated near the nuclear envelope in the center of the cytoplasm and subsequently spread along the nuclear envelope (Fig. 1, 2).
Assembly of the meiotic spindle. a Microtubules. b γ-Tubulin. c Chromosomes. Bar=5 μm. 1 Early prophase with off-center nucleus. Microtubules emanate from a cluster of γ-tubulin globules in the cytoplasm adjacent to the nucleus. 2 At a slightly later stage of prophase, microtubules emanate from γ-tubulin that has spread over the surface of the nucleus. 3 As prophase progresses, the nucleus returns to the center of the microsporocyte, which becomes slightly lobed. Microtubules and γ-tubulin are concentrated at forming poles, and a pattern of nearly parallel microtubules replaces the reticulate pattern of earlier stages. 4 The metaphase spindle with γ-tubulin distributed throughout the microtubule arrays
The first and second divisions of meiosis. a Microtubules. b γ-Tubulin. c Chromosomes. Bar=5 μm. 5 At anaphase I, γ-tubulin is still distributed throughout the spindle but is now clearly concentrated at poles. 6 At telophase I the γ-tubulin is concentrated, not at the polar regions, but rather at the proximal surfaces of the sister groups of chromosomes and in the interzonal array. Organelles (dark unstained bodies) form a peripheral ring at the equator. 7 In inframeiotic interphase, γ-tubulin outlines the nuclear surfaces, especially lining the proximal surfaces and is concentrated at the equatorial region of first meiosis. No cell wall is deposited. Instead, the organelles invade this area forming a band that delineates the dyad domains of the undivided cytoplasm. 8 The spindles of metaphase II are at right angles to each other with γ-tubulin distributed more or less throughout the spindles
Completion of meiosis II, cytokinesis, and the early spore tetrad. a Microtubules. b γ-Tubulin. c Chromosomes. Bar=5 μm. 9 At anaphase II, γ-tubulin is concentrated at poles. 10 At telophase II, γ-tubulin is weakly distributed throughout interzonal microtubules between pairs of sister nuclei. 11 Phragmoplasts form between all nuclei and fuse. Primary phragmoplasts between sister nuclei and secondary phragmoplasts between non-sister nuclei become indistinguishable once they fuse and form a six-sided complex that interconnects the four-spore nuclei. γ-Tubulin is distributed throughout the phragmoplast complex. Intersporal walls are deposited simultaneously along all six division planes. 12 Unusual encircling microtubules form in the distal hemispheres around each microspore of the tetrad
In later stages of prophase when the nucleus returns to the center of the microsporocyte, microtubules become increasingly concentrated around the nucleus. These microtubules, which are associated with γ-tubulin that is concentrated at several foci around the nucleus, give rise to bundles of microtubules that comprise the early multipolar spindle (Fig. 1, 3). The several poles gradually consolidate into two pointed polar regions. The mature metaphase spindle has a distinctive shape with narrow poles and a broad equatorial region (Fig. 1, 4a). γ-Tubulin becomes distributed throughout the spindle, but appears more concentrated at the poles (Fig. 1, 4b). In anaphase I (Fig. 2, 5), kinetochore bundles shorten, and the polar regions become broader than in metaphase. Numerous microtubules radiate from the sister groups of chromosomes and form caps on the distal surfaces. At anaphase, γ-tubulin is concentrated in the polar regions which appear to be comprised of a number of distinct foci (Fig. 2, 5b).
In telophase I (Fig. 2, 6), a phragmoplast forms in the interzonal region between the sister nuclei. The phragmoplast consists of two opposing dense brush-like sets of microtubules emanating from proximal faces of nuclear envelopes (Fig. 2, 6a). Microtubules of the phragmoplast are organized into cone-like phragmoplast fibers exactly opposite one another on either side of an unstained midzone (Fig. 2, 6). The γ-tubulin staining pattern differs from that of anaphase in that the concentrations at the distal poles have disappeared and γ-tubulin appears to be distributed along the length of radial microtubules and the bipolar arrays of the interzonal phragmoplast (Fig. 2, 6b). γ-Tubulin appears virtually absent from the plane of microtubule overlap in the middle of the phragmoplast. The larger organelles (amyloplasts, mitochondria, and small vacuoles), which are clearly visible in the immunofluorescence micrographs as dark unstained spheres, first form an equatorial collar surrounding the midzone of the spindle (Fig. 2, 6b) and then invade the interzone forming a distinct organelle band (e.g. Fig. 2, 7b, 8b) that defines the dyad domains throughout second meiosis.
There is no evidence of cell plate deposition or wall ingrowths in the equatorial region of first meiosis. In inframeiotic interphase an unusual microtubule array appears. The γ-tubulin becomes evenly distributed around the surface of the dyad nuclei. Microtubules enshroud the nuclei and radiate into the organelle band from the proximal surfaces of the nuclear envelopes. Suspended between them is a shortened brush-like set of microtubules embracing the organelle band (Fig. 2, 7a). No specific concentration of γ-tubulin was observed in association with the equatorial microtubules (Fig. 2, 7b), which disappear before second meiosis.
Second-division spindles develop simultaneously in the two hemispherical dyad domains on either side of the organelle band (Fig. 2, 8). Poles of second-division spindles are broader than in first division, but behavior of γ-tubulin and microtubules during chromosome separation is similar to first division (Figs. 2, 8; 3, 9). γ-Tubulin occurs throughout the metaphase spindles (Fig. 2, 8b) and becomes decidedly more concentrated at poles in anaphase (Fig. 3, 9b).
After chromosomes have moved to the poles of second division, well-defined interzonal arrays of microtubules form between the sister groups of chromosomes (Fig. 3, 10), and microtubules emanating from nonsister groups of chromosomes form secondary interactions (Fig. 3, 10). γ-Tubulin distribution is punctate throughout these populations of microtubules and slightly more concentrated around the periphery of the reforming nuclei (Fig. 3, 10b). Well-ordered phragmoplasts develop among the four nuclei, and phragmoplasts of primary (interzonal between sister nuclei) and secondary (adventitious between nonsister nuclei) origin soon become indistinguishable (Fig. 3, 11a) and fuse to form a complex six-sided structure. γ-Tubulin occurs in a punctate pattern throughout the phragmoplast but is more concentrated around the nuclei (Fig. 3, 11b). Intersporal cell plates that develop in the phragmoplasts fuse to separate the four spores of the tetrad (Fig. 3, 11). Cell plates seem to be laid down first in the centers of phragmoplasts and then expand to the periphery. There is no evidence of wall ingrowth or other structural marking of the position where the expanding intersporal walls will join the periphery. Concomitant with deposition of the intersporal wall in the first division plane, the organelles of the band disperse, resulting in an approximate equipartitioning of the amyloplasts and mitochondria to the four spores of the tetrad.
The tetrad members are typically in a tetrahedral arrangement (Fig. 3, 11 and 12) reflecting the mutually perpendicular orientation of second-division spindles. Nuclei of the young spores are located in the distal cytoplasm of tetrad members (Fig. 3, 12c). The microtubules are differentiated in the proximal and distal hemispheres of the tetrad members and form an unusual overall configuration of circumferential hoops most prominent over the nuclei (Fig. 3, 12a). A conspicuous punctate pattern of γ-tubulin is associated with the cap of microtubules (Fig. 3, 12b).
Discussion
Data from this investigation add to our understanding of the relationship of γ-tubulin and the microtubule organizing center (MTOC) in the organization of the anastral meiotic spindle of seed plants and the evolutionary position of microsporogenesis of Ginkgo in relationship to other spermatophytes. Meiosis in Ginkgo is clearly like that of other euphyllophytes in that microsporocytes are polyplastidic and the anastral spindle is organized in the perinuclear region. Spindle development is unlike the bryophytes and lycophytes, which undergo monoplastidic meiosis characterized by an initially quadripolar spindle that emanates from four plastids located in the future spore domains (Brown and Lemmon 1997). Recent work has shown that γ-tubulin is associated with the plastid MTOCs from which microtubules emanate (Shimamura et al. 2004). In Ginkgo, there is no indication of precocious cytoplasmic lobing into spore domains, clustering of plastids at polar regions, or other vestiges of quadripolarity in meiotic prophase.
The origin of anastral spindles in plants, so different from the formation of half spindles from centrosomes in animal cells, has remained enigmatic (Brown and Lemmon 1993; Palevitz 1993; Franklin and Cande 1999). Overwhelming evidence indicates that the microtubules of higher plants are organized at the nuclear envelope (Schmit et al. 1994; Stoppin et al. 1994; Hasezawa et al. 2000). Furthermore, the meiotic prophase spindle of plants is often strikingly multipolar rather than bipolar. The degree of subsequent polar consolidation varies. The meiotic spindle poles of some plants become sharply focused whereas those of others have broad polar regions comprising numerous minipoles, as is more typical of the mitotic plant spindle (De Mey et al. 1982). It is thought that poles are organized by a complex assemblage of molecular motors first associated with the chromosomes and subsequently moving to polar regions to organize the poles (Vernos and Carsenti 1995; Franklin and Cande 1999). Although the mechanism of spindle-pole formation in plants is unknown, spindle-pole aggregation is perturbed by certain drugs such as griseofulvin and chlorisophenylcarbamate (Brown and Lemmon 1992), which also disrupt centrosomal poles in animal cells. There are mutants in maize with faulty meiosis due to abnormal spindle development. In the mutant dv, nuclear events of prophase and metaphase appear normal but the spindle poles fail to converge into the narrow poles typical of maize (Staiger and Cande 1990). As a consequence, supernumerary nuclei are formed leading to multiple defects in meiosis. In a newly described and equally interesting mutant, bivalents remain scattered in the cytoplasm rather than forming a single metaphase plate (Caetano-Pereira and Pagliarini 2001). Instead of a single spindle, several minispindles form in association with the bivalents/groups of bivalents, and a varied number of telophase nuclei are formed. Taken together these results indicate that while subunits of the spindle are capable of moving chromosomes, polar organization is necessary to deliver the chromosomes into two groups in telophase. The phenomenon of polar consolidation is seen very clearly in Ginkgo where several foci of γ-tubulin initiate spindle development and then move to opposite poles consolidating the multipolar spindle into a distinctly bipolar structure by metaphase.
The ability to stain γ-tubulin, microtubules, and nuclei/chromosomes in a single preparation has provided data that allows us to investigate plant spindle origin and maturation from a new perspective. In lower plants, γ-tubulin consistently marks the foci of microtubule organization and is not detectable in the cell other than in association with microtubule arrays (Shimamura et al. 2004; Brown and Lemmon 2004). Polar organizers (POs) arise de novo at the onset of both mitosis (Brown et al. 2004) and meiosis of hepatics (Brown and Lemmon 2004) suggesting that fundamental aspects of spindle organization are basically similar in the two types of cell division. Although the hepatic PO contains a distinct concentration of γ-tubulin in prophase, the behavior of γ-tubulin and microtubule organization throughout the remainder of the cell cycle is like that in higher plants (Brown et al. 2004). The POs break up into minipoles along broad polar regions, γ-tubulin moves into the spindle at metaphase, and again becomes polar at anaphase. It is becoming increasingly apparent that γ-tubulin is indeed a motile entity in plant cells and that the plant MTOC is not restricted to a specific location as in centrosomal cells.
Discovery of a single cluster of γ-tubulin globules in the polarized cytoplasm of microsporocytes of Ginkgo during early prophase provides insight into the nature of the plant MTOC in meiosis of a spermatophyte without POs. The onset of plant meiosis is marked by a stage during which the nucleus is in an off-center position and the cytoplasm is unequally distributed around it, a phenomenon observed in all groups of plants from bryophytes to spermatophytes (e.g. Rodkiewicz et al. 1986; Brown and Lemmon 1987, 1991b). This off-center stage corresponds to the clustering of the chromosomes ends to form the bouquet stage of early prophase (Cowan et al. 2001), when polarity for meiosis is thought to be established. In some plants such as Impatiens (Rodkiewicz et al. 1986), the bouquet stage is accompanied by clustering of plastids at the cytoplasmic side of the nucleus in the same position as the γ-tubulin globules in Ginkgo. The γ-tubulin globules subsequently spread over the surface of the nucleus and appear to be foci of microtubules that form a reticulate pattern as prophase proceeds. The even distribution of γ-tubulin in the perinuclear area typical of the onset of spindle development in angiosperms begins only when the nucleus returns to center. As the γ-tubulin becomes more diffuse around the periphery of the nucleus, there are usually three or more regions of concentration, each with microtubule arrays that begin to be organized into cone-like arrays. The individual cones of microtubules remain distinct and give rise to a spindle that is initially multipolar. Thus, from the start of spindle initiation, cones of microtubules are nucleated at γ-tubulin, presumably with minus ends proximal and plus ends distal. During late prophase, the nucleus expands, moving poles outward. By metaphase, the poles have consolidated into two opposite regions and the meiotic spindle is bipolar. At this time γ-tubulin moves into the spindle itself, but at anaphase it becomes distinctly polar again, a phenomenon that has been reported in all plants studied thus far (Shimamura et al. 2004).
The γ-tubulin becomes concentrated at the tightly focused polar regions in anaphase-telophase, then moves from distal (polar) to proximal surfaces of the sister groups of chromosomes where the interzonal arrays of microtubules are organized. The interzonal array typically gives rise to a phragmoplast, but may fail to do so in many cases, as after first meiosis in plants undergoing successive division or in syncytial endosperm development (Brown and Lemmon 1991a, 2001). In Ginkgo, the central bipolar interzonal array, as well as radial microtubules emanating from sister groups of chromosomes/nuclei, effectively defines the two NCDs of the dyad but no walls are deposited. A peripheral collar of organelles invades the equatorial area and forms an organelle band between the two dyad domains. Instead of a typical phragmoplast, the interzonal microtubules emanating from γ-tubulin at the nuclear envelopes develop into a specialized array along which the organelles are in parallel orientation, at right angles to the equator of the first division spindle. This unusual array disappears before second meiosis. The organelle band is a widespread feature of sporogenesis by simultaneous cytokinesis (Brown and Lemmon 1991a, 2001; Furness et al. 2002).
Cytokinesis in microsporogenesis of Ginkgo occurs simultaneously after second meiosis. Typical primary phragmoplasts in the interzones between the two pairs of sister nuclei develop slightly in advance of secondary phragmoplasts, which develop adventitiously between nonsister nuclei. The organelle band between dyad domains disperses, and all phragmoplasts fuse into a complex six-sided structure in which the intersporal walls are deposited. This wall deposition, responsible for what is traditionally referred to as “spore cleavage” in the palynological literature, is a secretory plant-like process unlike the actomyosin-driven cleavage by which cytokinesis is accomplished in animal cells. Even in bryophytes where the cytoplasm is deeply lobed early in meiosis and in microsporogenesis of many angiosperms where meiotic cytokinesis involves infurrowing of cytoplasm as a result of centripetal wall deposition, the wall is formed by vesicle fusion and not cytoplasmic constriction. Wall deposition in sporogenesis has been described in a wide variety of plants (Barnes and Blackmore 1986; Brown and Lemmon 1991a) and has recently been substantiated by TEM tomography (Otegui and Staehelin 2004).
The unusual cortical hoop-like arrangement of microtubules in newly cleaved spore tetrad members is inexplicable, as no equivalent arrangement has been reported in the literature on sporogenesis or microsporogenesis. During formation of the proximal aperture in certain mosses a distinct radial system emanates from the site. It is possible that the parallel arrangements of microtubules seen in early microspores of Ginkgo represent an early manifestation of MTOC activity in anticipation of the subsequent development of two sperm in each spore/pollen grain. The sperm of Ginkgo and cycads are unique among plants in that each is very large and has a long coiled multilayered structure bearing about 1,000 flagella (Friedman and Gifford 1997). Since spermatogenous cells of the lower plants develop centrioles de novo (Vaughn and Harper 1998), it will be interesting to trace this phenomenon in development of the multiflagellate sperm of Ginkgo, especially as it may be possible to follow the transition of γ-tubulin from a motile diffuse entity to a concentrated animal-like MTOC.
References
Barnes SH, Blackmore S (1986) Some functional features in pollen development. In: Blackmore S, Ferguson IK (eds) Pollen and spores: form and function. Academic, London, pp 71–80
Brown RC, Lemmon BE (1987) Division polarity, development, and configuration of microtubule arrays in bryophyte meiosis. I. Meiotic prophase to metaphase I. Protoplasma 137:84–99
Brown RC, Lemmon BE (1991a) The cytokinetic apparatus in meiosis: control of the division plane in the absence of a preprophase band of microtubules. In: Lloyd C (ed) The cytoskeletal basis of plant growth and form. Academic, London, pp 259–273
Brown RC, Lemmon BE (1991b) Plastid polarity and meiotic spindle development in microsporogenesis of Selaginella. Protoplasma 161:168–180
Brown RC, Lemmon BE (1992) Control of division plane in normal and griseofulvin-treated microsporocytes of Magnolia. J Cell Sci 103:1031–1038
Brown RC, Lemmon BE (1993) Diversity of cell division in simple land plants holds clues to evolution of the mitotic and cytokinetic apparatus in higher plants. Mem Torrey Bot Club 25:45–62
Brown RC, Lemmon BE (1995) Methods in plant immunolight microscopy. Methods Cell Biol 49:85–107
Brown RC, Lemmon BE (1997) The quadripolar microtubule system in lower land plants. J Plant Res 110:93–106
Brown RC, Lemmon BE (2001) The cytoskeleton and the spatial control of cytokinesis in the plant life cycle. Protoplasma 215:35–49
Brown RC, Lemmon BE (2004) γ-Tubulin, microtubule arrays, and quadripolarity during sporogenesis in the hepatic Aneura pinguis (L.) Dumort. (Metzgeriales). J Plant Res 117:371–376
Brown RC, Lemmon BE, Nguyen H (2002) The microtubule cycle during successive mitotic waves in the syncytial female gametophyte of ginkgo. J Plant Res 115:491–494
Brown RC, Lemmon BE, Horio T (2004) γ-Tubulin localization changes from discrete polar organizers to anastral spindles and phragmoplasts in mitosis of Marchantia polymorpha L. Protoplasma 224:187–193
Caetano-Pereira CM, Pagliarini MS (2001) A new meiotic abnormality in Zea mays: multiple spindles associated with abnormal cytokinesis in both divisions. Genome 44:865–871
Cowan CR, Carlton PM, Cande WZ (2001) The polar arrangement of telomeres in interphase and meiosis. Rabl organization and the bouquet. Plant Physiol 125:532–538
De Mey J, Lambert A-M, Bajer AS, Moeremans M, De Brabander M (1982) Visualization of microtubules in interphase and mitotic plant cells of Haemanthus endosperm with the immuno-gold staining method. Proc Natl Acad Sci USA 79:1898–1902
Dibbayawan TP, Harper JDI, Marc J (2001) A γ-tubulin antibody against a plant peptide sequence localizes to cell division specific microtubule arrays and organelles in plants. Micron 32:671–678
Franklin AE, Cande WZ (1999) Nuclear organization and chromosome segregation. Plant Cell 11:523–534
Friedman WE, Gifford EM (1997) Development of the male gametophyte of Ginkgo biloba: a window into the reproductive biology of early seed plants. In: Hori T et al (eds) Ginkgo biloba-a global treasure. Springer-Verlag, Tokyo, pp 29–49
Furness CA, Rudall PJ, Sampson FB (2002) Evolution of microsporogenesis in angiosperms. Int J Plant Sci 163:235–260
Hasezawa S, Ueda K, Kumagai K (2000) Time-sequence observations of microtubule dynamics throughout mitosis in living cell suspensions of stable transgenic Arabidopsis-direct evidence for the origin of cortical microtubules at M/G1 interface. Plant Cell Physiol 41:244–250
Hori T, Ridge RW, Tulecke W, Del Tredici P, Trèmouillaux-Guîller J, Tobe H (eds) (1997) Ginkgo biloba: A global treasure. Springer-Verlag, Tokyo
Joshi HC, Palevitz BA (1996) γ-Tubulin and microtubule organization in plants. Trends Cell Biol 6:41–44
Kurmann MH (1990) Exine development in conifers. In: Blackmore S, Knox RB (eds) Microspores: evolution and ontogeny. Academic, London, pp 157–172
Liu B, Marc J, Joshi HC, Palevitz BA (1993) A γ-tubulin-related protein associated with the microtubule arrays of higher plants in a cell cycle-dependent manner. J Cell Sci 104:1217–1228
Mann MC (1924) Microsporogenesis of Ginkgo biloba L. with special reference to the distribution of plastids and to cell wall formation. Univ Cal Pub Agr Sci 2:243–248
Otegui MS, Staehelin LA (2004) Electron tomographic analysis of post-meiotic cytokinesis during pollen development in Arabidopsis thaliana. Planta 218:501–515
Ovenchkina Y, Oakley BR (2001) γ-Tubulin in plant cells. Methods Cell Biol 67:195–212
Palevitz B (1993) Morphological plasticity of the mitotic apparatus in plants and its developmental consequences. Plant Cell 5:1001–1009
Pennell RI (1988) Sporogenesis in conifers. Adv Bot Res 15:179–196
Rodkiewicz B, Bednara J, Mostowska A, Duda E, Stobiecka H (1986) The change in disposition of plastids and mitochondria during microsporogenesis and sporogenesis in some higher plants. Acta Bot Neerl 35:209–215
Royer DL, Hickey LJ, Wing SL (2003) Ecological conservatism in the “living fossil” Ginkgo. Paleobiology 29:84–104
Schmit A-C, Stoppin V, Chevrier V, Job D, Lambert A-M (1994) Cell cycle dependent distribution of a centrosomal antigen at the perinuclear MTOC or at the kinetochores of higher plant cells. Chromosoma 103:343–351
Shimamura M, Brown RC, Lemmon BE, Akashi T, Mizuno K, Nishihara N, Tomizawa K-I, Yoshimoto K, Deguchi H, Hosoya H, Horio T, Mineyuki Y (2004) γ-Tubulin in basal land plants: characterization, localization and implication in the evolution of acentriolar microtubule organizing centers. Plant Cell 16:45–59
Staiger CJ, Cande WZ (1990) Microtubule distribution in dv, a maize meiotic mutant defective in the prophase to metaphase transition. Dev Biol 138:231–242
Stoppin V, Vantard M, Schmit A-C, Lambert A-M (1994) Isolated plant nuclei nucleate microtubule assembly: the nuclear surface in higher plants has centrosome-like activity. Plant Cell 6:1099–1106
Vaughn KC, Harper JDI (1998) Microtubule-organizing centers and nucleating sites in land plants. Int Rev Cytol 181:75–149
Vernos I, Karsenti E (1995) Chromosomes take the lead in spindle assembly. Trends Cell Biol 5:297–301
Wolniak SM (1976) Organelle distribution and apportionment during meiosis in the microsporocyte of Ginkgo biloba L. Am J Bot 63:251–258
Acknowledgements
We thank Professor T. Horio, Tokushima University, Japan for the gift of the G9 antibody.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brown, R.C., Lemmon, B.E. γ-Tubulin and microtubule organization during microsporogenesis in Ginkgo biloba . J Plant Res 118, 121–128 (2005). https://doi.org/10.1007/s10265-005-0199-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10265-005-0199-1