Abstract
The predominance of the effector mechanisms by CD4 + T cells is a characteristic of inflammatory autoimmune diseases such as rheumatoid arthritis (RA). The CD40/CD40L costimulatory pathway contributes to these pathogenic mechanisms by promoting autoantibody production and inflammation. Aberrant expression of CD40 and CD40L in RA patients has been shown, the latter prevailing in females. However, contrasting results have emerged regarding the clinical associations of these findings. We determined the association of CD40 and CD40L expression with the clinical activity evaluated through DAS28 in RA patients. A total of 38 female RA patients and 10 age- and sex-matched control subjects were included. CD40 and CD40L mRNA expression was quantified by real-time qPCR, cell surface proteins were determined by flow cytometry, and protein soluble forms were determined by ELISA. The expansion of a CD4 + T cell subpopulation expressing CD40 was identified in the RA group. In addition, high frequencies of CD4 + CD40L + T cells expressing high levels of CD40L, increased levels of sCD40L and overexpression of CD40L mRNA were observed in these patients. Moreover, there was a gradual increase in CD40L when data were stratified according to DAS28, except for very active patients. No correlation was observed between the levels of mRNA, cell surface protein and soluble protein of CD40 and CD40L with the clinical features of RA patients. There is an altered expression of CD40L in female RA patients in association with clinical activity assessed by DAS28, these findings support the evidence that suggests CD40L as a marker of clinical activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory autoimmune disease characterized by synovial inflammation of arthrodial joints. This disease affects ~ 1% of the population worldwide with a predominance in females (female/male ratio 3:1) [1]. An important feature in the pathogenesis of RA is the overactivation and proliferation of autoreactive CD4 + T cells, which are directly involved in B cell activation and autoantibody production. In addition, the expansion of effector CD4 + T cells, mainly the Th1 and Th17 cell subsets, contributes greatly to the high levels of proinflammatory cytokines observed in these patients. In the long term, these are key events that maintain a chronic inflammatory state that culminates in cartilage and bone damage and systemic complications [2, 3]. One of the main costimulatory pathways involved in the positive feedback that maintains inflammation in RA is the CD40/CD40L engagement. CD40 is a well-characterized costimulatory molecule expressed by professional antigen-presenting cells that recognize CD40L, which is mainly expressed on activated CD4 + T cells. However, upon inflammatory stimulus, both molecules can be upregulated in several other cell types including other leukocytes, endothelial cells, fibroblasts and platelets [4]. In RA, CD40/CD40L signals promote autoantibody production and class switching as well as the expression of proinflammatory cytokines, chemokines, adhesion molecules and other costimulatory molecules. Moreover, this pathway is directly involved in tissue damage by stimulating the expression of matrix metalloproteinases (MMP) and the receptor activator of NF-kB ligand (RANKL) [5].
Like other members of the TNF family, CD40 and CD40L have been shown to be released in a soluble form [6,7,8,9,10]. Both molecules are cleaved by proteolytic mechanisms and soluble CD40 (sCD40) is also produced by alternative splicing [11], although the mechanisms regulating its expression are not well understood. It is suggested that sCD40 acts as a decoy receptor, whereas soluble CD40L (sCD40L) has cytokine-like properties.
Currently, overexpression of CD40L mRNA and cell surface protein has been reported in synovial tissue of patients with RA [12,13,14]. Likewise, high frequencies of CD4 + T cells expressing CD40L [15, 16] as well as high levels of sCD40L have been detected in the periphery of RA patients [17,18,19]. It is suggested that CD40L dysregulation may be exacerbated in female patients due to the coding of the CD40L gene on the X chromosome [20]. However, there are conflicting results regarding CD40L expression in association with the clinical features of RA patients, probably due to the heterogeneity of the patients studied.
High CD40 mRNA expression has also been reported in synovial tissue of RA patients [14]. However, to date, there are no studies assessing neither serum sCD40 levels nor the expression of the membrane-bound form in CD4 + T cells of RA patients. There are some studies suggesting that the assessment of these CD40 protein forms is relevant in autoimmune diseases. Elevated levels of sCD40 have been reported in patients with systemic lupus erythematosus [21], and the expansion of a population of CD4 + T cells expressing CD40 in patients with type 1 diabetes [22] and patients with multiple sclerosis [23] has been described. The assessment of mRNA, cell surface protein and soluble protein levels of CD40 and CD40L in a homogeneous group of patients is an approach that could yield useful data regarding the CD40/CD40L axis and its clinical relevance in RA.
In the present study, we determined mRNA, cell surface protein and soluble protein levels of CD40 and CD40L in association with clinical activity according to DAS28 and with the clinical features of RA patients.
Materials and methods
Subjects
A total of 38 female RA patients classified according to the 2010 American College of Rheumatology (ACR), and the European League Against Rheumatism (EULAR) criteria were recruited from Rheumatology Service of O.P.D. Hospital Civil de Guadalajara “Fray Antonio Alcalde,” Guadalajara, Jalisco, Mexico. Patients with other rheumatic, inflammatory and infectious diseases were excluded. The control subjects (CS) group was formed by a total of 10 gender- and age-matched healthy individuals.
Clinical assessment
All patients were evaluated by a rheumatologist, and the clinical and demographic data were obtained. Clinical activity was assessed by the Disease Activity Score 28 (DAS28) considering C reactive protein (CRP) values. According to DAS28 score, patients were stratified in remission (DAS28 < 2.6; n = 10), low activity (DAS28 ≥ 2.6 < 3.2; n = 9), moderate activity (DAS28 ≥ 3.2 < 5.1; n = 10) and high activity (DAS28 ≥ 5.1; n = 9). Functional disability was evaluated by the Spanish version of the Health Assessment Questionnaire-Disability Index (HAQ-DI). From a peripheral blood sample, the following clinical parameters were determined in all subjects: the erythrocyte sedimentation rate (ESR) was determined by the Wintrobe method; levels of CRP and rheumatoid factor (RF) were quantified using a turbidimetric assay (COD31029 and COD31030, respectively; Biosystems, Spain); anti-cyclic citrullinated peptide (anti-CCP) antibodies were quantified by a second-generation ELISA assay, following manufacturer’s instructions (FCCP600; DIASTAT, Axis-Shield Diagnostics, UK).
Quantitative mRNA expression analysis
The CD40 and CD40L mRNA relative expression was determined by real-time quantitative PCR (qPCR). Total RNA extraction was performed from peripheral blood leukocytes following the modified Chomczynski and Sacchi method [24] using TRIzol™ reagent (Thermo Fisher Scientific, Waltham, Massachusetts, USA). Complementary DNA (cDNA) was synthesized from 1 μg of total RNA using Oligo(dT) and M-MLV reverse transcriptase (Promega Corp., Madison, WI, USA), following manufacturer’s protocol; cDNA samples were stored at − 80 °C until use. PCR was carried out in a LightCycler® 96 (Roche Applied Science, Penzberg, Germany) instrument using FastStart Essential DNA Green Master kit (Roche Applied Science). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a reference gene in order to normalize CD40 and CD40L data. Primers used for each gene were GAPDH, forward: 5′ CAC TGC CAC CCA GAA GAC TGT G 3′, reverse: 5′ TGT AGG CCA TGA GGT CCA CCA C 3′; CD40, forward: 5′ CAG TCA GTG CTG TTC TTT GT 3′, reverse: 5′ GAT GGT GTC TGT TTC TGA GG 3′; CD40L, forward: 5′ CCT CAA ATT GCG GCA CA 3′, reverse: 5′ AAC AGA AGG TGA CTT GGG CAT A 3′. All PCR was run in duplicate. A melting curve analysis was performed after each PCR in order to determine the reaction’s specificity. The PCR efficiency for each gene was validated by running serial dilutions. The results obtained were analyzed by the 2−ΔCq comparative method [25].
Flow cytometry
Peripheral blood samples were obtained in tubes containing EDTA, and peripheral blood mononuclear cells (PBMC) were collected by a density gradient centrifugation protocol using Lymphoprep reagent (Axis-Shield Diagnostics, UK) and following manufacturer’s instructions. PBMC were suspended in RPMI 1640 medium (Life Technologies, Carlsbad, CA, USA; ATCC modified) with 20% fetal bovine serum (Life Technologies) and 10% dimethyl sulfoxide (Sigma-Aldrich Co., St. Louis, MO, USA) in a total volume of 1 mL and stored at − 80 °C until use.
For staining procedure, PBMC were thawed carefully and washed once with 9 mL of RPMI1640 medium (Life Technologies) followed by an additional wash with 4 mL of phosphate-buffered saline (PBS) solution 1 X (Sigma-Aldrich Co.). Cells were suspended in 1 mL of PBS 1 X and checked for viability in a Neubauer chamber using trypan blue (Sigma-Aldrich Co.). A 4-color flow cytometry staining protocol was performed to analyze the CD40 and CD40L expression on gated CD4 + T cells using the following panel of monoclonal antibodies (Biolegend Inc., San Diego, CA, USA): APC/Cy7 anti-CD3 (Clone UCTH1), Alexa 488 anti-CD4 (Clone RPA-T4), APC anti-CD40 (Clone 5C3) and PE anti-CD40L (Clone 24–31). Isotype control IgG antibodies conjugated with the same fluorochromes were used as a negative control (Biolegend Inc., San Diego, CA, USA). In order to accurately identify CD40- and CD40L-positive events, the fluorescence minus one experiment was included as an additional negative control. Samples were analyzed in the acoustic focusing Attune® NxT flow cytometer (Life Technologies). The data obtained were analyzed using FlowJo software v10.0 (Tree Star, Inc., Ashland, Oregon, USA). First, lymphocytes were gated according to their forward scatter (FC) and side scatter (SS) characteristics. Then, further gates were placed around CD3 + CD4 + T cells, and subsequently, the CD40 and CD40L expression was evaluated. Gating strategy and assessment of CD40L positive expression are shown in Fig. 1.
Quantification of sCD40 and sCD40L
Serum and plasma samples were obtained from peripheral blood of RA patients and control subjects in order to determine sCD40 and sCD40L levels, respectively. Samples were stored at − 20 °C until use. The sCD40 and sCD40L levels were quantified using a commercial enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems, Minneapolis, MN, USA), following the manufacturer’s protocol. The CD40 assay has a detection range of 19.5–1250 pg/mL and a sensitivity of 0.54 pg/mL, while the CD40L assay has a detection range of 62.5–4000 pg/mL and a sensitivity of 4.2 pg/mL.
Statistical analysis
Statistical analysis was carried out using STATA version 12.0 (StataCorp, College Station, TX, USA) and GraphPad Prism version 5.0 (GraphPad Software, San Diego, CA, USA) software. Variable distribution was determined using Shapiro–Wilk normality test. Variables with a normal distribution are presented in mean ± standard deviation (SD), and variables non-normally distributed are presented in median and 5–95th centiles. Categorical variables are presented in percentage and absolute frequency. Comparisons between groups were performed using Student’s t and ANOVA tests for parametric data or Mann–Whitney U and Kruskal–Wallis tests for nonparametric data, respectively. Linear correlation coefficients were determined using Spearman’s correlation test. A p value of < 0.05 was considered significant.
Results
Clinical features of RA patients
Clinical features of RA patients are summarized in Table 1. The mean age was 48.2 ± 10.8 years, and the median of disease evolution was 5.0 (0.9–22.5) years. The mean age in the control group was 49.5 ± 10.8 years. Inflammation markers were increased in RA patients [CRP = 6.4 mg/L (1.1–40.3); ESR = 29.8 ± 11.8 mm/h] with higher values of CRP according to clinical activity (p < 0.01); this was not the case for ESR. The CRP and ESR values in the control group were lower than those of RA patients [2.0 mg/L (0.4–3.8); p < 0.001 and 24 ± 8.6 mm/h; p = 0.15, respectively]. The RA patients presented mild disability according to the Spanish HAQ-DI score and an average moderate clinical activity according to DAS28 score (3.6 ± 1.5). Most of the patients showed positive and high positive values of RF and anti-CCP antibodies (71.1% and 94.7%, respectively), with a tendency to higher titers of RF in relation to the clinical activity. All patients were under treatment with one or more disease-modifying anti-rheumatic drugs (DMARDs) or biologics in addition to nonsteroidal anti-inflammatory drugs (NSAIDs) or low-dose steroids. Stratified according to clinical activity, RA groups were similar in age, time of disease evolution and treatment. RA patients with very active disease (DAS28 > 5.1) showed higher disability and the highest values of ESR and autoantibodies.
CD40 and CD40L mRNA gene expression in RA patients, association with clinical features and disease activity according to DAS28
Relative mRNA quantification of CD40 and CD40L was performed by qPCR, and data obtained were analyzed using 2−ΔCq comparative method. Regarding CD40 mRNA levels, there were no significant differences between RA patients and CS. Similarly, when CD40 mRNA levels were stratified according to DAS28 score, no differences were observed between the RA clinical activity groups (data not shown). On the other hand, as shown in Fig. 2a, RA patients showed significantly higher CD40L mRNA expression compared to CS (p < 0.0001). Furthermore, when RA patients were stratified according to DAS28 score and compared with CS, CD40L mRNA expression was higher as the clinical activity increased (remission p = 0.043; low activity p < 0.0001; moderate activity p = 0.0004; high activity p = 0.0068; Fig. 3a). Nevertheless, RA patients classified as high clinical activity showed a different behavior since CD40L mRNA expression was similar to RA patients in remission state, as depicted in Fig. 3a.
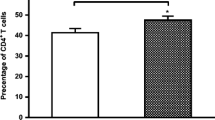
CD40L levels in RA patients and control subjects (CS). a Relative units of expression of CD40L mRNA; b representative dot plots of CD40L-expressing CD4 + T cells and graphics of CD4 + CD40L + T cell frequencies and CD40L MFI from CD4 + T cells; c levels of soluble CD40L (sCD40L). Data presented in mean ± SEM. Statistical comparisons between groups were determined using Mann–Whitney U test. * p < 0.05; ** p < 0.01; *** p < 0.0001. MFI: mean fluorescence intensity
CD40L levels in RA patients stratified according to DAS28. a Relative units of expression of CD40L mRNA; b representative dot plots of CD40L-expressing CD4 + T cells and graphics of CD4 + CD40L + T cell frequencies and CD40L MFI from CD4 + T cells; c levels of soluble CD40L (sCD40L). DAS28 groups: remission (< 2.6), low activity (≥ 2.6 < 3.2), moderate activity (≥ 3.2 < 5.1), high activity (≥ 5.1). Data presented in mean ± SEM. Statistical comparisons between groups were determined using Mann–Whitney U test. * p < 0.05; ** p < 0.01; *** p < 0.0001. CS: control subjects; MFI: mean fluorescence intensity
A linear correlation analysis was performed to identify an association between the clinical features of RA patients and the CD40 or CD40L mRNA levels. As shown in Table 2, no significant correlations were identified regarding the time of disease evolution, scores of clinical activity and disability, levels of inflammation markers and titers of autoantibodies.
CD40 and CD40L cell surface expression on CD4 + T cells in RA patients and its association with RA clinical features
Increased frequencies of the CD4 + CD40 + T cell population have been previously observed in autoimmune diseases [22, 23]. These and other studies suggest that this cell population has an increased cytokine-producing and cell activation capacity [26]. However, nowadays the frequency of CD4 + CD40 + T cells has not been reported for RA patients. In contrast, the subpopulation of CD4 + CD40L + T cells has been observed at low frequencies in peripheral blood of healthy subjects; however, previous reports have shown its expansion in some autoimmune diseases including RA [5, 15, 16]. Nonetheless, there is no evidence regarding CD4 + CD40L + T cell frequency in association with DAS28 stratification groups. In the present study, this approach was evaluated in PBMC of RA patients and CS by a multicolor flow cytometry assay.
The RA patients showed significantly higher frequencies of CD4 + CD40 + T cells compared to CS (21.49% ± 2.14 vs. 11.26% ± 2.18; p = 0.020) as well as an increased CD40 expression given by the MFI (4633 ± 94.13 vs. 4183 ± 96.63; p = 0.018). However, when stratified according to DAS28 score, no significant differences were observed between the clinical activity groups (remission: 17.84% ± 2.71; low activity: 26.25% ± 5.32; moderate activity: 16.89% ± 3.04; high activity: 25.89% ± 5.54; p = 0.41). Compared to CS, the frequency of CD4 + CD40 + T cells in the low activity group was significantly increased (p = 0.022).
Consistent with previous studies, the frequency of CD4 + CD40L + T cells was increased in RA patients compared to CS (9.07% ± 0.79 vs. 5.79% ± 0.78; p = 0.036). The CD40L expression in this cell population was also increased in the RA group (given by the MFI; p = 0.007; Fig. 2b). Moreover, RA patients showed a tendency toward higher frequencies of CD4 + CD40L + T cells as the DAS28 score increased (remission: 7.17% ± 1.03; low activity: 8.89% ± 1.44; moderate activity: 9.48% ± 1.47; high activity: 10.89% ± 2.24), as shown in representative dot plots depicted in Fig. 3b; however, the differences between the clinical activity groups were not significant (p = 0.52). Only a significant increase of CD4 + CD40L + T cells percentage was observed in the high activity group compared to CS (p = 0.028). Likewise, an increased expression of CD40L on CD4 + T cells was observed, being significant in low and moderate activity groups compared to CS (p = 0.022 and p = 0.015, respectively; Fig. 3b).
Considering a previous study reporting a higher frequency of CD4 + CD40L + T cells in patients with early RA compared with patients with established disease [16], this approach was evaluated in the present study. Compared with patients with established RA (> 2-year evolution, n = 30), early RA patients (< 2 years evolution, n = 8) presented higher frequencies of CD4 + CD40 + T cells (18.40% ± 2.19 vs. 29.79% ± 4.60) and CD4 + CD40L + T cells (7.98% ± 0.69 vs. 10.99% ± 1.68), being significant only for CD40-positive cells (p = 0.02 and p = 0.12, respectively). In addition, a linear correlation analysis of the CD4 + CD40 + and CD4 + CD40L + T cell frequencies with the clinical features of RA patients was performed. As shown in Table 2, no significant correlations were observed.
Levels of sCD40 and sCD40L in RA patients and its association with clinical features and disease activity
The soluble form of the CD40 receptor, sCD40, has been reported to be produced by alternative splicing mechanisms [11] as well as by proteolytic shedding [6]. Moreover, the sCD40 molecule has been suggested to act as a natural antagonistic decoy receptor. In order to identify a possible alteration regarding sCD40 in RA patients, the serum levels of sCD40 from RA patients and CS were determined. RA patients showed higher levels of sCD40 compared to CS, however, this difference was not significant (510.2 ± 17.15 pg/mL vs. 457.5 ± 26.39 pg/mL; p = 0.29). Likewise, no differences were observed when stratifying RA patients according to clinical activity (remission: 514.6 ± 31.11 pg/mL; low activity: 505.9 ± 53.91 pg/mL; moderate activity: 493.2 ± 13.0 pg/mL; high activity: 528.5 ± 36.45 pg/mL; p = 0.79).
CD40L is released by proteolytic shedding from the cell surface of CD4 + T cells [10] and activated platelets [7, 9]. In the present study, plasma sCD40L levels were quantified in RA patients and CS. Interestingly, as noted in mRNA and surface protein levels, sCD40L was also found increased in RA patients compared to controls (89.01 ± 7.23 pg/mL vs. 54.34 ± 2.33 pg/mL; p = 0.001), as shown in Fig. 2c. When stratified according to DAS28 score, all clinical activity groups showed higher levels of sCD40L compared to CS (remission: 74.43 ± 9.79 pg/mL, p = 0.049; low activity: 93.82 ± 14.18 pg/mL, p = 0.003; moderate activity: 118.4 ± 19.38 pg/mL, p = 0.001; high activity: 67.72 ± 4.67 pg/mL, p = 0.027). As observed with mRNA levels, higher levels of sCD40L were found in RA patients according to DAS28 scores increase, with the exception of the high activity group whose sCD40L levels were significantly lower compared to moderate clinical activity patients (p = 0.017; Fig. 3c).
Finally, a correlation analysis of sCD40 and sCD40L levels with the clinical features of RA patients was carried out. As shown in Table 2, a negative correlation between sCD40 and anti-CCP levels was observed (rs = − 0.340; p = 0.04). No significant associations were identified regarding sCD40L levels (Table 2).
Discussion
The CD40/CD40L pathway plays a pivotal role in the activation and progression of cellular and humoral adaptive immune response [4]. Several studies have implicated this pathway in the pathogenesis of various diseases with an autoimmune or inflammatory component. In RA, CD40/CD40L engagement is involved in the production of autoantibodies and molecules, as proinflammatory cytokines and RANKL, that promote inflammation and tissue damage [5]. However, there are currently no consistent results on the expression of CD40 and CD40L and their relationship with clinical activity and the clinical characteristics of RA patients.
As currently known, CD40 expression is mainly constitutive in antigen-presenting cells (APC); however, its expression can be upregulated in these and other cell types under inflammatory stimulus, including cells playing a direct role in tissue damage in RA [27,28,29]. In the present study, no alterations regarding CD40 mRNA expression in RA patients were found. However, previous studies, including one carried out by our group, have consistently reported a high CD40 mRNA expression in RA patients [14, 30]. These differences could be due to the design of the studies, the sample size, the type of tissue analyzed as well as the clinical characteristics of the patients included.
In contrast with the CD40 findings, an increased expression of CD40L mRNA was identified in patients with RA compared with CS, which is in accordance with previous evidence that suggests an altered expression of CD40L mRNA in patients with RA [12]. In particular, a higher expression of CD40L mRNA has been shown in CD4 + T cells from females with RA in association with low levels of CD40L promoter methylation [20]. In a study conducted by our group, it was observed a 4.5-fold increased expression of CD40L mRNA in peripheral blood leukocytes from a group of women with RA compared to healthy women [31]. A more recent work has reported a higher expression of CD40L mRNA in synovial tissue from early and established RA patients in contrast to synovial tissue from osteoarthritis patients or healthy individuals [14]. Taken together, all of these results suggest that there is a deregulation of CD40L expression at the transcriptional level both in the periphery and synovial tissue in patients with RA and this might be exacerbated in female individuals due to the influence of epigenetic factors. Interestingly, similar results have been described for other autoimmune diseases like systemic lupus erythematosus [32], multiple sclerosis [33] and systemic sclerosis [34]. Moreover, an increased expression of CD40L mRNA was found according to clinical activity, except for patients with very active disease (DAS28 > 5.1). Currently, the aim of the treatment in RA patients is based on the achievement of a remission state and this often involves an aggressive treatment with more than one DMARD or biological agents, especially in patients with persistent high activity. Furthermore, some studies have provided evidence about the ability of some treatments to modulate CD40L expression, which might explain the lowest CD40L mRNA levels observed in patients with high activity [35, 36]. This suggests that in patients with high activity disease, treatment could be helping to downregulate CD40L expression and that other molecules may be playing a key role in promoting a persistent inflammation. In the present study, an analysis considering DMARD status in association with levels of CD40L mRNA, cell surface protein and soluble protein was performed. No significant associations between the use of DMARDs and CD40L expression were found. However, this cannot be ruled out completely since RA patients without any type of treatment were not included in the study, which could represent a limitation.
In the present study, higher percentages of CD4 + CD40 + T cells and the overexpression of CD40 in this cell population were identified in RA patients compared to CS. This is the first study reporting CD4 + T cells expressing CD40 in peripheral blood of RA patients. Others have observed increased frequencies of CD4 + CD40 + T cells in type 1 diabetes [22] and multiple sclerosis [23], suggesting a possible implication in disease pathogenesis. Interestingly, the expression of CD40 in T cells has been reported to be as efficient as CD28 signals to induce T cell activation [26]. Although no associations were identified with the clinical parameters of RA patients, functional studies concerning this cell population could reveal new pathogenic molecular mechanisms involving the CD40/CD40L pathway in RA. An increased frequency of CD4 + CD40L + T cells, as well as an increased expression of CD40L according to clinical activity, was also identified in RA patients. These results support previous findings that have shown a high frequency of CD4 + CD40L + T cells in peripheral blood and synovial fluid of patients with RA [13, 15, 35]. Even though the frequency of CD4 + CD40L + T cells varies between studies, which could be due to differences in methodology design or clinical characteristics of the patients, these findings support the role that is suggested for CD40L as a marker of clinical activity in RA.
Another interesting finding was the increased frequencies of CD4 + T cells expressing CD40 and CD40L in RA patients with early disease. These results are consistent with that reported by Kosmaczewska et al. [16], who identified increased percentages of CD4 + CD40L + T cells in patients with early RA, suggesting that this could be related to the loss of CD28 expression followed by chronic T cell stimulation in RA patients with established disease [37, 38]. Since this is the first study demonstrating the expansion of the CD4 + CD40 + T cell subpopulation in RA patients, it remains to be determined whether this is also a consequence of chronic stimulation or a compensatory mechanism due to the loss of other costimulatory molecules like CD28. An interesting approach would be to determine whether the altered expression of CD40 and CD40L in T cells is a key factor in RA onset by stimulating the production of autoantibodies and proinflammatory molecules since the overexpression of CD40 and CD40L mRNA has been reported in synovial tissue from undifferentiated arthritis patients and early RA patients [14].
Like other members of the TNF receptor (TNFR) family, the CD40 receptor also has a soluble form. Although an antagonistic role has been attributed to this molecule, sCD40 serum levels in RA patients hadn’t been reported until now. No significant differences were found when comparing sCD40 levels between RA patients and CS as well as between the RA clinical groups. On the other hand, a negative correlation was identified between sCD40 and anti-CCP levels. It has been described that proteolytic cleavage and release of CD40 in its soluble form in B cells are dependent on the binding with CD40L of activated CD4 + T cells and have been proposed as a regulatory mechanism in T cell-dependent B cell activation [6, 39]. Moreover, an altered expression of CD40 isoforms mRNA has been reported in synovial tissue of RA patients [14]; however, it is still not well described how the expression of these isoforms is regulated and its function in antibody production. Taking together, it appears that CD40 protein and mRNA regulation are active molecular mechanisms in RA patients and that could be closely related to autoantibody production.
The soluble form of CD40L is produced by proteolytic shedding from the cell surface of activated CD4 + T cells and platelets and has been determined to function as a proinflammatory molecule. Soluble levels of CD40L were significantly higher in patients with RA compared to SC. These results are in accordance with previously published work reporting high levels of sCD40L in RA patients compared to healthy subjects [17,18,19]. In the present study, when RA patients were stratified by DAS28 score, as it was observed in mRNA and membrane protein expression, the sCD40L showed an increase according to the degree of clinical activity. A similar behavior regarding sCD40L levels has been reported for SLE patients [40]. In the study conducted by Pamuk et al., higher levels of sCD40L in patients with active RA (DAS28 > 5.1) compared to patients with inactive RA (DAS28 ≤ 5.1) were observed; however, as in our study, the differences between the groups of patients were not significant [19]. Taking all together, this suggests that sCD40L may be contributing to the degree of activity in RA patients. However, as observed with the expression of CD40L mRNA, patients with high activity presented lower levels of sCD40L compared with patients with moderate activity. Since the main cellular source of sCD40L is activated platelets, it is unlikely that treatment in these patients plays a direct role in the modulation of CD40L expression in these cells; however, an indirect role cannot be ruled out. On the other hand, this could be related to differences in the mechanisms involved in the CD40L shedding in this group of patients. High levels of MMP-2 and MMP-9 along with an increased enzymatic activity have been described in the circulation of RA patients compared to healthy subjects [41]. Previous studies have shown that these enzymes are the ones that mediate the proteolytic cleavage of CD40L from platelets [7,8,9]. The evaluation of sCD40L in association with the levels and enzymatic activity of MMP-2 and MMP-9 in patients with different clinical activity could yield interesting data, since it has been reported an increased platelet activation as well as an association between platelet counts and clinical activity in RA patients [19]. Currently, there is a report of the association between the levels of sCD40L and MMP-2 and MMP-9 in relation to the activity and the different clinical presentations in multiple sclerosis patients [42]. As observed in other studies, sCD40L values were not correlated with clinical features of RA patients. There is one study where sCD40L levels were positively correlated with RF factor titers; however, this association could have been influenced by the inclusion of RA patients with vasculitis [17].
Finally, it is worth to mention that, in order to exclude a possible influence of gender regarding CD40L expression, only female subjects were included. This could be a limitation since the associations observed were not determined considering male RA patients. In addition, a stratification analysis of CD40 and CD40L levels considering anti-CCP titers could have yield interesting data; however, it was not possible to perform this since most of the included patients presented high positive values of anti-CCP.
Taking together, the results in the present study show that there is a dysregulation in the molecular mechanisms around CD40L production (mRNA, cell surface protein and soluble protein) in females with RA in close association with the clinical activity. Interestingly, this was not the case for high activity patients. In addition, the expansion of CD4 + T cells expressing CD40 was demonstrated in the patients included in this study. Future studies are needed to validate the clinical utility of CD40L and to determine which are the molecules that could be overcoming CD40L downregulation and promoting a persistent inflammation in patients with very active disease.
References
Smolen JS, Aletaha D, Barton A, et al. Rheumatoid arthritis. Nat Rev Dis Primers. 2018;4:18001.
Firestein GS, McInnes IB. Immunopathogenesis of rheumatoid arthritis. Immunity. 2017;46:183–96.
McInnes IB, Buckley CD, Isaacs JD. Cytokines in rheumatoid arthritis—shaping the immunological landscape. Nat Rev Rheumatol. 2016;12:63–8.
Elgueta R, Benson MJ, de Vries VC, Wasiuk A, Guo Y, Noelle RJ. Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol Rev. 2009;229:189.
Peters AL, Stunz LL, Bishop GA. CD40 and autoimmunity: the dark side of a great activator. Semin Immunol. 2009;21:293–300.
Contin C, Pitard V, Itai T, Nagata S, Moreau JF, Déchanet-Merville J. Membrane-anchored CD40 is processed by the tumor necrosis factor-alfa-converting enzyme: implications for CD40 signaling. J Biol Chem. 2003;278:32801–9.
Menchén L, Marín-Jiménez I, Arias-Salgado EG, et al. Matrix metalloproteinase 9 is involved in Crohn’s disease-associated platelet hyperactivation through the release of soluble CD40 ligand. Gut. 2009;58:920–8.
Reinboldt S, Wenzel F, Rauch BH, et al. Preliminary evidence for a matrix metalloproteinase-2 (MMP-2)-dependent shedding of soluble CD40 ligand (sCD40L) from activated platelets. Platelets. 2009;20:441–4.
Choi WS, Jeon OH, Kim DS. CD40 ligand shedding is regulated by interaction between matrix metalloproteinase-2 and platelet integrin αIIbβ3. J Thromb Haemost. 2010;8:1364–71.
Yacoub D, Benslimane N, Al-Zoobi L, Hassan G, Nadiri A, Mourad W. CD154 is released from T-cells by a disintegrin and metalloproteinase domain-containing protein 10 (ADAM10) and ADAM17 in a CD40 protein-dependent manner. J Biol Chem. 2013;288:36083–93.
Eshel D, Toporik A, Efrati T, Nakav S, Chen A, Douvdevani A. Characterization of natural human antagonistic soluble CD40 isoforms produced through alternative splicing. Mol Immunol. 2008;46:250–7.
MacDonald KP, Nishioka Y, Lipsky PE, Thomas R. Functional CD40 ligand is expressed by T cells in rheumatoid arthritis. J Clin Invest. 1997;100:2404–14.
Liu MF, Chao SC, Wang CR, Lei HY. Expression of CD40 and CD40 ligand among cell populations within rheumatoid synovial compartment. Autoimmunity. 2001;34:107–13.
Guo Y, Walsh AM, Fearon U, et al. CD40L-dependent pathway is active at various stages of rheumatoid arthritis disease progression. J Immunol. 2017;198:4490–501.
Berner B, Wolf G, Hummel KM, Müller GA, Reuss-Borst MA. Increased expression of CD40 ligand (CD154) on CD4 + T cells as a marker of disease activity in rheumatoid arthritis. Ann Rheum Dis. 2000;59:190–5.
Kosmaczewska A, Ciszak L, Swierkot J, Szteblich A, Wiland P, Frydecka I. Alterations in both the activatory and inhibitory potential of peripheral blood CD4 + T cells in rheumatoid arthritis patients correlate with disease progression. Pathol Oncol Res. 2014;20:235–43.
Tamura N, Kobayashi S, Kato K, et al. Soluble CD154 in rheumatoid arthritis: elevated plasma levels in cases with vasculitis. J Rheumatol. 2001;28:2583–90.
Goules A, Tzioufas AG, Manousakis MN, Kirou KA, Crow MK, Routsias JG. Elevated levels of soluble CD40 ligand (sCD40L) in serum of patients with systemic autoimmune diseases. J Autoimmun. 2006;26:165–71.
Pamuk GE, Vural Ö, Turgut B, Demir M, Pamuk ÖN, Çakir N. Increased platelet activation markers in rheumatoid arthritis: are they related with subclinical atherosclerosis? Platelets. 2008;19:146–54.
Liao J, Liang G, Xie S, et al. CD40L demethylation in CD4 + T cells from women with rheumatoid arthritis. Clin Immunol. 2012;145:13–8.
Chen JM, Guo J, Wei CD, et al. The association of CD40 polymorphisms with CD40 serum levels and risk of systemic lupus erythematosus. BMC Genet. 2015;16:121.
Waid DM, Wagner RJ, Putnam A, et al. A unique T cell subset described as CD4loCD40 + T cells (TCD40) in human type 1 diabetes. Clin Immunol. 2007;124:138–48.
Waid DM, Schreiner T, Vaitaitis G, Carter JR, Corboy JR, Wagner DH. Defining a new biomarker for the autoimmune component of Multiple Sclerosis: Th40 cells. J Neuroimmunol. 2014;270:75–85.
Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–9.
Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–8.
Munroe ME, Bishop GA. A Costimulatory Function for T Cell CD40. J Immunol. 2007;178:671–82.
Cho CS, Cho ML, Min SY, et al. CD40 engagement on synovial fibroblast up-regulates production of vascular endothelial growth factor. J Immunol. 2000;164:5055–61.
Gotoh H, Kawaguchi Y, Harigai M, et al. Increased CD40 expression on articular chondrocytes from patients with rheumatoid arthritis: contribution to production of cytokines and matrix metalloproteinases. J Rheumatol. 2004;31:1506–12.
Lee HY, Jeon HS, Song EK, et al. CD40 ligation of rheumatoid synovial fibroblasts regulates RANKL-mediated osteoclastogenesis: evidence of NF-κB-dependent, CD40-mediated bone destruction in rheumatoid arthritis. Arthritis Rheum. 2006;54:1747–58.
Román-Fernández IV, Ávila-Castillo DF, Cerpa-Cruz S, et al. CD40 functional gene polymorphisms and mRNA expression in rheumatoid arthritis patients from western Mexico. Genet Mol Res. 2016;15:gmr15048775.
Román-Fernández IV, Sánchez-Zuno GA, Padilla-Gutiérrez JR, et al. The 3′-UTR (CA)n microsatellite on CD40LG gene as a possible genetic marker for rheumatoid arthritis in Mexican population: impact on CD40LG mRNA expression. Clin Rheumatol. 2018;37:345–53.
Lu Q, Wu A, Tesmer L, Ray D, Yousif N, Richardson B. Demethylation of CD40LG on the Inactive X in T Cells from Women with Lupus. J Immunol. 2007;179:6352–8.
Huang WX, Huang P, Hillert J. Systemic upregulation of CD40 and CD40 ligand mRNA expression in multiple sclerosis. Mult Scler. 2000;6:61–5.
Lian XR, Xiao R, Hu XH, et al. DNA demethylation of CD40L in CD4 + T cells from women with systemic sclerosis: a possible explanation for female susceptibility. Arthritis Rheum. 2012;64:2338–45.
Tung CH, Lu MC, Lai NS, Wu SF. Tumor necrosis factor-α blockade treatment decreased CD154 (CD40-ligand) expression in rheumatoid arthritis. PLoS ONE. 2017;12:1–12.
Wu SF, Chang CB, Hsu JM, et al. Hydroxychloroquine inhibits CD154 expression in CD4 + T lymphocytes of systemic lupus erythematosus through NFAT, but not STAT5, signaling. Arthritis Res Ther. 2017;19:183.
Valenzuela HF, Effros RB. Divergent telomerase and CD28 expression patterns in human CD4 and CD8 T cells following repeated encounters with the same antigenic stimulus. Clin Immunol. 2002;105:117–25.
Vallejo AN, Weyand CM, Goronzy JJ. T-cell senescence: a culprit of immune abnormalities in chronic inflammation and persistent infection. Trends Mol Med. 2004;10:119–24.
Van Kooten C, Gaillard C, Galizzi JP, et al. B cells regulate expression of CD40 ligand on activated T cells by lowering the mRNA level and through the release of soluble CD40. Eur J Immunol. 1994;24:787–92.
Vakkalanka RK, Woo C, Kirou KA, Koshy M, Berger D, Crow MK. Elevated levels and functional capacity of soluble CD40 ligand in systemic lupus erythematosus sera. Arthritis Rheum. 1999;42:871–81.
Chang YH, Lin IL, Tsay GJ, et al. Elevated circulatory MMP-2 and MMP-9 levels and activities in patients with rheumatoid arthritis and systemic lupus erythematosus. Clin Biochem. 2008;41:955–9.
Sanchooli J, Ramroodi N, Sanadgol N, Sarabandi V, Ravan H, Rad RS. Relationship between metalloproteinase 2 and 9 concentrations and soluble CD154 expression in Iranian patients with multiple sclerosis. Kaohsiung J Med Sci. 2014;30:235–42.
Acknowledgements
This research was supported by funding from the National Council of Science and Technology (CONACYT, Grant No. 180663), CONACYT-México-Universidad de Guadalajara, awarded to Muñoz-Valle JF. The funding source had no involvement in any phase of the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Ethical approval
All procedures were performed following the ethical guidelines established in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. The study was approved by Comité de ética en Investigación y Bioseguridad, Centro Universitario de Ciencias de la Salud, Universidad de Guadalajara (reference No. 0122017).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Román-Fernández, I.V., García-Chagollán, M., Cerpa-Cruz, S. et al. Assessment of CD40 and CD40L expression in rheumatoid arthritis patients, association with clinical features and DAS28. Clin Exp Med 19, 427–437 (2019). https://doi.org/10.1007/s10238-019-00568-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10238-019-00568-5