Abstract
Matrix metalloproteinase-2 (MMP-2) has been linked with tumor invasion and metastasis. However, the role of MMP-2 expression in ovarian cancer remains controversial. By searching the PubMed, Embase, Wanfang, and China National Knowledge Infrastructure databases, we conducted a meta-analysis to evaluate the pathological and prognostic significance of MMP-2 in ovarian cancer. Studies were pooled, and the odds ratio (OR) and its corresponding 95 % confidence interval (CI) were calculated. Version 11.0 STATA software was used for statistical analysis. Twenty-seven relevant articles were included for this meta-analysis study. The expression of MMP-2 in cancer tissue was significantly higher than that in benign or normal ovarian tissue [cancer vs. benign, OR 10.09 (95 % CI 6.95–14.64); P < 0.001; cancer vs. normal, OR 30.48 (95 % CI 17.19–54.05); P < 0.001; benign vs. normal, OR 1.88 (95 % CI 1.08–3.29); P = 0.025]. The expression of MMP-2 in stage III–IV or lymph node metastasis was significantly higher than that in stage I–II or that without metastasis, respectively [OR 5.83 (95 % CI 4.32–7.85); P < 0.001; OR 7.20 (95 % CI 4.75–10.91); P < 0.001]. MMP-2 was associated with histological types and grade of ovarian cancer [serous vs. mucinous, OR 1.67 (95 % CI 1.17–2.39); P = 0.004; grade 3 vs. 1, 2, OR 3.23 (95 % CI 2.29–4.55); P < 0.001]. However, the age of patients was not associated with MMP-2 expression [OR 1.25 (95 % CI 0.61–2.58); P = 0.546]. In conclusion, MMP-2 is related to the malignant degree, FIGO stage, histological types and grade, and lymph node metastasis of ovarian cancer. It may play a significant role in clinical guidelines for the treatment and prognostic evaluation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ovarian cancer is the fifth leading cause of cancer deaths in women and the main cause of gynecologic cancer mortality [1], and epithelial carcinoma is responsible for 90 % of the ovarian malignant tumors. Only 15 % of all ovarian cancers are found at stage I, when cure rates can approach 90 %. Cure rates for advanced-stage ovarian cancer are 30–35 %. As a result, 50 % of these patients die within 5 years [1, 2]. Therefore, it is necessary to identify the biological molecular makers to predict the outcomes of patients, which can effectively make strategies and improve the survival rate of ovarian cancer.
The matrix metalloproteases (MMPs) are zinc-dependent endopeptidases that degrade the extracellular matrix collagens and belong to a larger family of proteases known as the metzincin superfamily [3, 4]. The MMP-2 is one of the most important basement membrane, gelatin, type IV, V, VII, and X collagen-degrading enzymes of the MMP family in ovarian cancer [5]. MMP-2 is released in a pro-form and is activated through a unique extracellular proteolytic process by MT1-MMP (membrane type 1 matrix metalloproteinase) [6, 7]. Studies have shown that MMP-2 is overexpressed in some carcinoma tissues, e.g., the head and neck squamous cell carcinoma with higher ability of invasion and metastasis [8]. The expression of activated MMP-2 was significantly related to disease progression in epithelial ovarian carcinomas [9], and the MMP-2 expression was found to be significantly correlated with the histological grade and with the surgical stage of the malignant ovarian epithelial tumors [10, 11]. However, some researchers concluded that MMP-2 protein expression has no impact on the prognosis for ovarian cancer [12–14]. There is no meta-analysis study to reveal the association between MMP-2 expression and clinicopathological parameters of ovarian cancer. Therefore, we carried out the first meta-analysis to assess the potential association between MMP-2 expression and pathological and prognostic significance in ovarian cancer.
Materials and methods
Search strategy
We searched for relevant studies up to September 2014 through the PubMed, Embase, Wanfang, and China National Knowledge Infrastructure platform (CNKI) database with the following terms and their combinations: “ovarian cancer/ovarian carcinoma” and “matrix metalloproteinase-2/MMP-2.” All scanned abstracts, studies, and citations were reviewed. Moreover, references of the retrieved manuscripts were also manually cross-searched for further relevant publications.
Selection criteria
The inclusion criteria included: (1) the articles which had the association between MMP-2 expression and the clinicopathological significance of ovarian cancer; (2) the articles which had the association of MMP-2 expression and prognosis in patients with ovarian cancer; (3) the studies which utilized RT-PCR for detection of MMP-2 mRNA and immunohistochemical staining for tissue MMP-2 expression. The exclusion criteria included: (1) the studies which used the same population or overlapping database; (2) the studies of in vitro cell culture models.
Data extraction
All the available data were extracted from each study by two investigators independently according to the inclusion criteria listed above. The study characteristics were recorded as follows: (1) the first author, country, and article publication year; (2) the number of cancer cases, benign cases, and controls for positive MMP-2 expression (MMP-2 expression score ≥+), which was measured by semiquantitatively assessing the percentage of tumor cells expressing MMP-2, intensity of cell staining, and extent of staining; (3) the number of test cases (>60 years old, FIGO stage III–IV, and lymph nodes metastasis) and control cases (≤60 years old, FIGO stage I–II, and no lymph nodes metastasis) for positive MMP-2 expression; (4) the number of test cases (grade 3) and control cases (grades 1, 2); (5) the number of test cases (serous histological types) and test cases (mucinous histological types) for positive MMP-2 expression.
Statistical analysis
We estimated the odds ratio (OR) for clinicopathological variables (>60 vs. ≤60 years old, FIGO stage III–IV vs. I–II, lymph nodes metastasis vs. no lymph nodes metastasis, grade 3 vs. 1, 2, and serous histological types vs. mucinous histological types). The heterogeneity of the studies was assessed using the Cochran’s Q test (considered significant for P < 0.10) and was quantified by the I 2 statistic. Both Mantel–Haenszel fixed effects model, which weights the studies by the inverse of the variance of estimates, and Der Simonian and Laird random effects model, which weights the studies by the inverse of the sum of the individual sampling variance and the between studies’ variance, were used to combine the data. Relative influence of each study on the pooled estimate was assessed by omitting one study at a time for sensitivity analysis. Publication bias was evaluated by visual inspection of symmetry of Begg’s funnel plot and assessment of Egger’s test (P < 0.05 was regarded as representative of statistical significance). Statistical analyses were carried out in STATA software, version 11.0 (STATA Corp, College Station, TX, USA), and all tests were two-sided.
Results
Characteristics of the studies
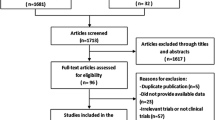
There were 157 papers relevant to the search words. Subsequently, 109 irrelevant articles were excluded. The remaining articles were systematically reviewed, and all 48 articles qualified for full-text reading. After full-text reading, 18 articles were deemed unsuitable and were therefore excluded, and 30 articles were identified to be included for qualitative analysis. In addition, another three studies were excluded due to overlapping data or not present the usable data after a more careful assessment of the remaining articles. Finally, 27 cohort studies composed of 6121 ovarian cancer samples were incorporated into the current meta-analysis. The flow chart of selection of studies and reasons for exclusion is presented in Fig. 1. The main characteristics of the eligible studies are shown in Table 1.
Quantitative synthesis
All 27 studies including 6121 patients explored the association between MMP-2 expression and clinicopathological variables of ovarian cancer. Table 2 summarizes the evaluations of association between MMP-2 expression and clinicopathological variables of ovarian cancer. We performed pooled analysis with available data on the association between MMP-2 expression and age, pathological type, histological type, FIGO stage, histological grade, and lymph node metastasis. We failed to find the association between MMP-2 expression and age (P = 0.546). The OR (95 % CI) was 1.25 (0.61–2.58) for age (>60 vs. ≤60). The positive MMP-2 expression was associated with pathological type, FIGO stage, histological grade, histological type, and lymph node metastasis (Figs. 2, 3, 4, all P < 0.05). The OR (95 % CI) was 10.09 (6.95–14.64), 30.48 (17.19–54.05), and 1.88 (1.08–3.29) for pathological type (cancer vs. benign, cancer vs. normal, and benign vs. normal); 3.23 (2.29–4.55) for histological grade (grade 3 vs. 1, 2); 5.83 (4.32–7.85) for FIGO stage (III–IV vs. I–II); 7.20 (4.75–10.91) for lymph node metastasis (yes vs. no); 1.67 (1.17–2.39) for histological type (serous vs. mucinous), respectively. Notably, the expressions of MMP-2 in cancer and benign tissues, FIGO III–IV stage, grade 3, and lymph node metastasis were significantly higher than those in normal ovarian tissue, FIGO I–II stage, grades 1, 2, and without lymph node metastasis.
Sensitive analysis
We further conducted sensitivity analyses to determine whether review conclusions were affected by the choice of single study; the finding suggested that no single study had the effect on the pooled ORs in the current meta-analysis (Fig. 5).
Publication bias
Finally, the Egger’s regression test showed no evidence of asymmetrical distribution in the funnel plot in MMP-2 expression in FIGO stage (Begg’s test P = 0.057; Egger’s test P = 0.061) and histological grade (Begg’s test P = 0.230; Egger’s test P = 0.197) (Fig. 6).
Discussion
Ovarian cancer is the leading cause of death from gynecologic malignancies, and because they usually do not cause symptoms until they have metastasized, patients with advanced disease account for more than two-thirds of cases [10]. Ovarian cancer therefore represents a major surgical challenge and needs intensive and often complex treatment [10, 11]. Patients frequently appear with advanced disease that is presumed to involve early and widespread metastasis mediated primarily by the transcelomic spread of tumor cells. The understanding of the degradation of basement membrane is essential to the understanding of cancer metastasis. Invasion and metastasis are triggered by degradation of basement membrane components by specific proteinases [38, 39].
Extracellular matrix degradation in the metastatic cascade is an important step, and it requires the involvement of active proteolytic enzymes such as serine proteases, cysteine proteases, and matrix metalloproteinases [10]. MMP-2 is a member of the matrix metalloproteinase families that have ability to degrade type IV collagen. MMP-2 is released in a pro-form, which can be activated by membrane-bound metalloproteinases [6, 7]. MMP-2 overexpression and activation have been associated with the invasive potential of ovarian, breast, lung, and cervical carcinomas, and MMP-2 has a key role in extracellular matrix invasion in ovarian carcinoma [10, 11].
This is, as far as we know, the first meta-analysis that explored the relationship between MMP-2 expression and clinicopathological variables of ovarian cancer. We observed that the expression of MMP-2 in cancer tissue was higher than that in benign or normal ovarian tissue, which is in agreement with previous finding [20, 26, 33]. Our meta-analysis also revealed the association between MMP-2 expression and malignant degree, FIGO stage, histological type and grade, and lymph node metastasis in ovarian cancer. This finding supports the assertion that MMP-2 can be considered as a hallmark of tumor progression in ovarian cancer. However, we failed to find the association between MMP-2 expression and the age of patients with ovarian cancer.
Several limitations of our meta-analysis should be addressed. First, there is potential publication bias in this study since we did not take some unpublished papers and abstracts and considering their data were not available to us. Secondly, language can also introduce a bias. Specifically, we only choose either English language or Chinese language and rule out other qualified research. A third potential limitation is that our study may be weakened to extract the original data from the included studies. Finally, the immunohistochemical method could affect the prognostic value due to the different detection antibodies and the application of different cutoff values for determining high MMP-2 levels. Despite the above limitations, this is the first example of a meta-analysis on the association of MMP-2 expression with the development of ovarian cancer. With the application of a statistical approach to combine the results from multiple studies in our meta-analysis and to achieve strong objectivity, all the research methods were carried out with strict inclusion and exclusion criteria, indicating the validity and significance of our conclusion.
In conclusion, despite the limitations of this meta-analysis, our study confirmed that the expression of MMP-2 in normal ovarian tissue was lower than in benign or cancer tissue of ovarian cancer. In addition, MMP-2 expression is associated with FIGO stage, histological type and grade, and lymph node metastasis, and there was no relationship between MMP-2 expression and the age of patient with ovarian cancer. Further studies with larger data set and well-designed models are required to validate our findings.
References
Siegel R, Naishadham D, Jemal A. Cancer statistics 2012. CA Cancer J Clin. 2012;62:10–29.
Chiang YC, Chen CA, Chiang CJ, et al. Trends in incidence and survival outcome of epithelial ovarian cancer: 30-year national population-based registry in Taiwan. J Gynecol Oncol. 2013;24:342–51.
Vincenti MP, White LA, Schroen DJ, Benbow U, Brinckerhoff CE. Regulating expression of the gene for matrix metalloproteinase-1 (collagenase): mechanisms that control enzyme activity, transcription, and mRNA stability. Crit Rev Eukaryo Gene Expr. 1996;6:391–411.
Galateau-Salle FB, Luna RE, Horiba K, et al. Matrix metalloproteinases and tissue inhibitors of metalloproteinases in bronchial squamous preinvasive lesions. Hum Pathol. 2000;31:296–305.
Collier IE, Wilhelm SM, Eisen AZ, et al. H-ras oncogene-transformed human bronchial epithelial cells (TBE-1) secrete a single metalloprotease capable of degrading basement membrane collagen. J Biol Chem. 1988;263:6579–87.
Nagase H, Woessner JF Jr. Matrix metalloproteinases. J Biol Chem. 1999;274:21491–4.
Kolkenbrock H, Hecker-Kia A, Orgel D, Ulbrich N, Will H. Activation of progelatinase A and progelatinase A/TIMP-2 complex by membrane type 2-matrix metalloproteinase. Biol Chem. 1997;378:71–6.
O-Charoenrat P, Khantapura P. The role of genetic polymorphisms in the promoters of the matrix metalloproteinase-2 and tissue inhibitor of metalloproteinase-2 genes in head and neck cancer. Oral Oncol. 2006;42:257–67.
Wu X, Li H, Kang L, Li L, Wang W, Shan B. Activated matrix metalloproteinase-2-a potential marker of prognosis for epithelial ovarian cancer. Gynecol Oncol. 2002;84:126–34.
Kamel H, Abdelazim I, Habib SM, El Shourbagy MA, Ahmed NS. Immunoexpression of matrix metalloproteinase-2 (MMP-2) in malignant ovarian epithelial tumours. J Obstet Gynaecol Can. 2010;32:580–6.
Abdelazim IA, Al-Kadi M. Immunoexpression of matrix metalloproteinase-2 (MMP-2) in epithelial ovarian cancers (EOCs). Asian Pac J Reprod. 2013;2:136–41.
Lin CK, Chao TK, Yu CP, Yu MH, Jin JS. The expression of six biomarkers in the four most common ovarian cancers: correlation with clinicopathological parameters. APMIS. 2009;117:162–75.
Manenti L, Paganoni P, Floriani I, et al. Expression levels of vascular endothelial growth factor, matrix metalloproteinases 2 and 9 and tissue inhibitor of metalloproteinases 1 and 2 in the plasma of patients with ovarian carcinoma. Eur J Cancer. 2003;39:1948–56.
Cai KQ, Yang WL, Capo-Chichi CD, et al. Prominent expression of metalloproteinases in early stages of ovarian tumorigenesis. Mol Carcinog. 2007;46:130–43.
Torng PL, Mao TL, Chan WY, Huang SC, Lin CT. Prognostic significance of stromal metalloproteinase-2 in ovarian adenocarcinoma and its relation to carcinoma progression. Gynecol Oncol. 2004;92:559–67.
Zhang H. Expression and clinical significance of GLUT-1 and MMP-2 in the tissue of human malignant ovarian tumor. Wanfang Master Thesis Database. 2005.
Wu JX, Zhao Y, Jia SJ, Li YY. Expressions of MMP-2, MMP-3, MMP-9 and CD147 in human ovarian cancer and their clinicopathological significance. J Chongqing Med Univ. 2007;32:1165–8.
Wang J. Expression and significance of osteopontin, MMp-2 in epithelial ovarian tumor. Wanfang Master Thesis Database. 2007.
Luo YH, He AB, Zhang YQ. The unbalanced expression of MMP-2 and TIMP-2 and its correlations with angiogenesis in epithelial ovarian neoplasm. An Hui Yi Xue. 2007;28:88–90.
Zhang Y, Chen Y, Wei L. Expressions of VEGF165 and MMP2 in epithelial ovarian cancer. J Clin Res. 2008;25:1968–70.
Pan Y, Zhu D, Si N, Zhang W. Expressions of RhoC and MMP-2 proteins in epithelial ovarian cancer tissues and their clinical significances. J Jilin Univ. 2008;34:1042–5.
Liu Y, Zhang Y, Kuang H, Kong C. Expression of MMP2 protein in epithelial ovarian cancer tissue. China Trop Med. 2008;8:1694–5.
Zhu G. Expression and significance of OPN, CD44v6, MMp-2 in epithelial ovarian tumor. Wanfang Master Thesis Database. 2008.
Li H, Su Y, Wu J, Zhang J, Dou Z, Li Y. Expression and clinical significance of MMP-2 and VEGF in ovarian carcinoma. Chin J Lab Diagn. 2009;13:726–9.
Sun H, Yan X, Li R, Wang X, Zhang Y. Expression and significance of CXCR4 and MMP-2 in human ovarian epithelial tumors. Zhong Liu Fang Zhi Yan Jiu. 2009;36:747–9.
Wang T. Explore the relationship between VEGF and MMP-2 and ovarian cancer ascites and abdominal metastases. Pract Prev Med. 2009;16:1531–2.
Liu Y, Lv J, Chen Y, Lin M. Expressions of claudin-4 and MMP-2 in epithelial ovarian tumor. Oncol Progr. 2009;7:563–9.
Zeng X, Peng Y. Expression and clinical significance of VEGF, MMP-2 and MMP-9 in ovarian carcinoma. Prog Mod Biomed. 2010;10:2322–4.
Guo J. The expression of HGF, IGF-I and MMP-2 in epithelial ovarian cancer. Wanfang Master Thesis Database. 2010.
Yu L, Wang W, Gan H, Wu S, Song W, Guo B. Expression and clinical significance of MMP-2 and MMP-7 in ovarian carcinoma. Chin J Histochem Cytochem. 2011;20:325–30.
Hu J. Expression of MMP2 and MMP9 in tissue of epithelial tumor of ovary and its clinical significance. Chin Med Mod Distance Educ China. 2011;9:208–10.
Su M, Zhang Y, Huang H, Shi G, Sun F, Qiu Y. Expression of HIF-1A and MMP-2 in vasculogenic mimicry of ovarian cancer. Prog Obstet Gynecol. 2011;2:83–7.
Shen L, Tang L, Zhang J, Zhou S. Expression and significance of WAVE1, MMP-2 and MMP-9 in ovarian cancer. J Third Mil Med Univ. 2013;35:1406–9.
Wang L, Jin X, Lin D, et al. Clinicopathologic significance of claudin-6, occludin, and matrix metalloproteinases -2 expression in ovarian carcinoma. Diagn Pathol. 2013;8:190.
Grelewski PG, Bar JK. The role of p53 protein and MMP-2 tumor/stromal cells expression on progressive growth of ovarian neoplasms. Cancer Invest. 2013;31:472–9.
Li S, Mei J. Clinical significance of MMP-2 and COX-2 expression in epithelial ovarian cancers. J Wannan Med Coll. 2013;32:283–6.
Wu D, Yang J, Yao Y, Zhou Q. Expression and significance of MMP- 2 and its inhibitors in epithelial ovarian carcinoma. Proc Clin Med. 2013;22:650–2.
Brown MR, Blanchette JO, Kohn EC. Angiogenesis in ovarian cancer. Baillieres Best Pract Res Clin Obstet Gynaecol. 2000;14:901–18.
Arnold JM, Cummings M, Purdie D, Chenevix-Trench G. Reduced expression of intercellular adhesion molecule-1 in ovarian adenocarcinomas. Br J Cancer. 2001;85:1351–8.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, C. Pathological and prognostic significance of matrix metalloproteinase-2 expression in ovarian cancer: a meta-analysis. Clin Exp Med 16, 375–382 (2016). https://doi.org/10.1007/s10238-015-0369-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10238-015-0369-y