Abstract
An early event in melanocytic tumor growth is the upregulation of Notch signaling. When an active form of Notch1 is overexpressed in primary human melanocytes, it increases cell growth, survival and invasive properties, promoting melanoma progression. Recent evidence suggested that tumor initiation and growth are driven by a subset of tumor-initiating cells termed cancer stem cells. Notch1 plays a predominant role in the maintenance of melanoblasts, including melanocyte stem cells, by preventing initiation of apoptosis. Moreover, the importance of Notch1 in the regulation of tumor angiogenesis is supported by growing evidence in various cancers. Nestin has been widely used as a marker for melanocyte stem cells as well as an angiogenic marker to evaluate neovascularity of endothelial cells in tumors. To gain an insight into the impact of Notch1 activation on the maintenance of melanocyte stem cells and angiogenesis in melanoma, the expression levels of activated Notch1 and nestin were analyzed by immunohistochemistry in 114 primary cutaneous melanomas and 35 lymph node metastases. Activated Notch1 and nestin expression was also evaluated in four dysplastic melanocytic nevi. This study provides evidence that activated Notch1 is overexpressed in cutaneous melanoma, in tumor cells as well as in microvessel endothelium, and that it can promote tumor angiogenesis. Indeed, the overexpression of activated Notch1 in both tumor and vascular endothelial cells was significantly associated with microvascular density in melanoma samples. Thus, activated Notch1 inhibitors may provide a therapeutic strategy in the treatment of melanoma by blocking tumor-associated vascularization.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Melanoma is a neoplasia with adverse prognosis and no cure in advanced stages. The identification of molecular players in melanoma progression may provide prognostic and therapeutic tools against such an unpredictable tumor. An early event in melanocytic tumor growth is the upregulation of Notch signaling that may sustain melanoma progression [1]. Several groups showed that amplified Notch signaling increases the aggressiveness of primary melanoma cell lines [2, 3]. In particular, a significant upregulation of Notch1, 2 and 4 was found in both melanoma lesions and cell lines compared to normal melanocytes. These findings suggest that Notch alone can be a transforming oncogene in human melanocytes and a “driving” event in melanocytic transformation [4]. It is likely that the metastatic potential that Notch activation confers to primary melanoma cells is, at least in part, mediated by the change in adhesion properties of melanoma cells [2]. Indeed, Notch upregulates the expression of N-cadherin, whose expression is highly correlated with melanoma progression and metastasis [5].
Recent evidence suggested that tumors are composed of heterogeneous cell types and that tumor initiation and growth are driven by a subset of cells termed tumor-initiating cells or cancer stem cells (CSCs) [6]. Notch signaling is implicated in the maintenance of the stem/progenitor cells of a variety of stem cell systems [7]. It seems that genetic alterations in critical signaling pathways that govern stem cells, such as in the Notch one, would allow stem cells to become independent of growth signals or resist antigrowth signals and would lead them to undergo uncontrolled proliferation and tumorigenesis [6]. In particular, Moriyama et al. [8] demonstrated that Notch1 plays a predominant role in the maintenance of melanoblasts (Mbs), including melanocyte stem cells (MSCs), by preventing initiation of apoptosis. Moreover, Notch activates the transcription of nestin, a marker of melanocyte stem cells, directly acting on nestin-regulatory region, in glioma cell cultures. Nestin expression also correlates with the activation of the Notch pathway in vivo [9]. Nestin, as well as being widely used as a marker for stem/progenitor cells, has been reported to be an angiogenic marker in animal models and a marker to evaluate neovascularity of endothelial cells in tumors [10, 11]. At the same time, the importance of Notch signaling in the regulation of tumor angiogenesis is supported by growing evidence [12, 13]. In particular, it has been suggested that tumor-associated growth factors stimulate the direct interaction between tumor cells and endothelial cells through the mitogen-activating protein kinase (MAPK) and Notch signaling pathways, promoting tumor neovascularization and tumor growth in vivo [14]. To date, no studies exploring the relationship between Notch1, nestin and tumor angiogenesis in cutaneous melanoma have been conducted. In this study, we first evaluated the impact of Notch1 activation on the maintenance of melanocyte stem cells and on tumor-associated vascularization, by using the marker nestin for MSCs and CD31 for microvascular density. Second, the expression of activated Notch1 was correlated with the full clinicopathological data of patients.
Materials and methods
Patients and tumor specimens
Archival tissue blocks (114 sporadic primary cutaneous melanomas and 35 lymph node metastases) were obtained from 137 patients: Only the primary tumor was obtained from 102 patients, only the lymph node metastasis from 23 patients, and both the primary tumor and the lymph node metastasis from 12 patients. The patients underwent observation at the Businco Oncologic Hospital, Cagliari, Italy, and at the Department of Pathology, Cancer Center of Solca, Cuenca, Ecuador, from November 1995 through June 2009, and were selected for further study according to the following criteria: melanoma with vertical growth phase and complete clinical data including follow-up until July 2009. Lymph node status and the presence of metastases were verified by a clinical and pathological examination. The clinicopathological characteristics of the 137 Stage I-IV melanoma patients are shown in Table 1 [15, 16]. Following surgical resection, each tumor was fixed in 10 % buffered formalin and paraffin-embedded. Tumoral areas were identified on H&E-stained sections and on adjacent sections immunohistochemically stained for melanoma-associated antigens, including S-100 protein, melan A and HMB-45. An independent histopathological analysis was performed by two pathologists (C.F. and E.M.). The study protocol was approved by the Research Ethics Committee of our Institutions, and informed consent was obtained from all the patients.
The study also included 4 dysplastic melanocytic nevi, one junctional and three compound that were formalin-fixed and paraffin-embedded for the immunohistochemical analysis.
Immunohistochemistry
Serial microtome sections (5-μm) were treated for the immunohistochemical demonstration of activated Notch1 (NIC, Notch intracellular domain), melanocyte stem cell markers nestin and CD133, endothelial cell marker CD31, and melanoma-associated antigens S-100, melan A and HMB-45, using the alkaline phosphatase-streptavidin method. Antigen retrieval was performed by heating at 95 °C for 40 min in 10 mM citrate buffer (pH 6.0) for the demonstration of activated Notch1, CD31 and melan A, and by immersion in 0.1 % trypsin in phosphate-buffered saline (PBS, pH 7.4), at 37 °C for 10 min for CD133, S-100 protein and the HMB-45 antigen. No antigen retrieval was used for nestin immunostaining. Nonspecific binding was blocked with 10 % normal goat or normal horse serum for 45 min. Mouse monoclonal antibodies to human nestin (clone 10C2, 1:1000; Novus Biologicals, Littleton, CO, USA), to human CD31 (clone JC70A, 1:50; Dakopatts, Glostrup, Denmark), to human melan A (clone A103, 1:100; Dakopatts), and to human HMB-45 (clone HMB-45, 1:100; Dakopatts), rabbit polyclonal antibodies to human activated Notch1 (1:100; Abcam plc, Cambridge, UK), to human CD133 (1:1000; Abcam plc) and to bovine S-100 protein (1:1000; Dakopatts) were used as primary antisera. Biotinylated anti-mouse and anti-rabbit immunoglobulins G (1:800 and 1:200, respectively; Vector Laboratories, Burlingame, CA, USA) were used as secondary antisera. The sections were further incubated in alkaline phosphatase-streptavidin (1:1000; Vector Laboratories) for 30 min at room temperature. The liquid permanent red substrate-chromogen system (Dakopatts) was used to develop the alkaline phosphatase reaction product. The sections were counterstained with Carazzi’s hematoxylin. Melanoma specimens that strongly expressed activated Notch1, nestin and CD133 were used as positive controls for activated Notch1, nestin and CD133 immunostaining, respectively. Negative controls were established by replacing the primary antibodies with normal serum. Skin specimens from the eyelid and external auditory canal of healthy donors were used as normal tissues.
Evaluation of immunohistochemical staining
Tumor specimens were examined separately by two pathologists (C.F. and E.M.) under double-blinded conditions without prior knowledge of the clinical status of the patients. For each analyzed section, the two estimates were then averaged to provide the final value of the staining. Because a less mature population of malignant cells with a higher invasive potential accumulates in the advancing edge of the tumor and in the peripheral area of the multiple small nodules within the tumor mass, at the tumor-normal tissue interface [10, 17], we evaluated activated Notch1 immunoreactivity in these areas separately and in combination with the immunoreactivity in the center and throughout the whole tumor. Moreover, activated Notch1 staining was evaluated in endothelial cells of microvessels. To count tumor cells positive for activated Notch1 in the nucleus, in the cytoplasm, or both, and positive endothelial cells, the entire tumor and the advancing edge of each case were microscopically examined through ×200 magnification fields and the evaluation was then confirmed through adjacent microscopic fields at ×400 magnification. For each case, the average of the single counts of positive tumor cells per field was considered. An immunoreactivity score system based on the proportion and intensity of positive tumor cells was applied [18]. (A) Number of positive stained cells ≤25 % (scored 0), 26–50 % (1) and >50 % (2). (B) Intensity of staining: colorless/weak (0), moderate (1) and strong (2). The staining score was obtained by multiplying (A) and (B) and stratified as absent/weak (0–1 score) and moderate/strong (2–4 score). Tumors with moderate/strong immunostaining were classified as positive (activated Notch1 overexpression), whereas tumors with absent/weak immunostaining were classified as negative.
Corresponding microscopic fields on adjacent sections from the same samples were evaluated for nestin immunoreactivity. Cases with nestin-positive tumor cells showing moderate/strong staining intensity and with >10 % of labeled tumor cells were defined as positive; otherwise, they were defined as negative. The 10 % cut-off was chosen because of the possibility that even a relatively small number of nestin-positive tumor cells may play a role in melanoma tumorigenesis. To confirm that nestin-positive tumor cells were melanocyte stem cells, adjacent sections were stained for CD133 and used as control.
The samples were scored as positive for the expression of activated Notch1 or nestin in microvessels (capillaries and small venules) when a moderate/strong staining intensity was found in microvessels throughout the whole tumor or in the peripheral area of small nodules. To confirm that activated Notch1- or nestin-positive microvessels were blood vessels, adjacent sections were stained for CD31 and used as control.
The microvascular density was evaluated in terms of CD31-immunostained microvessels accordingly to the modified Weidner’s method [19]. CD31-positive microvessels were counted in the five ×400 fields with the highest microvascular density. The median value of CD31-positive microvessels was used as a cut-off to distinguish between low and high microvascular density in the tumor tissue.
Double immunofluorescence
Depending on the different properties of each individual antibody, a simultaneous procedure was used for the staining of activated Notch1 and nestin. Following deparaffinization, rehydration, antigen retrieval and blocking of nonspecific binding, sections were incubated overnight at 4 °C with a mixture of rabbit antihuman activated Notch1 (1:25; Abcam plc) and mouse antihuman nestin (clone 10C2, 1:500; Novus Biologicals) antibodies. Donkey Alexa Fluor 488 anti-rabbit IgG (H + L) and Alexa Fluor 594 anti-mouse IgG (H + L) (1:200; Invitrogen Life Technologies, Paisley, UK) were used for immunofluorescence detection. The sections were mounted in Vectashield mounting medium with 4′,6-diamidino-2-phenylindole to visualize nuclear detail (Vector Laboratories). A Zeiss Axioplan 2 (HBO 100 illuminator; mercury vapor, short arc lamp) was used for sample analysis and image processing.
Statistical analysis
Data were computed with the Statistical Package for the Social Sciences (SPSS) 15.0 software. The correlations between the expression of activated Notch1, nestin and microvascular density, or between activated Notch1 and the clinicopathological characteristics of Stage I-IV melanoma patients were assessed by Fisher’s exact or Pearson’s chi-square test. The tests used were two-tailed. For multiple comparisons, the p values were adjusted by using the Bonferroni method.
Results
Activated Notch1 and nestin expression by immunohistochemistry
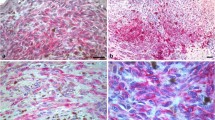
Immunostaining for activated Notch1 revealed a diffuse moderate/strong cytoplasmic staining and scattered or absent positive nuclei (Fig. 1a and b) in most tumor samples. Other cases showed a moderate to strong immunoreactivity both in nuclei and cytoplasm (Fig. 1c, d), or a strong nuclear positivity and a weak or absent cytoplasmic immunostaining (Fig. 1e, f). Positive cells were homogeneously distributed in the center/throughout the tumor and at the invading front of the tumor. Altogether, 32 % of Stage I–II and 51.2 % of Stage III–IV primary tumors strongly expressed activated Notch1 in the nuclei of tumor cells, considering both the center and the front of the tumor. Cytoplasmic activated Notch1 (in the tumor center and/or front) was detected in 58.6 and in 76.9 % of Stage I–II and Stage III–IV primary tumors, respectively. A strong positivity (nuclear and/or cytoplasmic) for activated Notch1 was observable in the center/throughout the tumor in 72 % of Stage I–II and in 89.7 % of Stage III–IV primary tumors. At the same time, the edge of the tumor and the peripheral areas of the small tumor nodules were positive for activated Notch1 (in nuclei and/or in the cytoplasm) in 72 % of Stage I–II and in 84.6 % of Stage III-IV primary tumors. In both Stage I–II and Stage III–IV primary melanomas, there was no difference between the expression of activated Notch1 in the center and at the invading front of the tumor (p = 1.000 and p = 0.736, respectively). For this reason, we considered activated Notch1 (nuclear and/or cytoplasmic) expression in the center and at the advancing edge of the tumor together (total activated Notch1) for further analyses. Regarding lymph node metastases, nuclear activated Notch1 was observed in 10 of 35 (28.5 %) cases and cytoplasmic activated Notch1 in 22 of 35 (62.8 %) samples. Twenty-seven of 35 (77.1 %) nodal metastases were found to be positive for total (nuclear and/or cytoplasmic) activated Notch1 throughout the infiltrating tumor. No significant difference in total activated Notch1 expression was found between primary tumors and lymph node metastases (p = 1.000). Activated Notch1 expression was observed in the endothelium of microvessels (Fig. 2a, b) in 46.6 % of Stage I–II, in 53.8 % of Stage III–IV primary tumors and in 37.1 % of lymph node metastases. In most tumors, activated Notch1-positive microvessels were also immunostained for the endothelial cell marker CD31. The frequencies of the expression of activated Notch1 in tumor cells and microvessels are shown in Table 2. Pigmented melanoma cells were negative for activated Notch1 in the majority of cases, although some cases showed pigmented cells with a weak to strong immunostaining.
Representative immunohistochemical staining for activated Notch1 in melanoma samples. a, b Diffuse moderate/strong staining in the cytoplasm of tumor cells and scattered or absent positive nuclei (original magnification: a ×400; b ×630). c, d Moderate to strong immunoreactivity both in nuclei and cytoplasm (original magnification: c ×400; d ×630). e, f Strong nuclear positivity and weak or absent cytoplasmic staining (original magnification: e ×200; f ×400)
A nuclear and/or cytoplasmic positivity for activated Notch1 was observed in the majority of melanocytes in all dysplastic melanocytic nevi, including junctional and compound ones. Activated Notch1 expression was observed in the endothelium of microvessels in all the compound nevi, but not in the junctional nevus.
Nestin expression was observable in the cytoplasm of tumor nonpigmented cells and in vascular endothelial cells. Nestin-positive tumor cells and microvessels were present throughout the tumor and increased in the peripheral, invasive areas of primary tumors. Cases positive for nestin in tumor cells included 56 % of Stage I–II, 53.8 % of Stage III–IV primary tumors and 60 % of lymph node metastases. Nestin-positive microvessels were observable in 42.6 % of Stage I–II, in 48.7 % of Stage III–IV primary tumors and in 48.5 % of nodal metastases, respectively (Table 2).
Nestin-positive melanocytes were observed in all the compound nevi, but not in the junctional nevus, while nestin-positive microvessels were present in the junctional nevus and in 2 out of 3 compound nevi.
Activated Notch1 and nestin expression by double immunofluorescence
Activated Notch1-positive cells were located in the same tumor areas where nestin-positive cells were distributed (Fig. 3a). Tumor cells mostly showed a colocalization of activated Notch1 and nestin in the cytoplasm (Fig. 3b), although a codistribution of nuclear activated Notch1 and cytoplasmic nestin was also observable (Fig. 3c). Nestin-positive microvessels were distributed close to tumor cells showing positivity for activated Notch1 in the cytoplasm and mostly in the nucleus (Fig. 3d), and they often showed a codistribution of activated Notch1 and nestin in endothelial cells (Fig. 3b).
Double-label immunofluorescence staining for activated Notch1 and nestin in melanoma samples. a Activated Notch1-positive cells (green) in the same tumor areas where nestin-positive cells (red) are distributed (original magnification: ×400). b Colocalization of activated Notch1 and nestin (yellow) in the cytoplasm of tumor cells (arrows) and codistribution of activated Notch1 and nestin in the same blood vessels (arrowheads; original magnification: ×400). c Codistribution of nuclear activated Notch1 and cytoplasmic nestin in tumor cells (arrows; original magnification: ×630). d Blood vessels with nestin-positive endothelium (arrowheads) are distributed close to tumor cells stained for activated Notch1 (arrows; original magnification: ×400). The nuclei were counterstained with DAPI (blue)
Activated Notch1 expression in normal tissues
In normal skin, activated Notch1 was diffusely expressed in the cytoplasmic compartment of basal and suprabasal epidermal keratinocytes, while nuclear activated Notch1 was confined to suprabasal epidermal keratinocytes (Fig. 4a). In areas of apparently normal skin adjacent to melanoma, a weak/moderate cytoplasmic and a strong nuclear immunostaining were diffused in basal and suprabasal epidermal keratinocytes, while normal epidermal melanocytes were negative (Fig. 4b). A high expression level of activated Notch1 was consistently observed in adnexal structures, including hair follicle epithelium, sebaceous (Fig. 4c) and sweat glands (Fig. 4d).
Immunohistochemical staining for activated Notch1 in normal skin. a Activated Notch1 expression in normal skin is observed in the cytoplasm of basal and suprabasal epidermal keratinocytes, while nuclear activated Notch1 is confined to suprabasal epidermal keratinocytes (original magnification: ×400). b In areas of apparently normal skin adjacent to melanoma, a weak/moderate cytoplasmic and a strong nuclear immunostaining are diffused in basal and suprabasal epidermal keratinocytes, while normal epidermal melanocytes are negative (original magnification: ×400). High expression of activated Notch1 in adnexal structures, including (c) hair follicle epithelium, sebaceous and (d) sweat glands (original magnification: c, d ×400)
Associations between activated Notch1 and nestin expression
After the p values for multiple comparisons were adjusted by using the Bonferroni method, total activated Notch1 expression in tumor cells was significantly associated with nestin expression in vascular endothelial cells (p < 0.0003) but not with nestin expression in tumor cells (p = 0.087). The number of microvessels positive for activated Notch1 was increased in the cases that overexpressed total activated Notch1 in tumor cells. Moreover, activated Notch1-positive microvessels were more frequent in the tumors that showed nestin-positive microvessels. However, these differences did not reach statistical significance (p = 0.141) (Table 3).
Associations of activated Notch1 and nestin expression with microvascular density
The extent of angiogenesis in the tumors, evaluated as microvascular density, was statistically correlated with the expression of total activated Notch1 both in tumor and in vascular endothelial cells (p = 0.006 and p = 0.004, respectively) as well as to nestin expression in the endothelium of microvessels (p = 0.004) (Table 4).
Table 5 shows the expression of total activated Notch1 in relation to the clinicopathological characteristics of patients, even though they did not result significantly associated (p > 0.007).
Discussion
When an active form of Notch1 (NIC) is overexpressed in primary human melanocytes, it significantly accelerates cell growth in vitro and increases melanocyte survival in growth-limiting conditions. Interestingly, in association with these acquired growth/survival characteristics, melanocytes infected with NIC show morphological changes reminiscent of vasculogenic mimicry and increased invasion, suggesting that Notch1 promotes the malignant transformation of melanocytes. Activated Notch1 specifically enhances vertical growth phase (VGP) melanoma cell proliferation. Morphologic changes in VGP melanoma cells might reflect an increased cell adhesion capability, conferring a more aggressive phenotype to the disease [2].
Notch1, acting through the hairy and enhancer of split 1 (Hes1) transcription factor, participates in the maintenance of melanocyte stem cells, by preventing initiation of apoptosis [8]. The Notch pathway has been shown to play a particular role in mammary stem cell expansion and to promote breast cancer progression, by supporting epithelial-to-mesenchymal transition [20]. Even though the difference did not reach statistical significance, probably because of a low number of patients analyzed, most of the tumors that overexpressed activated Notch1 in tumor cells also showed a high number of nestin-positive tumor cells. This may suggest that Notch1 contributes to the maintenance of MSCs in melanoma. Thus, activated Notch1 inhibitors might target cells, such as MSCs, with tumor-initiating features and may provide a therapeutic strategy for eliminating surviving cells, hence preventing tumor recurrence and improving long-term survival in melanoma patients. Indeed, recent studies reported that Notch inhibition by therapeutic Notch1 monoclonal antibodies may have an anti-CSC activity with a potential clinical benefit in the treatment of breast cancer [21]. Even in glioblastoma, Notch blockade by γ-secretase inhibitors reduces the percentage of cells expressing the CSC markers nestin and CD133 and depletes stem-like cancer cells through reduced proliferation and increased apoptosis [22].
The involvement of Notch signaling has also been demonstrated in the regulation of physiological as well as tumor angiogenesis, one of the requirements for tumor growth and metastasis [12, 13, 23]. The current model of endothelial angiogenesis centers on the interplay between “tip” and “stalk” cell characters, and Notch signaling is central to the establishment of these identities, sustaining stalk cells development [24–26]. In head and neck squamous cell carcinoma, Jagged1 is highly expressed and is induced through the MAPK pathway. The elevated Jagged1 expression levels on tumor cells trigger Notch activation in neighboring endothelial cells and promote the network formation [14]. In breast cancer, estrogen-upregulated Jagged1 and Notch1 expression in MCF7 breast cancer cells and in endothelial cells promotes sprouting in endothelial cells. Indeed, Notch1- and Jagged1-expressing cells or a constitutive activated form of Notch1 results in differentiation of endothelial cells into vessel-like structures. Moreover, Notch1 gene interestingly clusters with the hypoxia-inducible factor 1α (HIF1α) gene, a transcription factor stabilized during hypoxia that activates several angiogenic genes acting in a paracrine manner in endothelium [27].
This study is the first to examine the relationship between Notch1 activation and angiogenesis in cutaneous melanoma. We provided evidence that the expression of activated Notch1 both in tumor and in vascular endothelial cells is associated with the extent of angiogenesis. This may suggest that tumor-associated growth factors stimulate an interaction between tumor cells and endothelial cells also in melanoma, with Notch1 activating the transcription of angiogenic genes and promoting angiogenesis in a paracrine manner. Moreover, the expression of activated Notch1 in endothelial cells of microvessels confirms its key role in tumor angiogenesis, accordingly to previous findings. The correlation between the extent of angiogenesis and nestin expression in the endothelium of microvessels may confirm that, also in melanoma, nestin can be used as a marker of endothelial proliferation, as suggested by previous studies [11].
In conclusion, our results indicate that activated Notch1 is overexpressed in cutaneous melanoma and that it can promote tumor angiogenesis. Understanding the mechanisms of melanoma angiogenesis provides a basis for the development of antiangiogenic therapies. In light of our findings, activated Notch1 appears to be a potential drug target for disrupting tumor-associated vasculature and indicates Notch1 inhibition as a promising and effective avenue for the treatment of melanoma.
References
Massi D, Tarantini F, Franchi A, et al. (2006) Evidence for differential expression of Notch receptors and their ligands in melanocytic nevi and cutaneous malignant melanoma. Mod Pathol 19: 246–254. Erratum in: Mod Pathol 19: 616.
Liu ZJ, Xiao M, Balint K, et al. Notch1 signaling promotes primary melanoma progression by activating mitogenactivated protein kinase/phosphatidylinositol 3-kinase-Akt pathways and up-regulating N-cadherin expression. Cancer Res. 2006;66:4182–90.
Bedogni B, Warneke JA, Nickoloff BJ, Giaccia AJ, Powell MB. Notch1 is an effector of Akt and hypoxia in melanoma development. J Clin Invest. 2008;118:3660–70.
Pinnix CC, Lee JT, Liu ZJ, et al. Active Notch1 confers a transformed phenotype to primary human melanocytes. Cancer Res. 2009;69:5312–20.
Panelos J, Massi D. Emerging role of Notch signaling in epidermal differentiation and skin cancer. Cancer Biol Ther. 2009;8:1986–93.
Ailles LE, Weissman IL. Cancer stem cells in solid tumors. Curr Opin Biotechnol. 2007;18:460–6.
Molofsky AV, Pardal R, Morrison SJ. Diverse mechanisms regulate stem cell self-renewal. Curr Opin Cell Biol. 2004;16:700–7.
Moriyama M, Osawa M, Mak SS, et al. Notch signaling via Hes1 transcription factor maintains survival of melanoblasts and melanocyte stem cells. J Cell Biol. 2006;173:333–9.
Shih AH, Holland EC. Notch signaling enhances nestin expression in gliomas. Neoplasia. 2006;8:1072–82.
Kim HS, Kang HS, Messam CA, et al. Comparative evaluation of angiogenesis in gastric adenocarcinoma by nestin and CD34. Appl Immunohistochem Mol Morphol. 2002;10:121–7.
Brychtova S, Fiuraskova M, Hlobilkovà A, et al. Nestin expression in cutaneous melanomas and melanocytic nevi. J Cutan Pathol. 2007;34:370–5.
Koch U, Radtke F. Notch and cancer: a double-edged sword. Cell Mol Life Sci. 2007;64:2746–62.
Rehman AO, Wang CY. Notch signaling in the regulation of tumor angiogenesis. Trends Cell Biol. 2006;16:293–300.
Zeng Q, Li S, Chepeha DB, et al. Crosstalk between tumor and endothelial cells promotes tumor angiogenesis by MAPK activation of Notch signaling. Cancer Cell. 2005;8:13–23.
Greene FL, Page DL, Fleming ID, et al. American Joint Committee on Cancer Staging Manual. 6th ed. Philadelphia: Springer; 2002.
Clark WH Jr, Elder DE, Guerry D IV, et al. Model predicting survival in stage I melanoma based on tumor progression. J Natl Cancer Inst. 1989;81:1893–904.
Piras F, Perra MT, Murtas D, et al. The stem cell marker nestin predicts poor prognosis in human melanoma. Oncol Rep. 2010;23:17–24.
Chu D, Zhou Y, Zhang Z, et al. Notch1 expression, which is related to p65 Status, is an independent predictor of prognosis in colorectal cancer. Clin Cancer Res. 2011;17:5686–94.
Ranieri G, Ammendola M, Patruno R, et al. Tryptase-positive mast cells correlate with angiogenesis in early breast cancer patients. Int J Oncol. 2009;35:115–20.
Sethi N, Kang Y. Notch signalling in cancer progression and bone metastasis. Br J Cancer. 2011;105:1805–10.
Qiu M, Peng Q, Jiang I, et al. Specific inhibition of Notch1 signaling enhances the antitumor efficacy of chemotherapy in triple negative breast cancer through reduction of cancer stem cells. Cancer Lett. 2013;328:261–70.
Fan X, Khaki L, Zhu TS, et al. NOTCH pathway blockade depletes CD133-positive glioblastoma cells and inhibits growth of tumor neurospheres and xenografts. Stem Cells. 2010;28:5–16.
Yin L, Velazquez OC, Liu ZJ. Notch signaling: emerging molecular targets for cancer therapy. Biochem Pharmacol. 2010;80:690–701.
Tung JJ, Tattersall IW, Kitajewski J. Tips, stalks, tubes: notch-mediated cell fate determination and mechanisms of tubulogenesis during angiogenesis. Cold Spring Harb Perspect Med. 2012;2:a006601. doi:10.1101/cshperspect.a006601.
Ribatti D, Crivellato E. “Sprouting angiogenesis”, a reappraisal. Dev Biol. 2012;372:157–65.
Beets K, Huylebroeck D, Moya IM, et al. Robustness in angiogenesis: notch and BMP shaping waves. Trends Genet. 2013;29:140–9.
Soares R, Balogh G, Guo S, et al. Evidence for the notch signaling pathway on the role of estrogen in angiogenesis. Mol Endocrinol. 2004;18:2333–43.
Acknowledgments
This work was supported by grants from Ministero Affari Esteri (MAE) and Fondazione Banco di Sardegna (to PS and MTP). The study was also supported by Regione Autonoma della Sardegna, Master and Back Program (grant to DM). Particular thanks are due to Itala Mosso and Massimo Annis for their expert technical assistance.
Ethical standards
The study protocol was approved by the Research Ethics Committee of our Institutions, and all the patients gave their informed consent prior to their inclusion in the study.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Murtas, D., Piras, F., Minerba, L. et al. Activated Notch1 expression is associated with angiogenesis in cutaneous melanoma. Clin Exp Med 15, 351–360 (2015). https://doi.org/10.1007/s10238-014-0300-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10238-014-0300-y